Abstract
A role for the cerebellum in cognition has been proposed based on studies suggesting a profile of cognitive deficits due to cerebellar stroke. Such studies are limited in the determination of the detailed organisation of cerebellar subregions that are critical for different aspects of cognition. In this study we examined the correlation between cognitive performance and cerebellar integrity in a specific degeneration of the cerebellar cortex: Spinocerebellar Ataxia type 6 (SCA6). The results demonstrate a critical relationship between verbal working memory and grey matter density in superior (bilateral lobules VI and crus I of lobule VII) and inferior (bilateral lobules VIIIa and VIIIb, and right lobule IX) parts of the cerebellum. We demonstrate that distinct cerebellar regions subserve different components of the prevalent psychological model for verbal working memory based on a phonological loop. The work confirms the involvement of the cerebellum in verbal working memory and defines specific subsystems for this within the cerebellum.
Keywords: SCA-6, Cerebellum, Cognition, MRI, VBM, Neurodegeneration
1. Introduction
The traditional role of the cerebellum is one of motor control and coordination. Recently this role has been augmented with a function in higher-order cognitive processes such as language, verbal memory, visuospatial functions and verbal and non-verbal executive functions (e.g. Chiricozzi, Clausi, Molinari, & Leggio, 2008; Durisko & Fiez, 2010; Fabbro et al., 2004; Gottwald, Wilde, Mihajlovic, & Mehdorn, 2004; Hayter, Langdon, & Ramnani, 2007; Konczak & Timmann, 2007; Leggio et al., 2008; Ravizza et al., 2006; Riva & Giogi, 2000; Silveri, Di Betta, Filippini, Leggio, & Molinari, 1998; Tavano et al., 2007).
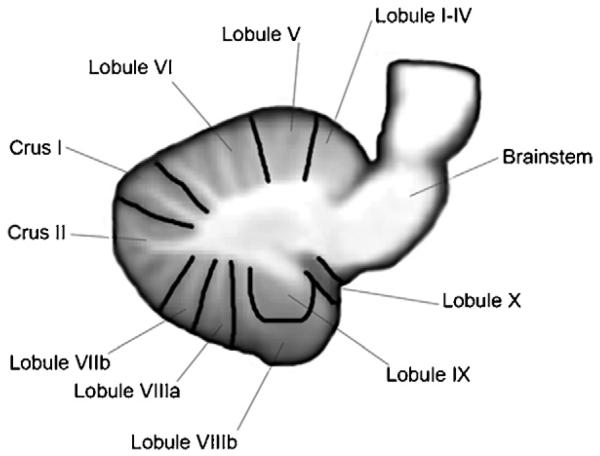
The cortex and the cerebellum are connected via a two stage feedforward and feedback loop. The feedforward loop consists of projections (corticopontine) from the cortex to the pontine nucleus and then from the pontine nucleus to the cerebellum nuclei. The cerebellum in turn projects back to the cortex via the thalamus. Both anatomical and functional studies in animals and humans show that the cerebellum is not only connected to the motor areas of the cortex but also receives inputs and project back to the associative and prefrontal areas of the cortex (Schmahmann, 1996; Schmahmann & Pandya, 1997). In fact tract tracing using diffusion tensor imaging (DTI) in humans show that proportion of connections to the cerebellum from the frontal areas is greater than the proportion from motor areas (Ramnani et al., 2006). Moreover, connections between the cerebellum and the cortex are topographically organised with the anterior cerebellum (lobules I–IV) (Adrian, 1943; Schmahmann, Lober, & Hurwitz, 2002; Schoch, Dimitrova, Gizwiski, & Timmann, 2006; Snider & Eldred, 1952) receiving majority of its input from the sensory motor area whereas the posterior lobe of the cerebellum is connected more to the associative and prefrontal areas of the cortex (see Fig. 1 for cerebellar anatomy). This pattern of connectivity therefore points to a role of the cerebellum in sensory and cognitive tasks in addition to its motor-based tasks.
Fig. 1. Anatomy of the cerebellum.
Sagittal view (x = 14) showing lobules of the cerebellum using the nomenclature introduced by Schmahmann, Doyon, Toga, Petrides, and Evans (2000). Image of the cerebellum from the SUIT atlas developed by Diedrichsen (2006).
Theories detailing how the cerebellum contributes to motor control are well established; however, little is known about the theoretical model underlying the cognitive role of the cerebellum. The homogeneous cytoarchitecture of the cerebellum allows a similar computation to be performed upon all information passing through it (Ramnani, 2006; Schmahmann, 2004). Models of motor control processing might therefore be applied to understand how the cerebellum might process information. Ramnani (2006) details a model of cerebellar cognitive function based upon an internal model of cerebellar motor control via error correction: cerebellar forward models. Information exchanged across cortical networks is transmitted as an efference copy to specific target areas (forward models) within the cerebellum via the cerebrocerebellar inter-connections. The receiving cerebellar forward model ‘simulates’ information processing in the cortical target, allowing it to identify and correct errors within the cortical network via feedback. This theory predicts that specific areas of the cerebellum will represent individual forward models, dependent on the cortical origin of the received projection; however, the precise organisation of the cerebellar components for cognitive functions is unknown.
The current study investigated this by correlating cerebellar grey matter volume and cognitive performance in a group of patients with a pure cerebellar degeneration: Spinocerebellar Ataxia type 6 (SCA-6). SCA-6 is a slowly progressive, genetic neurodegenerative condition where damage is restricted to the Purkinje cells of the cerebellum (Zhuchenko et al., 1997). The degeneration is characterised by a stereotyped, dorso-ventral progression in the cerebellar cortex (Butteriss, Chinnery, & Burchall, 2005), with usually no significant white matter loss or degeneration in extracerebellar areas including cortical areas (Ishikawa et al., 1999; Lukas et al., 2006; Nanri et al., 2010; Schlos et al., 1998; Schulz et al., 2010; Zhuchenko et al., 1997). Interpretation of previous studies has been limited by the use of heterogeneous patient groups with lesions (especially strokes) in which the cerebellum is not affected in the same way across patients, and where extracerebellar damage may also occur. The confinement of degeneration to the cerebellar cortex in SCA-6 makes this an excellent model to investigate the contributions of particular cerebellar areas to specific cognitive processes, using Voxel Based Morphometry.
