Abstract
Purpose
Autosomal dominant optic atrophy (DOA) is a major cause of visual impairment in young adults and it is characterized by selective retinal ganglion cell loss. In order to define the prevalence and natural history of this optic nerve disorder, we performed a population-based epidemiological and molecular study of presumed DOA cases in the north of England.
Design
Case series
Participants
Seventy-six affected probands with a clinical diagnosis of DOA were identified from our neuro-ophthalmology and neurogenetics database.
Methods
OPA1 genetic testing was performed using a PCR-based sequencing strategy. OPA1 negative cases were then screened for large-scale OPA1 re-arrangements and OPA3 mutations. Additional affected family members identified through contact tracing were examined and longitudinal visual data was analyzed.
Main Outcome Measures
The prevalence and molecular characteristics of DOA in the north of England. Visual function and disease progression among patients with OPA1 positive mutations.
Results
The detection rate of OPA1 mutations was 57.6% among probands with a positive family history of optic atrophy (19/33) and 14.0% among singleton cases (6/43). About two-thirds of our DOA families harbored OPA1 mutations (14/22, 63.6%), and five novel OPA1 mutations were identified. Only one family carried a large-scale OPA1 rearrangement and no OPA3 mutations were found in our optic atrophy cohort. The minimum point prevalence of DOA in the north of England was 2.87 per 100,000 (95% CI 2.54-3.20), or 2.09 per 100,000 (95% CI 1.95-2.23) when only OPA1 positive cases were considered. Snellen visual acuity varied markedly between OPA1 positive cases with a mean of 20/173 (Range 20/20-hand movement), and visual function worsened in 67.4% of patients during follow-up. The mean rate of visual loss was 0.032 LogMAR/years but some patients experienced much faster visual decline (Range = 0–0.171 LogMAR/years). OPA1 missense mutations were associated with a significantly worse visual outcome compared to other mutational subtypes (P = 0.0001).
Conclusions
DOA causes significant visual morbidity and affects at least 1 in 35,000 of the general population.
Keywords: Dominant optic atrophy, epidemiology, mitochondrial DNA, optic neuropathy, OPA1, prevalence
Introduction
Autosomal dominant optic atrophy (DOA) is an important cause of inherited visual failure and the primary pathological process selectively targets retinal ganglion cells (RGCs) resulting in optic nerve degeneration. The majority of DOA families harbor pathogenic mutations in the OPA1 gene (3q28-q29, OMIM 165500) and the characteristic features observed in this condition are an insidious onset in early childhood, reduced colour vision, central field defects and a marked inter- and intra-familial variation in disease severity.1, 2 Over 200 different OPA1 mutations have been reported including single nucleotide changes within the coding regions (44%), splice site variants (27%) and small exonic deletions and duplications (29%).3 In a recent study using a multiplex ligation probe amplification (MLPA) assay, large scale OPA1 rearrangements were identified in 8/42 (19%) probands with no previously identified OPA1 mutations by conventional screening methods.4 Heterozygous mutations in the OPA3 gene (19q13.2-q13.3, OMIM 165300) have also been implicated in two French families segregating bilateral optic atrophy in association with premature cataracts,5 a more benign phenotype compared to the generalised neurodegenerative process seen in the autosomal recessive form of the disease, 3-methylglutaconic aciduria type III or Costeff’s syndrome (OMIM 258501).6, 7 Interestingly, both Opa1 and Opa3 are inner mitochondrial membrane proteins and pathogenic mutations exert their deleterious effects on RGC survival via several, likely interacting, mechanisms, namely impairment of mitochondrial oxidative phosphorylation, disruption of the delicate balance regulating mitochondrial fusion and fission, and an increased susceptibility to apoptotic cell death.8, 9
Although the mutational spectrum and natural history of DOA have been described in several case series,3, 10-15 there are currently no robust prevalence figures of molecularly confirmed cases in the general population, so the true impact of this disorder is not known. The latter reflects the methodological limitations of these studies which were performed by specialist research centers with often wide and overlapping patient catchment areas. We therefore performed an extensive clinical and molecular study of DOA in the north of England, both to address these important epidemiological issues and to clarify the burden of OPA1 and OPA3 mutations in this patient group.
Patients and Methods
Study Population
The north of England has been relatively stable in terms of migratory flux and based upon the mid-2007 United Kingdom (UK) national census, it consists of a predominantly white, Caucasian population of 3,061,400 individuals. As a result of historical clinical links and the nature of healthcare provision in our region, patients with unexplained visual failure and/or possible inherited optic neuropathies are referred to the neuro-ophthalmology (PYWM, MPC and PGG) and neurogenetics (RH and PFC) services in Newcastle upon Tyne for further assessment. This includes molecular testing provided by the Mitochondrial Diagnostic Laboratory since 1990 and with UK National Commissioning Group status for “Rare Mitochondrial Diseases” since 2007 (http://www.mitochondrialncg.nhs.uk/, Accessed 10-12-09). Over the past two decades, these referral patterns have enabled us to establish a comprehensive research database of patients with a likely genetic basis for their optic atrophy, both in the north of England and from outside the region. This study had the relevant institutional approval from the County Durham and Tees Valley 1 Research Ethics Committee and complied with the Declaration of Helsinki.
