Abstract
The generalist genes hypothesis implies that general cognitive ability (g) is an essential target for understanding how genetic polymorphisms influence the development of the human brain. Using 8791 twin pairs from the Twins Early Development Study, we examine genetic stability and change in the etiology of g assessed by diverse measures during the critical transition from early to middle childhood. The heritability of a latent g factor in early childhood is 23%, while shared environment accounts for 74% of the variance. In contrast, in middle childhood, heritability is 62% and shared environment accounts for 33%. Despite increasing importance of genetic influences and declining influence of shared environment, similar genetic and shared environmental factors affect g from early to middle childhood, as indicated by a cross-age genetic correlation of 0.57 and a shared environmental correlation of 0.65. These findings set constraints on how genetic and environmental variation affects the developing brain.
Keywords: Cognitive Ability, Genetics, Intelligence, Childhood Development
General cognitive ability, also known as g, is the emergent first principal component of diverse test batteries designed to measure a range of cognitive abilities, from matrix reasoning and picture completion to vocabulary and general knowledge (Deary, 2000; Jensen, 1998). Despite differences between test batteries, the g factor consistently accounts for around 40% of the variance and correlates highly between batteries (Carroll, 1993). It also remains stable for much of adult life and is predictive of a wide range of important life outcomes (Gottfredson, 2004). g is one of the most consistently replicated findings in individual differences psychology. Similarly, neuroimaging techniques have established that brain measures such as total volume, regional volumes and gray matter density all correlate with g (Toga & Thompson, 2005).
Whether g reflects a single underlying cognitive or neurological process or is an emergent property of a wide range of physiological and psychological factors, it is clear that understanding childhood g and its genetic and environmental etiology is essential to our understanding of the developing brain (Plomin & Spinath, 2004). As a first step towards addressing these questions, quantitative genetics allows us to explore the structure of nature and nurture as it affects individual differences in development at the level of mind and brain (Plomin, DeFries, McClearn, & McGuffin, 2008). For example, beyond telling us that g and brain morphology are both highly heritable, recent quantitative genetic studies using multivariate analysis have calculated genetic correlations to show that the vast majority of genetic influences on a range of cognitive abilities and disabilities are shared (Plomin & Kovas, 2005), as are genetic influences on g and brain measures (Posthuma et al., 2002). These findings further underline the importance of g to research into the genetics of the developing brain.
One of the most fascinating hints from research on the genetics of g is that heritability appears to increase during development (Plomin, 1986; McGue, Bouchard, Jr., Iacono, & Lykken, 1993). Increases in heritability have been reported most often during the critical neurocognitive transition from infancy to early childhood (Petrill et al., 2004; Bartels, Rietveld, van Baal, & Boomsma, 2002); understanding these changes and their timing will prove crucial for choosing between competing models of neurocognitive development. However, very large sample sizes are required to accurately estimate the variance components. For the full genetic model, the typical sample size of 200 pairs (80 monozygotic and 120 dizygotic) in previous studies gives a 95% confidence interval of 0.05–0.70 for a heritability estimate of 0.40. Moreover, the demands for statistical power are greatly increased when comparing heritability estimates across age.
Earlier publications from our large, longitudinal Twins Early Development Study (TEDS) have focused on g in early (Spinath, Ronald, Harlaar, Price, & Plomin, 2003) or middle childhood (Davis, Arden, & Plomin, 2008). The aim of the present paper is to use the TEDS sample to explore changes in etiological influences during the transition between early and middle childhood. To do this we used a latent variable for early and middle childhood, which allows us to focus on the differences between these developmental periods, rather than on differences between individual measures or measurement occasions. Combining different measurements in this way also allows us to capitalize on the reliability of measurement afforded by a latent factor approach. Charting such natural genetic and environmental variation is likely to prove crucial as a frame for understanding the developmental neurobiology of the human brain in the context of the early school years.
METHODS
Participants
TEDS recruited families of twins born in England and Wales in 1994, 1995 and 1996 (Oliver & Plomin, 2007). Since then, the sample has remained representative of the UK population (ascertained by comparison with census data from the Office of National Statistics; Kovas, Haworth, Dale, & Plomin, 2007). Although twins have the option of participating or not during each phase of data collection, the pairs that do participate remain representative of the larger sample. Informed consent is obtained by post or online consent forms, and a test administrator is then assigned who telephones the family to assist or encourage. Ethical approval is provided by the Institute of Psychiatry ethics committee (05/Q0706/228).