Voxel Based Morphometry (VBM) is a method of brain image analysis that essentially allows detection of local changes in the composition of brain tissues (grey and white matter or CSF) which may also be correlated with behavioural performance (Ashburner & Friston, 2000). A group of 15 SCA-6 patients participated in a comprehensive battery of internationally standardized, age corrected, neuropsychometric tests measuring current intellectual function, auditory and visual memory, and verbal and non-verbal executive functions. These patients then underwent high resolution structural MRI. The particular aim of this study was to compare the amount of cerebellar degeneration with cognitive performance. Therefore, the correlational design of this study, excludes the need for a control group of healthy, age-matched subjects.
This method allows the test of the hypotheses that the grey matter volume of the superior (lobules V–crus I) and posterior-inferior (crus II–lobule X) cerebellar lobes is correlated with cognitive performance. Based on previous work investigating the cognitive role of the cerebellum in healthy volunteers, cerebellar stroke and tumour patients, we predicted performance on language dependent tasks to correlate with grey matter density in the right superior-posterior cerebellum (lobules V–VII) (Lidzba, Wilke, Staudt, Krageloh-Mann, & Grood, 2008; Schmahmann et al., 2002), verbal working memory bilaterally in lobules V, VI, VII and VIII (Chen & Desmond, 2005; Chiricozzi et al., 2008; Desmond & Fiez, 1998; Ravizza et al., 2006) and non-verbal, spatial function with the left posterior lobules VI–X (Konczak & Timmann, 2007; Leggio et al., 2008).
2. Methods
2.1. Subjects
A group of 15 patients with genetically confirmed SCA-6 took part in this study: 5 males, 10 females, age range 47–75 (mean = 64 years, standard deviation = 8 years), with a mean disease duration of 8 years (standard deviation = 5 years). Supplemental Table 1 (see supplemental material) provides demographic details for each individual patient. Two subjects had not yet developed the motor symptoms associated with this disease, and so were asymptomatic at the time of the scan. No lesion or atrophy was noted in any of the subjects in supratentorial structures, with degeneration confined to the cerebellum. Within this group, cerebellar degeneration was found to be correlated with disease duration with the left superior and posterior lobe and the right posterior lobe (see Supplemental Table 2 for further details), consistent with the studies mentioned above. Furthermore, we have carried out a correlational analysis to show areas of atrophy within the whole brain, as a function of disease duration. No supratentorial areas were demonstrated. Subjects received travel and expenses reimbursement. This study was approved by the Newcastle and North Tyneside 2 Research Ethics Committee. All subjects gave informed consent.
2.2. Neuropsychological assessment
All 15 patients participated in a comprehensive neuropsychological assessment including assessment of current intellectual function using the UK edition of the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1999a); memory using the UK edition of the Wechsler Memory Scale-III (WMS-III; Wechsler, 1999b) and executive functioning using the Brixton Test of Spatial Anticipation (Burgess & Shallice, 1997) and the Delis and Kaplin Executive Functions System (D-KEFS; Delis, Kaplin, & Kramer, 2001). The tests were administered according to the protocols described in the manuals. The assessment was run over 2–3 sessions not lasting more than 2 h each to limit fatigue in the participants. Most patients were scanned within a month of their neuropsychological assessment; however, for 3 patients there was a gap of just over 1 year between their assessment and the scan. This disease (SCA6) is characterised by a slowly progressive disorder, with symptoms developing over 20-30 years (Zhuchenko et al., 1997). Therefore one year represents a very small proportion of the time over which symptoms develop, suggesting this gap will have a limited impact upon the results. In the case of the D-KEFS subtests, not all subjects could complete some of the tests of executive functions due to motor difficulties. Therefore we present here results for the tasks that do not depend on motor skill and speed (D-KEFS subtests of Verbal Fluency, Colour/Word Interference, Tower, Word in Context and Card Sorting) for the 14 patients who could complete these subtests. See Supplemental Tables 3-8 for cognitive performance of individual patients.
2.3. MRI acquisition
Scans were acquired from all subjects using a 1.5 T Philips NT Intera scanner and a 5 channel phased array head coil. The subject’s head was held in place by a head brace to prevent movement. High resolution anatomical scans (3D T1 gradient echo, 180 slices, 0.94 mm × 0.94 mm × 0.94 mm plane resolution and slice thickness,TR = 14 ms, TE = 6.5 ms, flip angle = 22°) were acquired for each subject.
2.3.1. Image analysis
Image analysis was carried out using the Statistical Parametric Mapping software, SPM5 (http://www.fil.ion.ucl.ac.uk/spm) running on Matlab R2007b (The Mathworks, Natick, MA).
2.3.2. Image pre-processing
Image pre-processing followed the DARTEL based VBM which was developed to achieve a more accurate inter-subject registration of brain images (Ashburner, 2007). In addition, the DARTEL based analysis is considered to avoid potential biases within the cerebellum (D’Agata et al., 2011).
All images were re-orientated so that the origin coordinates lay over the anterior commissure. The images were then segmented into grey matter, white matter and CSF tissues using the unified segmentation procedure (Ashburner & Friston, 2005). The segmented grey matter images of all the subjects were used to create a grey matter template using an iterative and nonlinear registration implemented in the DARTEL toolbox. After the template was registered to the MNI template, individual grey matter images were normalised to this template. As normalisation can cause volumes of certain areas to grow or shrink, the spatially normalised grey matter signal of each segmented image was modulated by multiplying the relative volume before and after normalisation. This means that the total amount of grey matter signal in the normalised images is preserved. These images were finally smoothed using an isotropic Gaussian kernel of 6 mm Full Width Half Maximum (FWHM).
2.4. Statistical analysis
Correlations between cerebellar grey matter volume and performance on the neuropsychological tests were sought using a small volume correction for the cerebellum. The small volume correction mask was applied using the maximum probabilistic map of the human cerebellum in MNI space (Diedrichsen, Balsters, Flavell, Cussans, & Ramnani, 2009).