Optic Atrophy Cohort
We identified 76 affected probands with clinical features suggestive of DOA in our database and these were initially screened for possible OPA1 mutations as part of their diagnostic work-up. All affected individuals had previously been investigated to exclude compressive, inflammatory and infiltrative causes for their bilateral optic neuropathy, and a complete ophthalmological examination had also been performed including: (i) intraocular pressure (IOP) measurement by applanation tonometry, (ii) visual field assessment by confrontation, static (Humphrey) and/or kinetic (Goldmann) perimetry, (iii) colour discrimination with the Ishihara pseudochromatic plates, (iv) dilated slit lamp biomicroscopy, (v) measurement of the peripapillary retinal nerve fibre layer (RNFL) thickness by optical coherence tomography (OCT), and (vi) visual electrophysiology when appropriate. Genomic DNA samples were extracted from peripheral blood leukocytes using established methods.
Visual Acuity
For the purpose of longitudinal analysis, additional clinical information and visual acuity data were obtained from archived records held in the patients’ local ophthalmology units (PWS, LG, DAK). In the majority of cases, best corrected visual acuity (BCVA) was measured using the Snellen chart and for the purpose of statistical analysis, Snellen ratios were converted to LogMAR (Logarithm of the minimum angle of resolution) decimal values. A LogMAR value of 0 is equivalent to 20/20 Snellen vision and a value of 1.0 is equivalent to 20/200 Snellen vision, the largest optotype on standard Snellen charts. Patients with visual acuities reduced to counting fingers (CF) were assigned a LogMAR value of 2.0, whilst those with only hand movement (HM) perception were given a LogMAR value of 2.3.16, 17
Molecular Genetics
The entire coding region of the OPA1 and OPA3 genes was amplified by the polymerase chain reaction (PCR) (Primer oligodeoxynucleotide sequences are available on request) and sequenced with BigDye™ terminator cycle chemistries on an ABI3100 Genetic Analyzer (Applied Biosystems). Sequence chromatograms were directly compared with the appropriate Genbank reference sequence using SeqScape™ software v2.1 (Applied Biosystems): OPA1 accession number AB011139 according to mRNA transcript variant 1, NM_011560, and OPA3 accession numbers NM_001017989.2 (Transcript 1) and NM_025136.2 (Transcript 2).
OPA1 Sequence Variants
All genetic variants were confirmed by reverse sequencing and checked against the eOPA1 database (http://lbbma.univ-angers.fr/eOPA1/, Accessed 10-12-09) to determine whether they had been described in previous mutational reports. Bioinformatic analysis and the functional significance of intronic OPA1 nucleotide changes were determined using the publicly available Fruitfly predictor software (http://www.fruitfly.org/seq_tools/splice.html and http://www.fruitfly.org/seq_tools/promoter.html, Accessed 10-12-09). Novel OPA1 mutations were also screened in a panel of 186 age-matched, white Caucasian controls from the north of England using a multiplex primer extension assay and product analysis with matrix-associated laser desorption/ionization time of flight mass spectrometry (MALDI-TOF) (Sequenom, San Diego, CA).
MLPA Assay
All optic atrophy patients with a family history suggesting an autosomal dominant pattern of inheritance, which was defined as affected family members in two or more generations, and no pathogenic OPA1 mutations with direct sequencing were subsequently screened for possible large scale OPA1 rearrangements using a previously described MLPA kit (MRC-Holland).4
Statistical Analysis
Statistical analysis was performed using GraphPad™ v.4 statistical software (San Diego, CA). Fisher’s exact test, χ2 analysis, independent sample t-test, and one-way ANOVA were used for group comparisons, as required.
Results
Frequency of OPA1 Mutations
In our initial molecular screen, OPA1 mutations were identified in 25/76 probands (32.9%), 17 families were from the north of England and 8 families were from outside the region. The detection rate was significantly higher among those referred by ophthalmologists (19/37, 51.4%) compared to referrals from neurologists and geneticists (6/39, 15.4%) (Odds ratio (OR) = 5.8, 95% confidence interval (CI) = 2.0-17.2, P = 0.0013). Similarly, there was a greater likelihood of identifying an OPA1 mutation among probands with a positive family history of optic atrophy (19/33, 57.6%) compared to singleton cases (6/43, 14.0%) (OR = 8.4, 95% CI = 2.8–25.3, P < 0.0001). For the remainder of this epidemiological study, the 8 OPA1 positive families that were referred from outside the region were not included.