We excluded from the analyses children with severe current medical problems and children who had suffered severe problems at birth or whose mothers had suffered severe problems during pregnancy. We also excluded twins whose zygosity was unknown or uncertain or whose first language was other than English. Finally, we included only twins whose parents reported their ethnicity as ‘white’, which is 93% of this UK sample. The present analyses are based on 8791 twin pairs (2979 monozygotic pairs, 2942 same-sex dizygotic and 2870 opposite-sex dizygotic). Subsets of this sample were assessed at each age, depending on the funding available.
Measures
General cognitive ability was assessed at each age through direct administration of nonverbal and verbal cognitive test batteries. Because of the difference in mean levels of ability between early and middle childhood, the large sample sizes involved and changes in technology over time, different subtests and modes of testing were used to assess general cognitive ability. Even though direct tests assessing a range of abilities were used at each age, it is possible that the varying subtests measured a different psychological construct. Fortunately, phenotypic g has proved amazingly robust to differences in the subtests used to assess it. For example, Johnson et al. (2004) found high correlations approaching unity among g factors extracted from three different test batteries in a large sample of 436. Recently, Johnson and colleagues constructively replicated this finding in a second, very different sample of 500 using five even more diverse measures of g (Johnson, te Nijenhuis & Bouchard, 2008); the g factors were again highly correlated (r > 0.95 among balanced batteries that assessed a range of skills). This argument for the equivalence of the g factor is further reinforced by the strong genetic correlations from early to middle childhood in the current study, which suggest that, despite the dramatic increase in heritability, substantially the same genes are involved during both developmental periods. This congruence of the genetic architecture implies that the construct measured at each age is the same g.
Early childhood
In early childhood, participants were administered verbal and nonverbal tests at each age. The g score used in the analysis was calculated as the standardized sum of the standardized verbal and nonverbal scores. Oliver et al. (2002) validate the measures against standard direct tests administered by a trained tester.
Nonverbal performance
Nonverbal cognitive performance was assessed using age-appropriate versions of the Parent Report of Children’s Abilities (PARCA; Oliver et al., 2002; Saudino et al., 1998). The PARCA is an hour-long test comprising three types of parent-administered tasks: a “find the pair” task, a drawing task, and a matching task. Some items are novel; others are adapted from previously well-validated tests such as the McCarthy Scales of Children’s Abilities (McCarthy, 1972) or the Bayley Scales of Infant Development (BSID-II; Bayley, 1993). Together, the administered items are designed to assess number, shape, size, conceptual grouping and orientation skills. This parent-administered component is supplemented by a small number of parent report items anchored on concrete behaviors and requiring simple yes or no answers. Some of these items are novel; others are, again, adapted from previously well-validated assessments such as the Minnesota Child Development Inventory (MCDI; Ireton & Thwing, 1974) and the Ages and Stages Questionnaires (Bricker, Squires, & Mounts, 1995).
Verbal performance
The verbal component of the early childhood battery comprises the CDI-III, an extension of the short form of the MacArthur Communicative Development Inventories: Words and Sentences (Fenson et al., 2000). The inventory is anchored in concrete instances of behavior, requiring a yes or no answer. The MCDI has been shown to have excellent internal consistency and test–retest reliability, as well as concurrent validity with tester-administered measures (Fenson et al., 2000).
Middle childhood
In middle childhood, participants were administered four mental ability tests at each age. As the factor loadings were similar for each of the subtests at each age, the g score used in the analysis was calculated as the standardized sum of the standardized subtest scores. These unit-weighted scores and factor scores derived from the first principal component correlated .99 at all ages.
Measures at age 7
Seven-year-olds were tested by telephone (Petrill, Rempell, Oliver, & Plomin, 2002). Prior to the telephone call, parents were sent a booklet of test items along with instructions indicating, for example, that the test booklet should not be opened prior to the telephone interview and that the twins should not be in the same room for the duration of the call. The booklet contained two tests of verbal cognitive abilities and two nonverbal tests. The verbal tests consisted of the Similarities subtest and the Vocabulary subtest from the Wechsler Intelligence Scale for Children (WISC-III-UK; Wechsler, 1992). The nonverbal tests were the Picture Completion subtest from the WISC-III-UK and Conceptual Grouping from the McCarthy Scales of Children’s Abilities (McCarthy, 1972).