Statistical Parametric Maps (SPM) were created for each composite index score of the WAIS-III and WMS-III, the Brixton standard score and the subtests of the D-KEFS, using a statistical level of p ≤ 0.001 (uncorrected for multiple comparisons) using a small volume correction for the cerebellum. It is appropriate to not correct for multiple comparisons here due to the presence of a priori hypotheses for each correlation. To avoid a Type II error, a conservative extent threshold of 100 voxels was applied. Total intracranial volume was included as a nuisance variable. Cerebellar areas were identified using the probabilistic cerebellar atlas in MRIcron (Diedrichsen et al., 2009). Results are displayed using the SUIT template of the cerebellum (Diedrichsen, 2006).
3. Results
3.1. Neuropsychological performance
Summary statistics of the group performance on each cognitive test are reported in Tables 1-3. In terms of intellectual and memory ability, group performance on most indices falls within the range of achievement expected by 82.2% of the general population (defined by an index score within the range of 80–120, Wechsler, 1999a, 1999b), with a very small number of patients (n = 1–2) falling within the impaired range for these tasks. The remaining patients scored within, or above, the 80–120 index score range on the cognitive indices measured by the WAIS-III and WMS-III (see Tables 1 and 2). A higher number of patients (maximum n = 5) show impaired performance on the tests of executive function (see Table 3). This pattern of cognitive performance has previously been characterised in SCA-6 patients (Cooper et al., 2010; Garrard, Martin, Giunti, Cipolotti, 2008; Globas et al., 2003; Kawai et al., 2008; Suenga et al., 2008).
Table 1. Mean, standard deviation (SD) and range of group performance on WAIS-III composite scores.
Normal mean standardized score = 100, SD= 15. Number of patients impaired defined by an index score < 80 (Wechsler, 1999a, 1999b).
| IQ/index scale | n | Mean(SD) | Range | Number impaired |
|---|---|---|---|---|
| Verbal Comprehension | 15 | 99.73 (10.92) | 78–118 | 1 |
| Perceptual Organisation | 15 | 107.13(14.46) | 89–138 | 0 |
| Verbal Working Memory | 15 | 99.53(14.18) | 80–130 | 0 |
| Processing Speed | 15 | 94.40(10.72) | 79–114 | 1 |
Table 2. Mean, standard deviation (SD) and range of group performance on WMS-III composite scores.
Normal mean standardized score = 100, SD= 15. Number of patients impaired defined by an index score < 80 (Wechsler, 1999a, 1999b).
| Index scale | n | Mean (SD) | Range | Number impaired |
|---|---|---|---|---|
| Auditory | 15 | 99.40(18.21) | 62–130 | 2 |
| Immediate | ||||
| Visual Immediate | 15 | 100.60(18.95) | 57–127 | 1 |
| Immediate Memory | 15 | 98.53 (20.56) | 51–130 | 2 |
| Auditory Delayed | 15 | 98.73 (16.92) | 58–128 | 2 |
| Visual Delayed | 15 | 101.87(14.99) | 72–132 | 1 |
| Auditory | 15 | 100.67(11.93) | 80–120 | 0 |
| Recognition | ||||
| Delayed | ||||
| General Memory | 15 | 101.07(17.12) | 63–137 | 2 |
| Working Memory | 15 | 107.07(14.35) | 88–127 | 0 |
Table 3. Mean, standard deviation (SD) and range of group performance on D-KEFS subtest scores and Brixton test of Spatial Anticipation scores.
D-KEFS: normal mean standardized score = 10, SD = 3; Brixton Test of Spatial Anticipation: normal mean standardized score = 100, SD= 15. Number of patients impaired defined by a standard score < 7 (D-KEFS) or < 80 (Brixton Test of Spatial Anticipation).
| Executive function subtest | Subtest condition | n | Mean (SD) | Range | Number impaired |
|---|---|---|---|---|---|
| Letter Fluency | 14 | 8.93 (4.09) | 4–18 | 4 | |
| Verbal Fluency (D-KEFS) | Category Fluency | 14 | 9.93 (4.20) | 4–19 | 3 |
| Category Switching Accuracy | 14 | 10.93 (3.39) | 4–16 | 2 | |
| Colour Naming | 14 | 7.79 (4.68) | 1–14 | 6 | |
| Colour/Word Interference (D-KEFS) | Colour Word Reading | 14 | 8.43 (4.05) | 1–14 | 5 |
| Inhibition | 14 | 8.00 (4.10) | 1–14 | 5 | |
| Free Sort | 14 | 10.64(2.79) | 6–16 | 1 | |
| Card Sort (D-KEFS) | Free Sort Description | 14 | 9.86 (3.46) | 5–17 | 3 |
| Sort Recognition | 14 | 8.07 (1.94) | 5–12 | 3 | |
| Word in Context (D-KEFS) | Number Consecutively Correct | 14 | 9.86 (2.35) | 5–13 | 2 |
| Tower (D-KEFS) | Total Achievement | 14 | 9.71 (2.67) | 6–15 | 3 |
| Brixton test of Spatial Anticipation | Number of Errors | 15 | 92.43(22.11) | 41–131 | 4 |
3.2. Correlation of cerebellar grey matter volume with working memory
Verbal working memory was assessed using four subtests from the WAIS-III: Forwards Digit Span (recall a string of numbers immediately after presentation); Backwards Digit Span (recall a string of numbers in reverse immediately after presentation); Letter/Number Sequencing (recall a mixed string of letters and numbers, with the letters in alphabetical order, followed by the numbers in numerical order) and a task of mental arithmetic. Visuospatial working memory was assessed using the Block Tapping Test (recall the sequence of blocks tapped by the examiner (a) in order and (b) in reverse order) from the WMS-III.