North of England Cohort
OPA1 positive families from the north of England harbored 13 different pathogenic mutations: missense (2/13, 15.4%), nonsense (2/13, 15.4%), splice site (5/13, 38.4%) and deletions (4/13, 30.8%) (Table 1, Figure 1). Of these, 5 were novel variants not present among 186 normal controls: c.636_637delAG in exon 6, c.876-878delTGT and c.889C>T in exon 9, c.1198C>T in exon 12, and c.2818+5g>a in intron 27. The c.636_637delAG, c.876-878delTGT and c.889C>T mutations are predicted to result in premature termination codons. The c.1198C>T mutation in exon 12 was identified in a singleton case (OA-17) with typical ophthalmological features of DOA, but it was not found in his unaffected father and two unaffected siblings (Figure 2). The mother was not thought to be visually affected but no DNA sample was available for analysis. The C to T substitution at nucleotide position 1198 is predicted to change a highly conserved amino acid proline to serine (p.P400S) in the critical GTPase domain of the Opa1 protein. The c.2818+5g>a splice site mutation in intron 27 segregated with affected status in a three-generation family (OA-6), all affected family members sharing the same disease haplotype. Previous pathogenic mutations have been described at positions +2 and + 6 (http://lbbma.univ-angers.fr/eOPA1/, Accessed 10-12-09) and the c.2818+5g>a substitution is predicted to completely abolish the functionality score of the splice donor site from 0.88 to 0.00, supporting the pathogenic status of this intronic variant.
Table 1. Molecular and Clinical Characteristics of OPA1 Positive Pedigrees Identified in the North of England.
| Pedigree | cDNA Change | Consequence | Affecteda N = |
Examined N = |
Age of Onset (Yrs) | Snellen BCVA | ||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Mean | Range | Mean | Range | |||||
| OA-1 | c.1212+3a>t | Splicing defect | 3 | 3 | 4.7 | 4.0-5.0 | 20/417 | 20/160-CF |
| OA-2 | c.870+5g>a | Splicing defect | 4 | 3 | 10.0 | 8.0-12.0 | 20/210 | 20/30 - CF |
| OA-3b, c | c.876-878delTGT* | p.V294fsX667 | 3 | 1 | 5.0 | N/A | CF | N/A |
| OA-4b, c | c.876-878delTGT* | p.V294fsX667 | 10 | 6 | 6.0 | 4.0-11.0 | 20/167 | 20/40 - CF |
| OA-5b, c | c.876-878delTGT* | p.V294fsX667 | 7 | 3 | 5.0 | 5.0 | 20/197 | 20/200 - 20/400 |
| OA-6 | c.2818+5g>a* | Splicing defect | 4 | 3 | 5.3 | 5.0-6.0 | 20/93 | 20/40 - 20/200 |
| OA-7 | c.2708_2711delTTAG | p.V903fsX3 | 2 | 1 | 5.0 | N/A | 20/200 | N/A |
| OA-8b, c | c.2713C>T | p.R905X | 3 | 3 | 14.0 | 11.0-16.0 | 20/273 | 20/60 - CF |
| OA-9b, c | c.2713C>T | p.R905X | 2 | 1 | 5.0 | N/A | 20/80 | N/A |
| OA-10b, c | c.2713C>T | p.R905X | 3 | 2 | 5.0 | 5.0 | 20/200 | 20/200 |
| OA-11 | c.2613+1g>a | Splicing defect | 3 | 3 | 7.0 | 3.0-10.0 | 20/160 | 20/120 - CF |
| OA-12 | c.1516+1g>t | Splicing defect | 4 | 3 | 15.0 | 15.0 | 20/53 | 20/20 - 20/200 |
| OA-13 | c.794_797delTTGA | p.I265fsX42 | 3 | 1 | 15.0 | N/A | 20/40 | N/A |
| OA-14 | c.636_637delAG* | p.K214fsX2 | 3 | 1 | 8.0 | N/A | 20/40 | N/A |
| OA-15 | c.889C>T* | p.Q297X | 3 | 3 | 5.0 | 5.0 | 20/107 | 20/20 - CF |
| OA-16 | c.1635C>G | p.S545R | 3 | 3 | 3.7 | 1.0-5.0 | 20/1170 | 20/200 - HM |
| OA-17 | c.1198C>T* | p.P400S | 1 | 1 | 2.0 | N/A | HM | N/A |
| OA-18 | Exons 1 -5b deletion* | p.M1fsX208 | 3 | 4 | 7.3 | 5.0-9.0 | 20/130 | 20/60 - 20/200 |
|
| ||||||||
| Entire Study Cohort | 64 | 45 | 7.0 | 1.0-16.0 | 20/207 | 20/20 - HM | ||
Affected individuals resident in the north of England,
Shared OPA1 haplotype,
Novel OPA1 mutations,
BCVA: Best corrected visual acuity, CF: Counting fingers, HM: Hand movement, N/A: Not applicable, Yrs: Years.
Figure 1.
Figure 2.
Haplotype Analysis
Affected family members within pedigrees OA-3, OA-4 and OA-5 (c.876-878delTGT) harbored the same OPA1 disease haplotype, suggesting a shared common ancestor (Table 2). Haplotype analysis of pedigrees OA8, OA-9 and OA-10 also revealed a similar combination of non-pathogenic OPA1 single nucleotide polymorphisms (SNPs) in combination with the c.2713C>T mutation, again indicating a likely founder event (Table 3).