Measures at age 9
Nine-year-old participants received a test booklet containing two verbal and two nonverbal tests that, like the tests in early childhood, were administered under the supervision of the parent (guided by an instruction booklet). The verbal tests comprised vocabulary and general knowledge tests adapted from the multiple-choice version of the WISC-III-UK (Wechsler, 1992).
The nonverbal tests included a Puzzle test adapted from the Figure Classification subtest of the Cognitive Abilities Test 3 (CAT3; Smith, Fernandes, & Strand, 2001). The second nonverbal test was a Shapes test also adapted from the CAT3 Figure Analogies subtest that assesses inductive and deductive reasoning. Details are reported by Davis et al. (2008).
Measures at age 10
Children at age 10 participated in web-based testing. Widespread access to inexpensive and fast internet connections in the UK has made online testing an attractive possibility for collecting data on the substantial samples necessary for genetic research, especially for multivariate genetic research. The advantages and potential pitfalls of data collection over the internet have been reviewed in detail elsewhere (Birnbaum, 2004). For older children, most of whom are competent computer users, it is an interactive and enjoyable medium. Through adaptive branching, it allows the use of hundreds of items to test the full range of ability, while requiring individual children to complete only a relatively small number of items to ascertain their level of performance. In tests where it is appropriate, streaming voiceovers can minimize the necessary reading. In addition, the tests can be completed over a period of several weeks, allowing children to pace the activities themselves, although they are not allowed to return to items previously administered. Finally, it is possible to intersperse the activities with games. All of these factors help to maintain children’s engagement with the tests. Participants at age 10 were tested on two verbal tests: WISC-III-PI Multiple Choice Information (General Knowledge) and WISC-III-PI Vocabulary Multiple Choice (Wechsler, 1992). Two nonverbal reasoning tests were also administered: WISC-III-UK Picture Completion (Wechsler, 1992) and Raven’s Standard Progressive Matrices (Raven, Court, & Raven, 1996). Details are reported in Haworth et al. (2007).
Statistical analyses
According to the quantitative genetic model (Plomin et al., 2008), twins reared together resemble each other due to the additive effects of shared genes (A) or shared (common) environmental factors (C). For identical or monozygotic (MZ) twins, the correlation between their genes is 1.00, whereas for non-identical or dizygotic (DZ) twins, the correlation is .50 because DZ twins on average share half of their segregating alleles. The correlation between twins for shared environment is, by definition, 1.00 for both MZ and DZ twins growing up in the same family, while non-shared environmental influences (E) are uncorrelated and contribute to differences between twins. For the twin analyses, standardized residuals correcting for age and sex were used because the age of twins is perfectly correlated across pairs, which means that, unless corrected, variation within each age group at the time of testing would contribute to the correlation between twins and be misrepresented as shared environmental influence. The same applies to the sex of the twins, since MZ twins are always of the same sex. The assumptions of the classical twin model, and their validity, have been discussed in detail elsewhere (Boomsma, Busjahn, & Peltonen, 2002).
As well as examining twin correlations in R (http://www.r-project.org), we used standard ACE model-fitting analysis in Mx (Neale, Boker, Xie, & Maes, 2006) where ACE stands for additive genetic influences (A), shared or common environmental influences (C), and non-shared environmental (E) influences, as above. Model-fitting analysis specifies a correlational structure (a model) using matrix algebra. This model is a hypothesis about the structure of the dataset, and is derived from what we know about how MZ and DZ twins are related to each other (see above). By fitting the model to the data using an iteration process, we can derive its ‘goodness of fit’ and derive parameter estimates for the contributions of A, C and E.