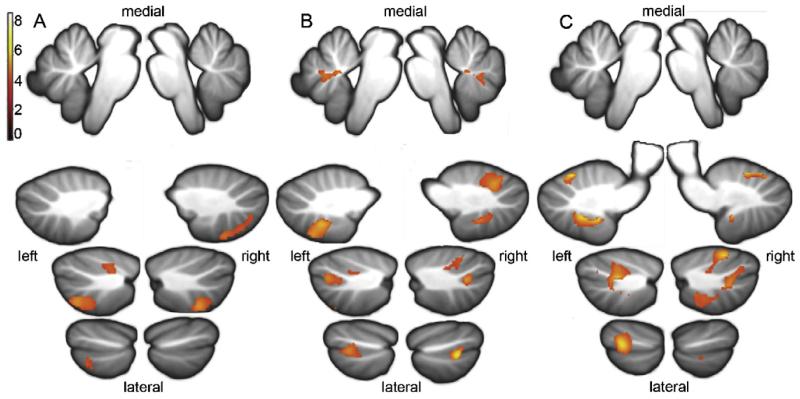
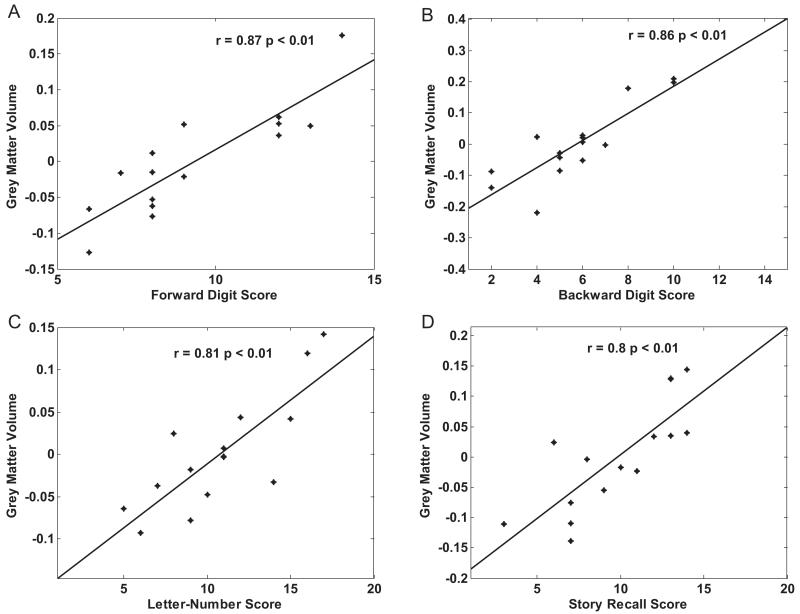
Here we present correlations of the verbal and visuospatial working memory tasks with cerebellar grey matter volume. A small volume correction of the cerebellum was applied using the maximum probabilistic map of the human cerebellum (Diedrichsen et al., 2009). Correlations are displayed in Fig. 2 using the SUIT template of the cerebellum (Diedrichsen, 2006). Regression plots detailing the striking correlations between cerebellar grey matter volume and individual working memory scores are displayed in Fig. 4.
Fig. 2. Correlation of cerebellar grey matter volume with working memory.
Anatomical location of correlations between cerebellar grey matter density and performance on subtests of verbal working memory (from WAIS-III): p ≤ 0.001 (uncor), k = 100, n = 15. (A) Forward Digit Span subtest from top to bottom and left to right: x = −2, x = 2, x = −20, x = 20, x = −29, x = 29, x = −39, x = 39; (B) Backward Digit Span: from top to bottom and left to right: x = −2, x = 2, x = −17, x = 17, x = −27, x = 27, x = −37, x = 37; (C) Letter/Number Sequencing: from top to bottom and left to right x = −2, x = 2, x = −14, x = 14, x = −29, x = 29, x = −42, x = 42 (MNI coordinates).
Fig. 4. Correlation of cerebellar grey matter volume and individual memory subtests.
Regression plots of localised maxima of cerebellar grey matter volume and individual memory subtest score: (A) Forward Digit Span (MNI coordinates: −35, −70, −56); (B) Backward Digit Span (MNI coordinates: 35, −68, −43); (C) Letter/Number Sequencing (MNI coordinates: 37, −53, −55); (D) Story Recall (MNI coordinates: 35, −73, −36).
3.2.1. Verbal working memory
Significant positive correlations were observed between cerebellar grey matter volume and performance on three out of the four verbal working memory subtests from the WAIS-III (Forward Digit Span, Backward Digit Span and Letter-Number Sequencing—see Fig. 2A–C respectively). No correlations were found for the Mental Arithmetic subtest.
Positive correlations were found between the cerebellar grey matter volume and performance on the Forward Digit Span task in superior left lobule VI, bilateral lobule VIIb and right posterior crus II and lobule VIIIa (see Table 4 and Fig. 2A).
Table 4. Anatomical localisation of correlation clusters between cerebellar grey matter density and Working Memory subtest scores.
Coordinates are in MNI space (p ≤0.001 uncor, k = 100, n =15). T values represent the strength of the correlation.