Table 2. Haplotype Analysis of Pedigrees OA-3, OA-4 and OA-5.
| Location | OA-3, 111-3 | OA-4, III-1 | OA-4, III-2 | OA-5, I-1 | OA-5, II-3 | OA-5, III-1 | Haplotype |
|---|---|---|---|---|---|---|---|
| Exon 4 | c.473A>G | c.473A>G | c.473A>G | c.473A>G | c.473A>G | c.473A>G | c.473A>G |
| Intron 4 | c.557-19t>c | c.557-19t>c | c.557-19t>c | ||||
| Exon 5 | c.575C>T | ||||||
| Intron 8 | c.870+4c>t | c.870+4c>t | c.870+4c>t | c.870+4c>t | c.870+4c>t | c.870+4c>t | c.870+4c>t |
| Intron 8 | c.870+32t>c | c.870+32t>c | c.870+32t>c | ||||
| Exon 9 | c.876-878delTGT | ||||||
| Intron 11 | c.1141-55t>a | ||||||
| Intron 14 | c.1443+23g>a | ||||||
| Exon 17 | c.1608A>C | ||||||
| Intron 18 | c.1770+16t>g | c.1770+16t>g | |||||
| Intron 18 | c.1770+51t>g | c.1770+51t>g | c.1770+51t>g | ||||
| Exon 21 | c.2109C>T | c.2109C>T | c.2109C>T | c.2109C>T | c.2109C>T | c.2109C>T | c.2109C>T |
| Intron 26 | c.2707+25t>a | c.2707+25t>a | c.2707+25t>a |
Homozygous OPA1 SNPs are highlighted in blue and OPA1 mutations are highlighted in red.
Table 3. Haplotype Analysis of Pedigrees OA-8, OA-9 and OA-10.
| Location | OA-8, II-1 | OA-8, III-1 | OA-8, III-2 | OA-9, II-1 | OA-IO, II-1 | OA-IO, II-2 | Haplotype |
|---|---|---|---|---|---|---|---|
| Exon 4 | c.473A>G | c.473A>G | |||||
| Intron 4 | c.557-19t>c | c.557-19t>c | c.557-19t>c | c.557-19t>c | c.557-19t>c | c.557-19t>c | c.557-19t>c |
| Intron 8 | c.870+32t>c | c.870+32t>c | c.870+32t>c | c.870+32t>c | c.870+32t>c | c.870+32t>c | c.870+32t>c |
| Intron 18 | c.1770+16t>g | c.1770+16t>g | c.1770+16t>g | c.1770+16t>g | c.1770+16t>g | c.1770+16t>g | c.1770+16t>g |
| Intron 18 | c.1770+51t>g | c.1770+51t>g | c.1770+51t>g | c.1770+51t>g | c.1770+51t>g | c.1770+51t>g | c.1770+51t>g |
| Exon 21 | c.2109C>T | c.2109C>T | c.2109C>T | ||||
| Intron 26 | c.2707+25t>a | c.2707+25t>a | c.2707+25t>a | c.2707+25t>a | c.2707+25t>a | c.2707+25t>a | c.2707+25t>a |
| Exon 27 | c.2713C>T |
Homozygous OPA1 SNPs are highlighted in blue and OPA1 mutations are highlighted in red.
OPA1 Rearrangements
Family OA-18 is a large three-generation family showing clear paternal transmission of early onset, bilateral optic atrophy. The clinical diagnosis of DOA was robust and when OPA1 sequencing failed to identify a pathogenic change, DNA samples were sent for further linkage analysis at the Institute for Ophthalmic Research in Tübingen, Germany. A deletion encompassing exons 1 to 5b was subsequently identified in family OA-184 and we therefore wished to determine the frequency of large scale OPA1 re-arrangements in other DOA families in the north of England without OPA1 mutations using our PCR-based sequencing protocol (N = 8). No additional rearrangements were found with the same MLPA kit (MRC-Holland) used by Fuhrmann and colleagues,4 giving an overall frequency of 11.1% (1/9) in this particular subgroup.
OPA3 Screen
OPA3 sequencing was finally performed in the remaining 50 probands in our optic atrophy cohort with no confirmed molecular diagnosis, a heterogeneous group consisting of families with affected individuals in two or more generations (N = 11), more than one affected individual but in only one generation (N=2), and singleton cases (N=37), none of whom had a history of premature cataracts. No OPA3 mutations were identified and 18 probands harbored a previously reported c.231T>C SNP in exon 2.18 A previously reported6 intronic c.1-38g>a SNP in the 5′ untranslated region was found in 3 probands but with no predicted effect on splicing at the acceptor site or on putative transcriptional promoters upstream of the start codon.
Prevalence Data
Our molecular investigations have established 14 genetically distinct OPA1 positive pedigrees in the north of England, with 64 living affected family members resident in the region at the time of contact tracing (Mean age = 39.5 years, standard deviation (SD) = 17.8 years, range 6.0– 78.0 years) (Table 1). There was no gender bias among affected individuals with a male to female ratio of 1.06:1. An additional 8 families (8/22, 36.4%) with a total of 24 affected family members had an autosomal dominant pattern of inheritance but no OPA1 or OPA3 mutations (OPA1 negative group) (Mean age = 40.1 years, SD = 16.6 years, range 12.0– 72.0 years). Including these OPA1 negative cases with a positive family history, the minimum point prevalence of DOA in the north of England was therefore estimated at 2.87 per 100,000 (95% CI 2.54-3.20), about 1 in 35,000 of the general population. The minimum point prevalence of patients with visual failure secondary to a confirmed OPA1 mutation was 2.09 per 100,000 (95% CI 1.95–2.23).