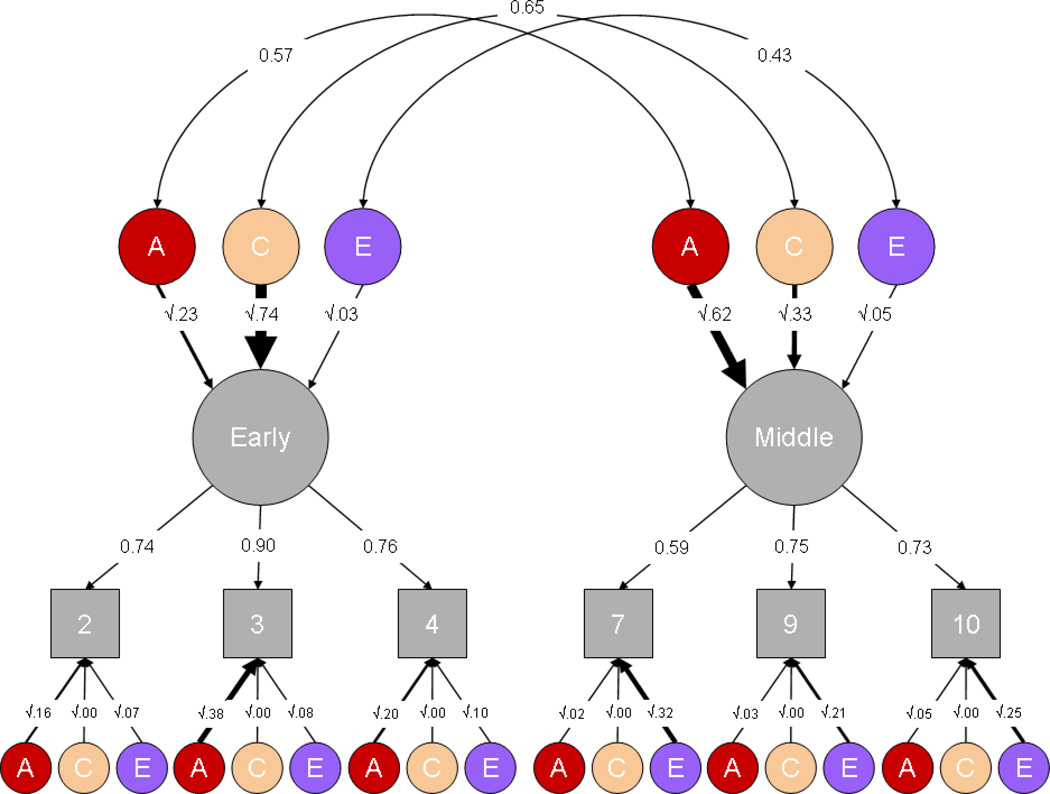
To explore the transition between early and middle childhood, we fitted a common pathway model to raw data (Figure 1; Neale et al., 2006). This model derives latent factors for early and middle childhood using maximum-likelihood factor analysis. It fixes the variance of these latent factors at 1 and partitions them into A, C and E components. It also partitions the covariance between the latent factors in the same way. Similarly, residual variance at each age is partitioned into A, C and E components. Earlier studies indicated very little difference in ACE estimates between males and females (Spinath et al., 2003; Davis et al., 2008), so we combined DZ same-sex and DZ opposite-sex twin pairs for this analysis.
Figure 1.
Longitudinal common pathway model showing genetic and environmental influences on g in early and middle childhood. Squares represent measures of g at each age. Single-headed arrows represent factor loadings; curved double-headed arrows represent correlations between latent factors. Variance is partitioned into additive genetic (A), shared (common) environmental (C), and non-shared environmental (E) influences. The latent factors (circles) at the top represent influences general to early or middle childhood; latent factors at the bottom represent residual variance specific to each age. The numbers and line weights represent parameter estimates, which are presented with confidence intervals in Table 2.
RESULTS
Univariate genetic analyses
Intraclass correlations (twin similarity coefficients) are presented in Table 1 for the MZ and DZ twins at each age, and for first principal component scores representing early and middle childhood. Correlations between MZ twins were consistently higher than those between DZ twins, suggesting a genetic contribution to g at each age. The significance of genetic influence is indicated by the lack of overlap between the confidence intervals for MZ and DZ twins. As a first estimate of the effect size (heritability), doubling the difference between the MZ and DZ correlations yields moderate and increasing heritability estimates of 26%, 32%, 30%, 38%, 36% and 46% at the individual ages and 26% and 54% for early and middle childhood. Shared environmental influences are estimated as the extent to which MZ resemblance exceeds heritability: 65%, 59%, 58%, 28%, 39% and 26% at each age and 68% and 28% for the first principal components. The remainder of the variance is attributed to non-shared environmental influences (plus error of measurement): 9%, 9%, 12%, 34%, 25%, 28% at each age and 6% and 18% for early and middle childhood. As shown in Table 2, ACE model-fitting results are consistent with estimates based on the twin correlations in Table 1.