| Subtest | Cerebellar region | Cluster size | T score | x | y | z |
|---|---|---|---|---|---|---|
| Forward Digit Span | Left lobule VI | 201 | 5.10 | −34 | −46 | −32 |
| 4.31 | −24 | −60 | −30 | |||
| Left lobule Vllb | 466 | 6.06 | −35 | −70 | −56 | |
| Right crus II | 593 | 4.64 | 15 | −81 | −47 | |
| 4.32 | 21 | −80 | −50 | |||
| Right lobule VIIb | 4.63 | 23 | −73 | −56 | ||
| 4.29 | 8 | −75 | −45 | |||
| 4.11 | 35 | −73 | −52 | |||
| Right lobule VIIIa | 5.58 | 27 | −65 | −56 | ||
| 4.89 | 19 | −62 | −59 | |||
| Backward Digit Span | Left lobule VI | 674 | 5.29 | −35 | −54 | −29 |
| 4.34 | −29 | −59 | −36 | |||
| Left crus I | 5.23 | −34 | −67 | −40 | ||
| 4.38 | −45 | −60 | −36 | |||
| 5.86 | −16 | −65 | −29 | |||
| Left crus II | 5.12 | −29 | −76 | −40 | ||
| Left lobule VIIb | 511 | 4.51 | −27 | −77 | −56 | |
| Left lobule VIIIa | 5.29 | −18 | −67 | −57 | ||
| Left lobule VIIIb | 6.07 | −13 | −62 | −50 | ||
| Right lobule VI | 1001 | 5.39 | 19 | −68 | −25 | |
| 5.32 | 35 | −57 | −27 | |||
| 4.45 | 10 | −68 | −18 | |||
| Vermis VIIIa | 5.54 | −5 | −60 | −30 | ||
| 5.38 | 6 | −62 | −30 | |||
| Right crus II | 316 | 316 | 35 | −68 | −43 | |
| Right lobule VIIIa | 203 | 4.62 | 19 | −65 | −48 | |
| Right lobule VIIIb | 5.13 | 16 | −59 | −50 | ||
| Right lobule IX | 4.15 | 13 | −51 | −48 | ||
| Letter/Number Sequencing | Left lobule VI | 1359 | 6.86 | −14 | −70 | −18 |
| 6.41 | −16 | −58 | −16 | |||
| 5.45 | −34 | −59 | −27 | |||
| 5.41 | −35 | −54 | −29 | |||
| 4.63 | −24 | −55 | −34 | |||
| 4.18 | −27 | −70 | −23 | |||
| 4.57 | −21 | −49 | −32 | |||
| Left crus I | 6.31 | −39 | −68 | −40 | ||
| 6.14 | −31 | −62 | −38 | |||
| 5.89 | −47 | −64 | −36 | |||
| 5.75 | −31 | −64 | −39 | |||
| Left crus II | 5.84 | −31 | −60 | −32 | ||
| 5.47 | −27 | −65 | −41 | |||
| Left lobule VIIb | 5.24 | −34 | −54 | −48 | ||
| Vermis VIIIa | 427 | 4.77 | −6 | −63 | −30 | |
| Left lobule VIIIa | 6.74 | −11 | −64 | −47 | ||
| 4.74 | −10 | −67 | −38 | |||
| Left lobule VIIIb | 6.49 | −18 | −54 | −48 | ||
| 5.99 | −16 | −59 | −47 | |||
| Left lobule IX | 7.05 | −14 | −51 | −46 | ||
| 5.18 | −11 | −56 | −38 | |||
| Right lobule IV | 707 | 7.01 | 24 | −70 | −21 | |
| 6.42 | 16 | −61 | −18 | |||
| Right crus I | 4.34 | 31 | −55 | −36 | ||
| Right crus I | 706 | 5.31 | 32 | −75 | −36 | |
| 4.24 | 27 | −79 | −31 | |||
| Right crus II | 4.43 | 35 | −68 | −43 | ||
| 4.32 | 42 | −59 | −46 | |||
| Right lobule VIIb | 4.91 | 32 | −64 | −46 | ||
| Right lobule VIIIa | 7.41 | 37 | −53 | −55 | ||
| 4.78 | 29 | −46 | −50 | |||
| Right lobule VIIIb | 4.72 | 26 | −45 | −53 | ||
| Right lobule VIIIb | 150 | 4.17 | 18 | −53 | −50 | |
| Right lobule IX | 6.81 | 13 | −52 | −46 | ||
| 5.03 | 10 | −59 | −45 | |||
| 4.79 | 7 | −64 | −47 | |||
| 4.22 | 11 | −56 | −41 |
Performance on the Backward Digit Span task and cerebellar grey matter volume correlated in bilateral lobules VI, crus I, VIIIa and VIIIb; left lobule VIIb, and right inferior lobule IX (see Table 4 and Fig. 2B).
The largest clusters of correlations were observed with the Letter/Number Sequencing task. Positive correlations were found in bilateral lobules VI, crus I, crus II and VIIIa and left lobules VIIb, VIIIb and IX (see Table 4 and Fig. 2C).
3.2.2. Visuospatial working memory subtest
No significant correlations between performance on the Spatial Span subtest from the WMS-III and cerebellar grey matter volume was found.
3.3. Correlation of cerebellar grey matter volume with auditory memory
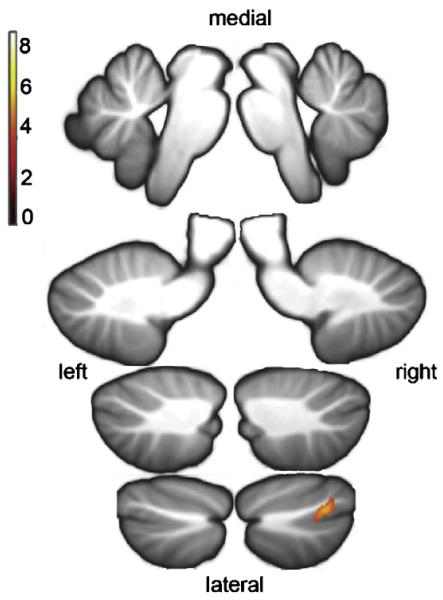
Auditory memory describes the ability to encode verbal information and repeat it back to the examiner immediately after presentation. This was assessed using two subtests from the WMS-III that assess the ability to encode complex verbal information (a story and a list of paired words) and to repeat this information back to the examiner immediately after presentation. Cerebellar grey matter was found to be correlated with the Story Recall subtest in right crus I (see Table 5 and Fig. 3) but no cerebellar correlations were observed for the Paired Word subtest.
Table 5. Anatomical localisation of correlation clusters between cerebellar grey matter density and scores on a test of auditory immediate memory (Story Recall).
Coordinates are in MNI space (p ≤0.001 uncor, k = 100, n = 15). T values represent the strength of the correlation.
| Test | Cerebellar region | Cluster size | T score | x | y | z |
|---|---|---|---|---|---|---|
| Story Recall | Right crus I | 118 | 5.69 | 34 | −73 | −36 |
| 4.06 | 29 | −81 | −29 |
Fig. 3. Correlation of cerebellar grey matter volume with auditory memory.
Anatomical localisation of correlations between cerebellar grey matter density and performance on a subtest of auditory immediate memory (from WMS-III): p ≤ 0.001 (uncor), k = 100, n = 15. Story Recall: from top to bottom and left to right: x = −2, x = 2, x = −14, x = 14, x = −24, x = 24, x = −34, x = 34 (MNI coordinates).