Fundal Appearance
The appearance of the optic nerve head was abnormal in all carriers with an OPA1 mutation, with total disc pallor observed in 32/82 (39.0%) eyes and a prominent temporal wedge of pallor in the remaining 50/82 (61.0%) eyes (Figure 2B). Other morphological changes were noted: (i) tilted discs (6/82, 7.3%), (ii) peripapillary optic atrophy (16/82, 19.5%), and (iii) cup to disc ratios > 0.5 (10/82, 12.2%) which was symmetrical between the 2 eyes, with normal IOPs and no family history of glaucoma. No significant macular or peripheral pigmentary changes were noted in all OPA1 cases.
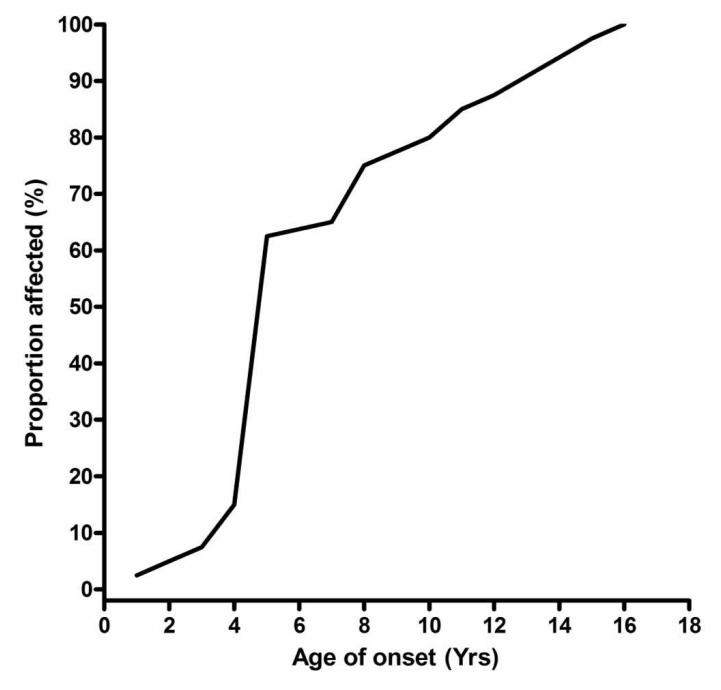
Visual Function
The mean age of onset of visual failure among OPA1 positive patients was 7.0 years (SD = 3.9 years, range 1.0-16.0 years) and 80% of affected individuals were symptomatic before the age of 10 years (Table 1, Figure 3). These figures do not include a small minority of patients (5/45, 11.1%) who were subjectively asymptomatic but were found to have mild reduction in visual acuity and subclinical optic neuropathy during contact tracing. Colour vision was abnormal in all but one patient, the daughter of proband OA-2, who had full Ishihara colour discrimination with 20/30 Snellen vision (OU) and only mild temporal disc pallor. Reliable Goldmann field perimetry was possible in 29 patients and 20/29 (69.0%) had a demonstrable field defect, a central scotoma in 15/29 cases (51.7%) and a centro-caecal scotoma in 5/29 cases (17.2%).
Figure 3.
At the time of their last ophthalmological assessment, the mean visual acuity for our north of England cohort was as follows: (i) all DOA cases; 0.94 LogMAR, 20/173 Snellen (N = 65), (ii) OPA1 positive group; 1.02 LogMAR, 20/207 Snellen (N = 45), and (iii) OPA1 negative group; 0.76 LogMAR, 20/117 Snellen (N = 20) (Table 1, Figure 4A). There was no significant difference in mean LogMAR visual acuity between these 3 groups (P = 0.1133), and there was a wide range in visual acuities (20/20-HM) both within and between families. Visual acuity was symmetrical between both eyes in the majority of patients (52/65, 80.0%), there was a difference of ≤ 2 LogMAR lines in 8/65 (12.3%) patients, and a difference of > 2 LogMAR lines in 5/65 (7.7%) patients. Among the latter group, 3/5 cases had childhood strabismus and they underwent horizontal recti surgery to improve ocular alignment.
Figure 4.
There was a significant difference in visual outcome between OPA1 mutational subtypes (Figure 5, P = 0.0018), with the mean LogMAR visual acuity for patients with missense mutations (Mean = 1.83, SD = 0.19) being worse compared to those harboring deletions, nonsense, and splice site mutations (Mean = 0.93, SD = 0.07, P = 0.0001). However, there was no significant difference in LogMAR visual acuity between OPA1 mutations when grouped according to their locations within the main functional gene domains (P = 0.6161).
Figure 5.