Table 1.
Twin intraclass correlations of general cognitive ability from 2 to 10 years and for early childhood (first principal component scores across 2, 3, and 4 years) and middle childhood (first principal component scores across 7, 9 and 10 years). (95% confidence intervals in parentheses.)
| Measures | MZ | N pairs | DZ | N pairs |
|---|---|---|---|---|
| 2 years | .91 (.91–.92) | 1633 | .78 (.77–.80) | 3208 |
| 3 years | .91 (.90–.92) | 1379 | .75 (.73–.77) | 2685 |
| 4 years | .88 (.87–.89) | 2078 | .73 (.72–.75) | 4050 |
| 7 years | .66 (.63–.68) | 1616 | .47 (.44–.50) | 2889 |
| 9 years | .75 (.72–.78) | 1045 | .57 (.54–.60) | 1767 |
| 10 years | .72 (.69–.76) | 838 | .49 (.45–.53) | 1440 |
| Early childhood | .94 (.93–.95) | 867 | .81 (.79–.82) | 1681 |
| Middle childhood | .82 (.80–.85) | 646 | .55 (.51–.59) | 1053 |
Table 2.
Longitudinal common pathway analysis (model shown in Figure 1). (95% confidence intervals in parentheses.) Analysis based on a total of 8791 pairs.
| Measures | Common | ||||
| A | C | E | |||
| Early childhood | .23 (.21–.26) | .74 (.72–.77) | .03 (.02–.03) | ||
| Middle childhood | .62 (.53–.72) | .33 (.25–.41) | .05 (.02–.07) | ||
| rA | rC | rE | rP | ||
| Early to middle childhood | .57 (.48–.66) | .65 (.56–.75) | .43 (.20–.71) | .55 (.52–.58) | |
| axayrA/rP | cxcyrC/rP | exeyrE/rP | |||
| Mediation of rP | .39 (.32–.46) | .58 (.52–.64) | .03 (.01–.04) | ||
| Specific | Factor Loading | ||||
| A | C | E | |||
| Early childhood | 2 years | .16 (.16–.17) | .00 (.00–.00) | .07 (.07–.08) | .74 (.72–.75) |
| 3 years | .38 (.36–.38) | .00 (.00–.00) | .08 (.07–.09) | .90 (.89–.91) | |
| 4 years | .20 (.19–.21) | .00 (.00–.00) | .10 (.10–.11) | .76 (.75–.78) | |
| Middle childhood | 7 years | .02 (.01–.02) | .00 (.00–.00) | .32 (.29–.34) | .59 (.57–.62) |
| 9 years | .03 (.03–.05) | .00 (.00–.00) | .21 (.19–.24) | .75 (.73–.78) | |
| 10 years | .05 (.03–.05) | .00 (.00–.00) | .25 (.22–.28) | .73 (.71–.76) | |
Common pathway model
Figure 1 represents the common pathway model used to investigate the transition from early to middle childhood. This model partitions variance into latent factors representing early or middle childhood and residual variance specific to each age. The variance is then further partitioned into additive genetic (A), shared (common) environmental (C) and non-shared environmental (E) influences. Confidence intervals for the estimates are presented in Table 2.
Considering the ‘early’ and ‘middle’ latent factors first, heritability increases significantly from 23% to 62% (chi-square=64.5, degrees of freedom=1, p=9.7×10−16). The genetic correlation is 0.57 between early and middle childhood, suggesting both genetic stability and change in cognitive development. As seen at the bottom of Figure 1, age-specific residual genetic effects are substantial in early childhood but they are minimal in middle childhood. The genetic correlation is independent of heritability of the latent factors; weighted by the heritabilities in early and middle childhood, genetics accounts for 39% of the phenotypic correlation of 0.55 between the latent factors.
Although our focus is on genetic influence, these same analyses provide interesting insights into environmental influence as well. For example, shared environmental influences account for much (74%) of the variance common across early childhood. The non-shared environment accounts for very little of the variance in the latent factor (3%). In middle childhood, shared environment accounts for much of the variance not attributable to genetic influences (33%). Similar to early childhood, the non-shared environment accounts for only 5% of variance common across middle childhood. The decrease in shared environmental influence is statistically significant (chi-square=102.1, df=1, p=5.3×10−24), but the change in the non-shared environment is not (chi-square=2.6, df=1, p=0.1). In terms of the residual variance specific to each age, although much of the residual influence in early childhood is accounted for by specific genetic influences (16%–38%), most of the residual variance in middle childhood is accounted for by the non-shared environment. There is a negligible amount of residual shared environment in either early or middle childhood.