3.4. Correlation of cerebellar grey matter volume with other measures of intelligence and executive function
No correlations were found between the amount of cerebellar grey matter and performance on the remaining subtests of intellectual ability (Verbal Comprehension, Perceptual Organisation and Processing Speed: WAIS-III); memory performance (Visual Immediate and Delayed Memory, Auditory Delayed Memory, Auditory Recognition Memory) or executive functions (from the D-KEFS: Verbal Fluency, Word in Context, Colour/Word Interference, Tower, Card Group Sorting, Group Description and Group Recognition, and the Brixton test of Spatial Anticipation).
4. Discussion
This study systematically tests the relationship between cerebellar integrity and cognitive performance. Positive correlations were found between cerebellar grey matter volume and measures of verbal working memory and verbal immediate memory. No correlations were found with other measures of intellect, memory or verbal and non-verbal executive functions.
The current findings are congruent with those from functional imaging studies of healthy subjects, in which cerebellar activation has been observed in verbal working memory tasks (Cairo, Liddle, Woodward, & Ngan, 2004; Chen & Desmond, 2005; Desmond & Fiez, 1998; Durisko & Fiez, 2010; Tomasi, Change, Caparelli, & Ernst, 2007) and with patient lesion studies that have shown damage to both the superior and inferior cerebellum to cause verbal working memory deficits (Chiricozzi et al., 2008; Fiez, Raife, Balota, Scharz, & Raichle, 1996; Ravizza et al., 2006; Silveri et al., 1998). The current study goes beyond both functional imaging studies and patient lesion studies in demonstrating detailed cerebellar substrates for cognitive tasks by finding correlations between grey matter density and cognitive performance in patients with cerebellar degeneration. The data suggest specific cognitive subsystems that are instantiated in different parts of the cerebellum.
This study demonstrates an important role for the cerebellum in verbal, but not spatial, working memory processes. This is supported by two previous studies that found damage to the cerebellum lead to verbal working memory deficits, whilst spatial working memory was spared (Kirschen et al., 2008; Ravizza et al., 2006), suggesting separate neural systems for visuospatial working memory processes.
A prominent model for working memory (Baddeley & Hitch, 1974) characterises this process as a central executive that coordinates information from two subsystems: the phonological loop and the visuospatial sketch pad. The phonological loop stores and rehearses speech-based information, whereas the visuospatial sketch pad manipulates visual images. In this study we show correlations within cerebellar grey matter for a score of verbal working memory (including measures of performance of both the phonological loop and central executive processes) and one of immediate verbal memory (a measurement solely of the phonological loop process). Correlations of cerebellar grey matter density with performance on the verbal working memory subtasks were observed bilaterally in the superior, posterior and inferior cerebellum, and in the right posterior cerebellum for performance on a task measuring ability in auditory memory. In imaging studies with healthy volunteers, cerebellar activation has been observed as part of a wider brain network during verbal working memory tasks (Cairo et al., 2004; Chen & Desmond, 2005; Desmond & Fiez, 1998; Durisko & Fiez, 2010; Hayter et al., 2007; Tomasi et al., 2007), and mild deficits have been found in patients with primarily right cerebellar hemisphere lesions (Chiricozzi et al., 2008; Fabbro et al., 2004; Gottwald et al., 2004; Ravizza et al., 2006; Silveri et al., 1998). Those findings are supported by the current results.
The Forward Digit Span and Story Recall subtests require the subject to hold phonological and verbal information in mind and repeat this back to the examiner immediately after presentation. Performance in such tasks is thought to rely on the storage and rehearsal mechanisms of the phonological loop. This study showed correlations between grey matter density and performance on the Forward Digit Span task in the left superior and right posterior cerebellum, and with performance on the Story Recall task in the right posterior cerebellum. Previous work suggests that the cerebellum may contribute to this mechanism of storing and rehearsing verbal information (Chen & Desmond, 2005; Chiricozzi et al., 2008; Kirschen et al., 2008; Silveri et al., 1998), in particular, the right posterior cerebellum (Cairo et al., 2004; Chen & Desmond, 2005), which is supported by the current data.
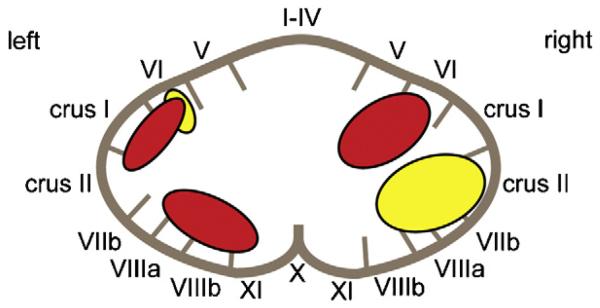
Both the Backward Digit Span and Letter/Number Sequencing tasks require the holding of information in mind and ‘online’ manipulation, processes which are controlled by the central executive system. The large clusters of voxels showing significant correlations between grey matter and performance on these two tasks suggest that the bilateral superior cerebellum and the left inferior cerebellum form part of the neural network subserving the central executive component of these verbal working memory tasks (Schumacher, Lauber, Awh, Jonides, & Smith, 1996). These areas are distinct from those implicated in maintenance of the phonological store described above, and Fig. 5 provides a summary of the proposed localisation of these two verbal working memory components within the cerebellum.
Fig. 5. Anatomical localisation of the components of the verbal working memory system.
Proposed anatomical localisation of the cerebellar contribution to the phonological loop mechanism (yellow) and the central executive (red) components of the verbal working memory system.
No correlations were found in the cerebellar grey matter for the Arithmetic subtest of the Verbal Working Memory composite score. This subtest involves online retention and manipulation of information, and therefore it is surprising that we have not found any correlations with the cerebellar grey matter similar to those found for the Backward Digit Span and Letter/Number Sequencing tasks. It can be argued that the early trials in this task can be achieved by accessing over-learned facts (i.e. ‘How much is £4 plus £5′) and that only the later, harder questions require online manipulation. Most of our participants did not answer these later questions because they had reached the discontinue criteria for this subtest (4 consecutively incorrect answers). This lack of variation in ability could explain why no correlation was found between ability and cerebellar grey matter density for this test.