Disease Progression
OPA1 positive patients > 40 years old had worse LogMAR visual acuities (Mean = 1.27, SD = 0.72) compared to those who were ≤ 40 years old (Mean = 0.73, SD = 0.48, P = 0.0001) (Figures 4B and 4C). Longitudinal visual acuity data was also available for 43 OPA1 positive patients with a mean follow-up duration of 18.0 years (SD = 14.7 years, range 1.2-53.0 years). BCVA worsened in 29/43 (67.4%) patients, with a reduction of ≤ 2 LogMAR lines (0.2 decimal value) in 4/43 (9.3%) patients and > 2 LogMAR lines in 25/43 (58.1%) patients. The mean rate of visual loss was 0.032 LogMAR/years (SD = 0.045 LogMAR/years), but some patients experienced much faster visual decline (Range = 0-0.171 LogMAR/years). There was also a significant overall reduction in LogMar visual acuities with disease duration when comparing baseline values (Mean = 0.68, SD = 0.53) with the patients’ most recent measurements (Mean = 1.06, SD = 0.67, P = 0.0001) (Figure 4D).
Discussion
This is the first population-based epidemiological study of DOA and in the north of England the minimum point prevalence of DOA was 2.87 per 100,000, about 1 in 35,000. About two-thirds of our families harbored OPA1 mutations (14/22, 63.6%) which is consistent with the average figure of 60% found in previous case series,2 and if only these molecularly-confirmed cases are considered, the minimum point prevalence of visual failure secondary to OPA1 mutations was 2.09 per 100,000. However, it must be emphasised that for the remaining families with no molecular diagnosis, the possibility of pathogenic variants within the OPA1 gene promoter and intronic regions has not been excluded, with putative roles in the regulation of OPA1 mRNA splicing or transcriptional activity. The prevalence of DOA is often quoted in the literature as 1 in 50,000 but this is only an estimate based upon one author’s personal views on the relative prevalence of DOA in comparison to other genetic ocular disorders.19 A higher figure of 1 in 12,000 in Denmark was derived from the Register of Hereditary Eye Diseases held at the National Eye Clinic in Hellerup, but this was based upon a clinical diagnosis of DOA in the pre-molecular era.20 Furthermore, the mutational spectrum of DOA in Denmark is more limited with about 42% of Danish pedigrees harboring the c.2708_2711delTTAG deletion in exon 27.21 This significant allelic over-representation has been attributed to an ancestral mutational event and this probably also accounts for the higher prevalence of DOA in this specific population. The prevalence of DOA in this study is comparable to that of Leber hereditary optic neuropathy (LHON) which was previously estimated at 3.22 per 100,000 (95% CI 2.47-3.97) in the north of England,22 further confirming the clinical impression that DOA and LHON are the two most common inherited optic nerve disorders in the general population. Unlike LHON, there is no gender bias in DOA and no cases of non-penetrance were identified in our cohort. The proportion of OPA1 carriers not manifesting optic nerve dysfunction varies between 0–57% in the literature,11, 12, 23 and this variation is likely due to a combination of factors such as the methods of case ascertainment, the clinical criteria used to define non-penetrance, and whether ancillary tests such as OCT and visual electrophysiology have been employed in the patient’s investigations.
As expected, the majority of OPA1 mutations were located in the GTPase, dynamin central region and terminal effector domains, and we have described 5 novel pathogenic variants extending the mutational spectrum of this highly polymorphic gene (Figure 1).3, 15 Although large scale OPA1 rearrangements should be ruled out when there is a high index of clinical suspicion supported by a positive family history, this mutational subtype probably accounts for only 10-20% of DOA cases found to be OPA1 negative with conventional PCR-based screening methods.4 OPA3 mutations have also been reported in two French families segregating both DOA and premature cataracts in an autosomal dominant mode of inheritance.5 However, no OPA3 mutations were identified in our study which suggests that these are likely rare causes of inherited optic atrophy especially in the absence of early-onset lenticular abnormalities.
Our clinical observations stress the importance of considering DOA in the differential diagnosis of patients with progressive onset of visual failure in early childhood, especially in the presence of prominent temporal optic disc pallor. The latter is a characteristic morphological feature seen in DOA and it was observed in 61% of our OPA1 positive cases, similar to other case series (41-52%).23, 24 It can be better appreciated with dilated, stereoscopic slit lamp biomicroscopy, reflecting the much higher OPA1 hit rate among cases referred by ophthalmologists (51.4%) compared to other specialties (15.4%). Although a positive family history of optic atrophy increases the diagnostic yield, in practice this can be difficult to ascertain and it is further compounded by the possibility of subtle visual deficits in asymptomatic family members.14, 25, 26 For example, the proband of OA-15 was initially thought to be a singleton case but examination of both her asymptomatic son and daughter revealed that they had subtle but definite temporal optic disc pallor, with visual acuities of 20/20 and 20/30 OU respectively, and abnormal visual electrophysiology. In one study, about half of all diagnosed OPA1 cases were “sporadic”, with the only caveat that this was conducted in a diagnostic center with no active contact tracing of other family members.3, 10-15 Nevertheless, OPA1 sequencing should be advocated in all cases presenting with clinical and ophthalmological features suggestive of DOA even in the absence of any apparent family history.