Examining the covariance between the environmental latent factors, the shared environmental correlation (0.65), like the genetic correlation, suggests both continuity and change; the non-shared environmental correlation is more modest (0.43). However, because non-shared environment accounts for such a small proportion of the latent factors, it is difficult to estimate this correlation accurately. The genetic and environmental correlations are independent of the strength of the genetic and environmental influences on the latent factors. However, it is also possible to estimate the genetic and environmental mediation of the phenotypic correlation of 0.55 between the latent factors – the proportion of the phenotypic correlation accounted for by genes and the environment (row 3 of Table 2). Because there is very little non-shared environmental influence on either latent factor and in spite of the moderate non-shared environmental correlation, non-shared environmental influences account for only 3% of the phenotypic correlation between early and middle childhood. In contrast the shared environment accounts for most (58%) of the correlation, with genetics accounting for the remainder (39%).
DISCUSSION
Four main findings are apparent from Figure 1: increasing heritability from early to middle childhood, diminishing shared environmental influence, genetic and environmental continuity and, conversely, genetic and environmental change.
Increasing heritability
Heritability of g increases dramatically (and significantly) from early to middle childhood. One possible explanation for this is active or evocative gene-environment correlation (Plomin, 1994; Jaffee & Price, 2007). Although early in life children have relatively little control over their environments, as they grow older, especially as they begin full-time education, children are freer to seek out environments or evoke reactions from teachers and peers correlated with their genetic propensities (Scarr & McCartney, 1983). This possibility of gene-environment correlation could increase heritability as environmentally mediated genetic influences impact on the development of cognition and, consequently, on the developing brain, reinforcing more direct genetic influences.
This increase in heritability could also reflect the changing relationship between g and the brain. For example, early developmental twin studies of structural magnetic resonance imaging suggest that in young children cortical thickness is uncorrelated with g, whereas by late childhood there is a modest positive correlation (Shaw et al., 2006). Results from other studies implicate the maturation of white matter tracts: the heritability of white matter volume increases through childhood and into adolescence, mirroring the increase in the heritability of g (Wallace et al., 2006).
Methodological explanations need also to be considered, such as the possibility that reliability of measures increase from early to middle childhood which would be reflected in increased heritability. The similar factor loadings on the latent g variables in early and middle childhood do not support this hypothesis. Moreover, a hypothesis of increasing reliability could not explain the next issue of decreasing shared environment.
Diminishing shared environment
An alternative explanation for the increase in heritability is that genetic influence increases because environmental variability decreases. Heritability and environmentality are measures of the proportions of the population variance accounted for by genetic and environmental factors, rather than measures of the raw variance attributable to these influences. So, for example, if the variance attributable to genetic factors increases while the variance attributable to the environment remains constant, this will be seen as an increase in heritability and a decrease in environmentality. The same is true if the variance attributable to environmental factors diminishes – heritability will appear to increase.
One major environmental change between early and middle childhood is the start of full time education. In the UK, where the national curriculum provides a relatively homogenous learning environment, it is possible that the start of school marks a decrease in the variability of the everyday environment between twin pairs, diminishing the importance of the shared environment for developing cognition.
Another reason for a decrease specifically in shared environmental variance could be differences in the uterine or perinatal environment. These could bring about early differences in g that diminish by middle childhood. In the twin model this process would be seen as diminishing shared environmental influence. Although twins whose mothers reported problems during pregnancy or birth and those twins with very low birth weight were excluded from this analysis, it is possible that enough variability remains to produce an effect that is still apparent in early childhood. However, we find very low correlations between g and perinatal variables such as birthweight (0.06) and gestation time (0.04) once twins with problematic pregnancies or births have been excluded.