Anatomical studies have shown cerebrocerebellar interconnections that suggest bases for cerebellar subspecialisation: cortical motor areas with the anterior cerebellum (lobules I-IV) (Adrian, 1943; Schmahmann et al., 2002; Schoch et al., 2006; Snider & Eldred, 1952), the pre-frontal cortex with the posterior cerebellar areas crus I and II (Kelly & Strick, 2003; Middleton & Strick, 2001; Schmahmann et al., 2002) and parietal cortical areas with the posterior areas crus I–II and lobule VIIB (Schmahmann et al., 2002). These highly organised connections between the superior and inferior cerebellar lobes and the frontal and parietal cortex suggest a neural network underlying the central executive mechanism that is not restricted to the frontal lobes (Schumacher et al., 1996).
The uniformity of the cellular structure throughout the cerebellum could allow similar computations to be performed upon all the information passing through it (Schmahmann, 2004). In the processing of motor information, the cerebellum integrates sensory information about the position of the limbs in space and in relation to the body with cortical instructions such as the goal of the movement. The cerebellum corrects and updates the movement trajectory to ensure no deviation from the goal occurs and that the movement is carried out in a fluid, continuous manner (Burt, 1993). Such motor function is operationally similar to psychological executive function, in which a central control mechanism is required to achieve a desired outcome where strategy might alter according to current state. We suggest that, although the cerebellum might have a generic mode of computation for different types of process, these processes are segregated not only between motor and cognitive modalities, but also between different cognitive processes.
The cerebellar cognitive affective syndrome (the CCAS: Schmahmann & Sherman, 1998) predicts that patients with cerebellar damage would develop a global cognitive disorder including executive dysfunction, visuospatial impairment, linguistic deficits and personality changes. The correlations between cerebellar grey matter density and certain aspects of cognitive performance shown here provide support for some aspects of the CCAS. The current results suggest that the bilateral superior and left inferior cerebellum are part of the neural network that subserves the central executive system. The processes controlled by the central executive (performing two or more tasks simultaneously and online manipulation) underlie many executive-type tasks such as planning, decision making and mental flexibility. Damage to the cerebellar part of this network would disrupt the supportive role the cerebellum provides to the frontal lobe in this network, leading to a disruption in executive function described by the CCAS.
Within the patient group that the CCAS was first described (Schmahmann & Sherman, 1998), those with anterior lesions were less affected by the CCAS than those with more posterior lesions. As the degeneration in SCA-6 progresses in a stereotypical way from the superior-anterior to posterior-inferior cerebellum (Butteriss et al., 2005), it is possible that we do not find cerebellar grey matter density correlations with language and visuospatial tasks due to the more anterior nature of the degeneration in this disease. However, the present group of patients included those with global cerebellar degeneration, and we have shown correlations with variation in the inferior cerebellar grey matter with verbal working memory performance. Hence, this is unlikely to be the sole explanation for why we have not shown a relationship between cerebellar grey matter density and performance on language and visuospatial tasks.
In conclusion, we have demonstrated a relationship between cerebellar grey matter degeneration and performance on tasks requiring phonological storage and retrieval and those requiring online manipulation and coordination of two or more verbal tasks at once. No correlations with cerebellar grey matter density were found for generalised intellectual and memory performance, or other tests of executive function. The data suggest a role for the cerebellum as part of the neural network subserving the verbal working memory system, with distinct areas supporting the phonological loop mechanism and the central executive system.
Supplementary Material
Acknowledgments
This study was funded by Ataxia UK. TG and MG are funded by the Welcome Trust. KvK is funded by the Max-Planck Society.
References
- Adrian ED. Afferent areas in the cerebellum connected with the limbs. Brain. 1943;66:289–315. [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working memory. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Academic Press; New York: 1974. pp. 47–89. [Google Scholar]
- Burgess PW, Shallice T. The Hayling and Brixton tests—Manual. Thames Valley Test Company/Harcourt Assessment; London: 1997. [Google Scholar]
- Burt AM. Textbook of neuroanatomy. W.B. Saunders Company; Philadelphia: 1993. [Google Scholar]
- Butteriss D, Chinnery P, Birchall D. Radiological characterization of spinocerebellar ataxia type 6. British Journal of Radiology. 2005;78:694–696. doi: 10.1259/bjr/73834093. [DOI] [PubMed] [Google Scholar]
- Cairo TA, Liddle PF, Woodward TS, Ngan ETC. The influence of working memory load on phase specific patterns of cortical activity. Cognitive Brain Research. 2004;21:377–387. doi: 10.1016/j.cogbrainres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE. Temporal dynamics of a cerbro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43:1227, 1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Chiricozzi FR, Clausi S, Molinari M, Leggio MG. Phonological short-term store impairment after cerebellar lesion: A single case study. Neuropsychologia. 2008;46:1940–1953. doi: 10.1016/j.neuropsychologia.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Cooper FE, Grube M, Elsegood KJ, Welch JL, Kelly TP, Chinnery PF, et al. The contribution of the cerebellum to cognition in spinocerebellar ataxia type 6. Behavioural Neurology. 2010;23:3–15. doi: 10.3233/BEN-2010-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agata F, Caroppo P, Boghi A, Coriasco M, Caglio M, Baudino B, et al. Linking coordinative and executive dysfunctions to atrophy in spinocerebellar ataxia 2 patients. Brain Structure and Function. 2011;216:275–288. doi: 10.1007/s00429-011-0310-4. doi:10.1007/s00429-011-0310-4. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) technical manual. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: Language, learning and memory. Trends in Cognitive Sciences. 1998;2:355–362. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Durisko C, Fiez JA. Functional activation in the cerebellum during working memory and simple speech tasks. Cortex. 2010;46:896–906. doi: 10.1016/j.cortex.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro F, Tavano A, Corti S, Bresolin N, De Fabritiis P, Borgatti R. Long term neuropsychological deficits after cerebellar infarctions in two young twins. Neuropsychologia. 2004;42:536–545. doi: 10.1016/j.neuropsychologia.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME. A positron emission tomography study of the short-term maintenance of verbal information. Journal of Neuroscience. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard P, Martin NH, Giunti P, Cipolotti L. Cognitive and social cognitive functioning in spinocerebellar ataxia: A preliminary characterization. Journal of Neurology. 2008;255:398, 405. doi: 10.1007/s00415-008-0680-6. [DOI] [PubMed] [Google Scholar]