Our DOA cohort has confirmed the salient clinical features in this condition, with the majority of patients experiencing visual loss in early childhood, and a marked inter- and intra-familial variability in disease severity. The legal definition of severe visual impairment in the UK is a BCVA < 10/200 Snellen (1.30 LogMAR) (http://www.dh.gov.uk/en/Healthcare/Primarycare/Optical/DH_4074843, Accessed 10-12-09), and registered individuals are entitled to a range of social care and financial benefits. It is therefore significant that patients with OPA1 missense mutations were much more likely to fall within this disability category compared to other mutational subtypes (OR = 12.4, 95% CI = 2.3-67.2, P = 0.0023). This has important clinical implications and although additional confirmatory studies are needed, it is tempting to speculate that missense OPA1 mutations are having a greater detrimental effect on RGC survival via a possible dominant-negative effect.
During follow-up, visual acuity worsened in two-thirds of patients and the rate of visual decline varied widely, being much faster in a subgroup of patients. In previous reports, including pre-molecular case series, 19-50% of patients experienced progressive,10, 13, 14, 27, 28 albeit slow, visual deterioration and 13-46% were registered legally blind.20, 23, 29 Although DOA has a relatively better visual prognosis compared to LHON,30 the majority of patients will still suffer a decline in visual function and some caution is therefore recommended when counselling OPA1 positive patients and their families. In conclusion, this study has defined the mutational spectrum and natural history of DOA in the north of England and our epidemiological data indicate similar prevalence figures for DOA and LHON in our region. However, these two mitochondrial optic neuropathies exhibit distinct clinical and ophthalmological features which can be helpful to clinicians in directing appropriate molecular investigations and subsequent genetic counselling. There is currently no proven treatment for LHON or DOA and given the central role played by mitochondrial dysfunction in the pathophysiology of both these disorders, future mechanistic studies will hopefully reveal disease pathways amenable to therapeutic interventions.
Supplementary Material
Acknowledgments
PYWM is an MRC Clinical Research Fellow and PFC is a Wellcome Trust Senior Fellow in Clinical Science. PFC also receives funding from the Parkinson’s Disease Society (UK), the Medical Research Council Translational Muscle Centre, and the UK NIHR Biomedical Research Centre in Ageing and Age related disease. RH is supported by the Deutsche Forschungsgemeinschaft (HO 2505/2-1), the Newcastle upon Tyne Hospitals NHS Charity, and the Academy of Medical Sciences. RWT is funded by the Wellcome Trust and the UK National Commissioning Group. We are grateful to our ophthalmology, neurology and genetics colleagues in the north of England for referring suspected cases of inherited optic neuropathies over the years and for their invaluable help with contact tracing.
Footnotes
Financial Disclosures: None of the authors have any financial interests to disclose.
References
- 1.Votruba M. Molecular genetic basis of primary inherited optic neuropathies. Eye. 2004;18(11):1126–32. doi: 10.1038/sj.eye.6701570. [DOI] [PubMed] [Google Scholar]
- 2.Yu-Wai-Man P, Griffiths PG, Hudson G, Chinnery PF. Inherited Mitochondrial Optic Neuropathies. J Med Genet. 2009;46(3):145–58. doi: 10.1136/jmg.2007.054270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferre M, Amati-Bonneau P, Tourmen Y, et al. eOPA1: An online database for OPA1 mutations. Hum Mutat. 2005;25(5):423–8. doi: 10.1002/humu.20161. [DOI] [PubMed] [Google Scholar]
- 4.Fuhrmann N, Alavi MV, Bitoun P, et al. Genomic rearrangements in OPA1 are frequent in patients with autosomal dominant optic atrophy. J Med Genet. 2009;46(2):136–44. doi: 10.1136/jmg.2008.062570. [DOI] [PubMed] [Google Scholar]
- 5.Reynier P, Amati-Bonneau P, Verny C, et al. OPA3 gene mutations responsible for autosomal dominant optic atrophy and cataract. J Med Genet. 2004;41(9):e110. doi: 10.1136/jmg.2003.016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anikster Y, Kleta R, Shaag A, et al. Type III 3-methylglutaconic aciduria (optic atrophy plus syndrome, or Costeff optic atrophy syndrome): Identification of the OPA3 gene and its founder mutation in Iraqi Jews. Am J Hum Genet. 2001;69(6):1218–24. doi: 10.1086/324651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleta R, Skovby F, Christensen E, et al. 3-methylglutaconic aciduria type III in a non-Iraqi-Jewish kindred: clinical and molecular findings. Mol Genet Metab. 2002;76(3):201–6. doi: 10.1016/s1096-7192(02)00047-1. [DOI] [PubMed] [Google Scholar]