Genetic and environmental continuity
In spite of these changes in the relative contributions of genetic and environmental factors, the high genetic and environmental correlations from early to middle childhood suggest that the particular genes and environmental influences involved remain relatively stable. Although this may seem at odds with such dramatic changes in heritability and environmentality, these changes signify relative changes in the variance attributable to variability in these sources and not necessarily to changes in the sources themselves. Differences in the environment largely explain variation between children in early g, whereas differences in genes explain more of the variation in middle childhood. However, it seems that although the relative scale of their impact changes, in general the same genes and environments remain important.
Genetic and environmental change
At the same time, because genetic and environmental correlations are far from 1.00, our results provide evidence of genetic and (particularly non-shared) environmental change. Environmentally, the start of compulsory education suggests a natural environmental breakpoint – the day-to-day social and physical environment of the twins shifts overnight as they adapt to new friends, adults and surroundings; it is not difficult to see that g-related environments are likely to change both between and within twin pairs.
In the same way, changes in the developmental neurobiology of the brain may give us some insight into how patterns of gene expression change. For example, during early childhood, cortical thickness and gray matter volumes increase (Shaw et al., 2006). This is accompanied by dendritic spine growth and the formation of new connections in response to the environment. In contrast, middle childhood coincides with the thinning of gray matter in a back-to-front wave over the cortex as it matures by pruning connections (Gogtay et al., 2004), a vital step in the formation of meaningful and efficient neural networks. It is likely that a different set of genes is active during this second developmental period, bringing into play a new range of genetic variants affecting cognition.
Conclusion
Modern quantitative genetic techniques used with large genetically informative samples allow us to go beyond simply asking whether and how much a trait is heritable, to answer important multivariate and longitudinal questions about the nature and nurture of cognition – ‘how’ rather than ‘how much’ (Anastasi, 1958). By addressing issues such as the developmental etiology of g from early to middle childhood, they give us a new perspective on questions that are becoming increasingly important in fields such as neuroscience and molecular genetics. Although studies such as ours paint with a broad brush, we predict that they will continue to play an important role by providing the map that will allow detailed studies of social environments, cellular processes and molecular mechanisms to meet in a more comprehensive understanding of the developmental biology of the brain.
Acknowledgements
We thank the parents of the twins in TEDS and the twins themselves for making this study possible. TEDS is supported by a program grant from the UK Medical Research Council (G500079), and this research is also supported by a grant from the US NICHD/OSERS (HD46167).
References
- Anastasi A. Heredity, environment, and the question how? Psychological Review. 1958;65:197–208. doi: 10.1037/h0044895. [DOI] [PubMed] [Google Scholar]
- Bartels M, Rietveld MJ, van Baal GC, Boomsma DI. Genetic and environmental influences on the development of intelligence. Behavior Genetics. 2002;32:237–249. doi: 10.1023/a:1019772628912. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. New York, NY: The Psychological Corporation; 1993. [Google Scholar]
- Birnbaum MH. Human research and data collection via the internet. Annual Review of Psychology. 2004;55:803–832. doi: 10.1146/annurev.psych.55.090902.141601. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Reviews Genetics. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities. New York: Cambridge University Press; 1993. [Google Scholar]
- Davis OSP, Arden R, Plomin R. g in middle childhood: Moderate genetic and shared environmental influence using diverse measures of general cognitive ability at 7, 9 and 10 years in a large population sample of twins. Intelligence. 2008;36:68–80. [Google Scholar]
- Deary I. Looking down on human intelligence: from psychometrics to the brain. Oxford: Oxford University Press; 2000. [Google Scholar]
- Fenson L, Pethick S, Renda C, Cox JL, Dale PS, Reznick JS. Short-form versions of the MacArthur Communicative Development Inventories. Applied Psycholinguistics. 2000;21:95–116. [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences US A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredson LS. Intelligence: is it the epidemiologists' elusive "fundamental cause" of social class inequalities in health? Journal of Personality and Social Psychology. 2004;86:174–199. doi: 10.1037/0022-3514.86.1.174. [DOI] [PubMed] [Google Scholar]