- Globas C, Bosch S, Zuhlke C, Daum I, Dichgans J, Burk K. The cerebellum and cognition. Intellectual functioning in spinocerebellar ataxia type 6 (SCA6) Journal of Neurology. 2003;250:1482–1487. doi: 10.1007/s00415-003-0258-2. [DOI] [PubMed] [Google Scholar]
- Gottwald B, Wilde B, Mihajlovic Z, Mehdorn HM. Evidence for distinct cognitive deficits after focal cerebellar lesions. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:1524–1531. doi: 10.1136/jnnp.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayter AL, Langdon DW, Ramnani N. Cerebellar contributions to working memory. Neuroimage. 2007;36:943–954. doi: 10.1016/j.neuroimage.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Fujugasaki H, Saegusa H, Ohwada K, Fujita T, Iwamoto H, et al. Abundant expression and cytoplasmic aggregations of [alpha]1A voltage-dependent calcium channel protein associated with neurodegeneration in spinocerebellar ataxia type 6. Human Molecular Genetics. 1999;8:118–1193. doi: 10.1093/hmg/8.7.1185. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Suenaga M, Watanabe H, Ito M, Kato K, Kato T, et al. Prefrontal hypoperfusion and cognitive dysfunction correlates in spinocerebellar ataxia type 6. Journal of the Neurological Sciences. 2008;271:68–74. doi: 10.1016/j.jns.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a non human primate. Journal of Neuroscience. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Davis-Ratner MS, Milner MW, Chen SHA, Schraedley-Desmond P, Fisher PG, et al. Verbal memory impairments in children after cerebellar tumour resection. Behavioural Neurology. 2008;20:39–53. doi: 10.3233/BEN-2008-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konczak J, Timmann D. The effect of damage to the cerebellum on sensorimotor and cognitive function in children and adolescents. Neuroscience and Biobehavioral Reviews. 2007;31:1101–1113. doi: 10.1016/j.neubiorev.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Tedesco AM, Chiricozzi FR, Clausi S, Orsini A, Molinari M. Cognitive sequencing impairment in patients with focal or atrophic cerebellar damage. Brain. 2008;131:1332–1343. doi: 10.1093/brain/awn040. [DOI] [PubMed] [Google Scholar]
- Lidzba K, Wilke M, Staudt M, Krageloh-Mann I, Grodd W. Reorganisation of the cerebro-cerebellar network of language production in patients with congential left-hemispheric brain lesions. Brain and Language. 2008;106:204–210. doi: 10.1016/j.bandl.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Lukas C, Schols L, Bellenberg B, Rub U, Przuntek H, Schmid G, et al. Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: A voxel-based morphometry study. Neuroscience Letters. 2006;408:230–235. doi: 10.1016/j.neulet.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. Journal of Neuroscience. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanri K, Koizumi K, Mitoma H, Taguchi T, Takeguchi M, Ishiko T, et al. Classification of cerebellar atrophy using voxel-based morphometry and SPECT with an easy z-score imaging system. Internal Medicine. 2010;49:535–541. doi: 10.2169/internalmedicine.49.2785. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system. Nature Reviews Neuroscience. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Johansen-Berg H, Richter MC, Pinsk MA, et al. The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from macaque monkeys and humans. Cerebral Cortex. 2006;16:811–818. doi: 10.1093/cercor/bhj024. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129:306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children treated for posterior fossa tumours. Brain. 2000;123:1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Schols L, Kruger R, Amoiridis G, Przuntek H, Epplen JT, Riess O. Spinocerebellar ataxia type 6: genotype and phenotype in German kindreds. Journal of Neurology, Neurosurgery, and Psychiatry. 1998;64:67–73. doi: 10.1136/jnnp.64.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: Anatomic substrates of cerebellar contribution to cognitive processing. Human Brain Mapping. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive effective disorder. The Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI atlas of the human cerebellum. Academic Press; San Diego: 2000. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Loeber RT, Hurwitz AS. Topographic organisation of sensorimotor and cognitive processing in the human cerebellum revealed by functional imaging studies. Neurology. 2002;58:A267. [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomic organisation of the basilar pontine projections from prefrontal cortices in rhesus monkey. Journal of Neuroscience. 1997;17:438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561, 579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schoch B, Dimitrova A, Gizewski ER, Timmann D. Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. Neuroimage. 2006;30:36–51. doi: 10.1016/j.neuroimage.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Borkert J, Wolf S, Schmitz-Hubsch T, Rakowicz M, Mariotti C, et al. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage. 2010;49:158–168. doi: 10.1016/j.neuroimage.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Lauber E, Awh E, Jonides J, Smith EE. PET evidence for an amodal verbal working memory system. Neuroimage. 1996;3:79–88. doi: 10.1006/nimg.1996.0009. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Di Betta AM, Filippini V, Leggio MG, Molinari M. Verbal short-term store-rehearsal system and the cerebellum. Evidence from a patient with a right cerebellar lesion. Brain. 1998;121:2175–2187. doi: 10.1093/brain/121.11.2175. [DOI] [PubMed] [Google Scholar]
- Snider RS, Eldred E. Cerebro-cerebellar relationships in the monkey. Journal of Neurophysiology. 1952;15:27–40. doi: 10.1152/jn.1952.15.1.27. [DOI] [PubMed] [Google Scholar]
- Suenaga M, Kawai Y, Watanabe H, Atsuta N, Ito M, Tanaka F, et al. Cognitive impairment in spinocerebellar ataxia type 6. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:496–499. doi: 10.1136/jnnp.2007.119883. [DOI] [PubMed] [Google Scholar]
- Tavano A, Grasso R, Gadliardi C, Triulzi F, Bresolin N, Fabbro F, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–2660. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli EC, Ernst T. Different activation patterns for working memory load and visual attention load. Brain Research. 2007;1132:158–165. doi: 10.1016/j.brainres.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd UK ed The Psychological Corporation; 1999a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd UK ed The Psychological Corporation; 1999b. [Google Scholar]
- Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the a-1A voltage dependent calcium channel. Nature Genetics. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.