- 8.Chan DC. Mitochondrial dynamics in disease. N Engl J Med. 2007;356(17):1707–9. doi: 10.1056/NEJMp078040. [DOI] [PubMed] [Google Scholar]
- 9.Carelli V, La Morgia C, Valentino ML, et al. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim Biophys Acta. 2009;1787(5):518–28. doi: 10.1016/j.bbabio.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Votruba M, Moore AT, Bhattacharya SS. Clinical features, molecular genetics, and pathophysiology of dominant optic atrophy. J Med Genet. 1998;35(10):793–800. doi: 10.1136/jmg.35.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toomes C, Marchbank NJ, Mackey DA, et al. Spectrum, frequency and penetrance of OPA1 mutations in dominant optic atrophy. Hum Mol Genet. 2001;10(13):1369–78. doi: 10.1093/hmg/10.13.1369. [DOI] [PubMed] [Google Scholar]
- 12.Thiselton DL, Alexander C, Taanman JW, et al. A comprehensive survey of mutations in the OPA1 gene in patients with autosomal dominant optic atrophy. Invest Ophthalmol Vis Sci. 2002;43(6):1715–24. [PubMed] [Google Scholar]
- 13.Puomila A, Huoponen K, Mantyjarvi M, et al. Dominant optic atrophy: correlation between clinical and molecular genetic studies. Acta Ophthalmol Scand. 2005;83(3):337–46. doi: 10.1111/j.1600-0420.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- 14.Cohn AC, Toomes C, Hewitt AW, et al. The Natural History of OPA1-related Autosomal Dominant Optic Atrophy. Br J Ophthalmol. 2008;92(10):1333–6. doi: 10.1136/bjo.2007.134726. [DOI] [PubMed] [Google Scholar]
- 15.Ferre M, Bonneau D, Milea D, et al. Molecular screening of 980 cases of suspected hereditary optic neuropathy with a report on 77 novel OPA1 mutations. Hum Mutat. 2009;30(7):E692–705. doi: 10.1002/humu.21025. [DOI] [PubMed] [Google Scholar]
- 16.Schulze-Bonsel K, Feltgen N, Burau H, et al. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–40. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 17.Lange C, Feltgen N, Junker B, et al. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT) Graefes Arch Clin Exp Ophthalmol. 2009;247(1):137–42. doi: 10.1007/s00417-008-0926-0. [DOI] [PubMed] [Google Scholar]
- 18.Neas K, Bennetts B, Carpenter K, et al. OPA3 mutation screening in patients with unexplained 3-methylglutaconic aciduria. J Inherit Metab Dis. 2005;28(4):525–32. doi: 10.1007/s10545-005-0525-8. [DOI] [PubMed] [Google Scholar]
- 19.Lyle WM. Genetic Risks. A Reference for Eye Care Practitioners. University of Waterloo Press; Waterloo, Ontario, Canada: 1990. [Google Scholar]
- 20.Kjer B, Eiberg H, Kjer P, Rosenberg T. Dominant optic atrophy mapped to chromosome 3q region .II. Clinical and epidemiological aspects. Acta Ophthalmol Scand. 1996;74(1):3–7. doi: 10.1111/j.1600-0420.1996.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 21.Thiselton DL, Alexander C, Morris A, et al. A frameshift mutation in exon 28 of the OPA1 gene explains the high prevalence of dominant optic atrophy in the Danish population: evidence for a founder effect. Hum Genet. 2001;109(5):498–502. doi: 10.1007/s004390100600. [DOI] [PubMed] [Google Scholar]
- 22.Man PY, Griffiths PG, Brown DT, et al. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am J Hum Genet. 2003;72(2):333–9. doi: 10.1086/346066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohn AC, Toomes C, Potter C, et al. Autosomal dominant optic atrophy: Penetrance and expressivity in patients with OPA1 mutations. Am J Ophthalmol. 2007;143(4):656–62. doi: 10.1016/j.ajo.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 24.Votruba M, Thiselton D, Bhattacharya SS. Optic disc morphology of patients with OPA1 autosomal dominant optic atrophy. Br J Ophthalmol. 2003;87(1):48–53. doi: 10.1136/bjo.87.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kline LB, Glaser JS. Dominant Optic Atrophy - Clinical Profile. Arch Ophthalmol. 1979;97(9):1680–6. doi: 10.1001/archopht.1979.01020020248013. [DOI] [PubMed] [Google Scholar]
- 26.Hoyt CS. Autosomal Dominant Optic Atrophy - a Spectrum of Disability. Ophthalmology. 1980;87(3):245–51. doi: 10.1016/s0161-6420(80)35247-0. [DOI] [PubMed] [Google Scholar]
- 27.Kjer B. Infantile optic atrophy with dominant transmission. Dan Med Bull. 1956;3:135–41. [PubMed] [Google Scholar]
- 28.Eliott D, Traboulsi EI, Maumenee IH. Visual Prognosis in Autosomal Dominant Optic Atrophy (Kjer Type) Am J Ophthalmol. 1993;115(3):360–7. doi: 10.1016/s0002-9394(14)73589-5. [DOI] [PubMed] [Google Scholar]
- 29.Votruba M, Fitzke FW, Holder GE, et al. Clinical features in affected individuals from 21 pedigrees with dominant optic atrophy. Arch Ophthalmol. 1998;116(3):351–8. doi: 10.1001/archopht.116.3.351. [DOI] [PubMed] [Google Scholar]
- 30.Kirkman MA, Korsten A, Leonhardt M, et al. Quality of life in patients with leber hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2009;50(7):3112–5. doi: 10.1167/iovs.08-3166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.