- Haworth CMA, Harlaar N, Kovas Y, Davis OSP, Oliver B, Hayiou-Thomas ME, et al. Online Internet Testing of Large Samples Needed in Genetic Research. Twin Research and Human Genetics. 2007;10(4):554–563. doi: 10.1375/twin.10.4.554. [DOI] [PubMed] [Google Scholar]
- Ireton H, Thwing E. The Minnesota Child Development Inventory. Minneapolis, MN: Behavior Science Systems; 1974. [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AR. The g factor: The science of mental ability. Wesport: Praeger; 1998. [Google Scholar]
- Johnson W, Bouchard TJ, Jr, Krueger RF, McGue M, Gottesman II. Just one g: consistent results from three test batteries. Intelligence. 2004;32:95–107. [Google Scholar]
- Johnson W, te Nijenhuis J, Bouchard TJ., Jr Still just 1 g: Consistent results from five test batteries. Intelligence. 2008;36:81–95. [Google Scholar]
- Kovas Y, Haworth CM, Dale PS, Plomin R. The genetic and environmental origins of learning abilities and disabilities in the early school years. Monographs of the Society for Research in Child Development. 2007;72:1–144. doi: 10.1111/j.1540-5834.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. McCarthy Scales of Children's Abilities. New York, NY: The Psychological Corporation; 1972. [Google Scholar]
- McGue M, Bouchard TJ, Jr, Iacono WG, Lykken DT. Behavioral genetics of cognitive ability: A life-span perspective. In: Plomin R, McClearn GE, editors. Nature, nurture, and psychology. Washington, DC: American Psychological Association; 1993. pp. 59–76. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 7th ed. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry; 2006. [Google Scholar]
- Oliver B, Dale PS, Saudino K, Petrill SA, Pike A, Plomin R. The validity of parent-based assessment of non-verbal cognitive abilities of three-year olds. Early Child Development and Care. 2002;172:337–348. [Google Scholar]
- Oliver BR, Plomin R. Twins Early Development Study (TEDS): A multivariate, longitudinal genetic investigation of language, cognition and behavior problems from childhood through adolescence. Twin Research and Human Genetics. 2007;10:96–105. doi: 10.1375/twin.10.1.96. [DOI] [PubMed] [Google Scholar]
- Petrill SA, Lipton PA, Hewitt JK, Plomin R, Cherny SS, Corley R, et al. Genetic and environmental contributions to general cognitive ability through the first 16 years of life. Developmental Psychology. 2004;40:805–812. doi: 10.1037/0012-1649.40.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrill SA, Rempell J, Oliver B, Plomin R. Testing cognitive abilities by telephone in a sample of 6-to 8-year olds. Intelligence. 2002;30:353–360. [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics (Fifth Edition) New York: Worth; 2008. [Google Scholar]
- Plomin R. Development, genetics, and psychology. Hillsdale, NJ: Erlbaum; 1986. [Google Scholar]
- Plomin R. Genetics and experience: The interplay between nature and nurture. Newbury Park, CA: Sage Publications; 1994. [Google Scholar]
- Plomin R, Kovas Y. Generalist genes and learning disabilities. Psychological Bulletin. 2005;131:592–617. doi: 10.1037/0033-2909.131.4.592. [DOI] [PubMed] [Google Scholar]
- Plomin R, Spinath FM. Intelligence: Genetics, genes, and genomics. Journal of Personality and Social Psychology. 2004;86:112–129. doi: 10.1037/0022-3514.86.1.112. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJC, Baare WFC, Pol HEH, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven's Progressive Matrices and Vocabulary Scales. Oxford: Oxford University Press; 1996. [Google Scholar]
- Saudino KJ, Dale PS, Oliver B, Petrill SA, Richardson V, Rutter M, et al. The validity of parent-based assessment of the cognitive abilities of two-year-olds. British Journal of Developmental Psychology. 1998;16:349–363. [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype --> environmental effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:619–620. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Smith P, Fernandes C, Strand S. Cognitive Abilities Test 3 (CAT3) Windsor: nferNelson; 2001. [Google Scholar]
- Spinath FM, Ronald A, Harlaar N, Price TS, Plomin R. Phenotypic 'g' early in life: On the etiology of general cognitive ability in a large population sample of twin children aged 2 to 4 years. Intelligence. 2003;31:195–210. [Google Scholar]
- Toga AW, Thompson PM. Genetics of brain structure and intelligence. Annual Review of Neuroscience. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Eric Schmitt J, Lenroot R, Viding E, Ordaz S, Rosenthal MA, et al. A pediatric twin study of brain morphometry. Journal of Child Psychology and Psychiatry. 2006;47:987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children - Third Edition UK (WISC-IIIUK) Manual. London: The Psychological Corporation; 1992. [Google Scholar]