Functional neuroimaging with positron emission tomography (PET) and functional MRI permits identification of neurophysiological correlates of distinctly human psychological functions such as language, conscious experience, and philosophical thought. In the enthusiasm to attribute regional brain activity in humans to high-order functions, low-level accounts may be underemphasized. It is widely appreciated that brainstem and hypothalamic nuclei have a critical role in regulating neuroendocrine cycles and controlling homeostatic autonomic reflexes and vegetative processes (1). However, psychologists frequently ascribe autoregulatory control only to subcortical “reptilian” (2) regions, whereas cortical areas emote, evaluate, and reason. In recent years, Damasio (3, 4) has reemphasized the dependence of higher-order functions on bodily states of arousal. In a recent issue of PNAS, the work of Teves et al. (5) represents an important reminder that cortical and thalamic activity is modulated directly by perturbations in peripheral homeostasis that evoke autonomic arousal. The authors undertook a PET study to examine changes in regional brain activity (reflected in regional cerebral blood flow) during states of low blood glucose (hypoglycemia). Hypoglycemia evoked sympathetic and parasympathetic autonomic arousal, apparent in a range of physiological and neuroendocrine measures. States of hypoglycemia and autonomic arousal were associated with enhanced activity in rostral anterior cingulate (ACC), orbitofrontal cortex, thalamus, and brainstem. One interpretation is that these activity increases reflect generation and control of autonomic responses to the low-level physiological challenge of hypoglycemia (5).
Anterior Cingulate Cortex
Historically, rostral ACC is implicated in autonomic control. Studies in animals (6) and humans (7) demonstrate that ACC stimulation may evoke a range of autonomic responses. ACC is interconnected with autonomic nuclei in hypothalamus and brainstem, and with orbitofrontal, insular, and medial temporal regions that also project these homeostatic centers (8). However, cognitive interpretations of ACC function predominate in recent neuroscience literature, where dorsal ACC is implicated in processes such as error monitoring, conflict processing (9), and response selection. The increasing preeminence of neuroimaging is responsible, in part, for this interpretative bias. ACC activity, especially in the dorsal supragenual region, is enhanced to most “challenging” situations, where a task (or stimulus) is contrasted with a low-grade control condition (10). Demanding cognitive processes are likely to elicit enhanced dorsal ACC activity. Difficult physical, psychological, or emotional challenges share the production of integrated arousal responses more obviously than a common cognition. The study of Teves et al. (5) demonstrates ACC activation by a low-level physiological challenge that evokes autonomic arousal without specific cognitive or emotional demands.
The ACC is a phylogenetically old “limbic” region [part of MacLean's “visceral brain” (2)]. The structure and cytoarchitecture of ACC are relatively consistent across mammalian species, perhaps with the exception of great apes (11), where the presence of atypical pyramidal cells may correlate with human evolutionary developments such as subjective consciousness or progress to bipedal gait. Damage to ACC may impair performance of self-initiated tasks and blunt emotional reactions to stimuli such as pain. Notably, ACC damage also impairs the generation of autonomic arousal responses (12, 13). These responses may serve to facilitate or interrupt ongoing behavior, hence their absence might contribute to decreased motivational performance. Akinetic mutism, an oft-cited manifestation of ACC damage, rarely occurs without involvement of supplementary motor cortex, thalamus, and/or basal ganglia (14). Performance in a range of psychometric “executive tasks” can be intact despite marked bilateral ACC damage (13). These observations suggest that ACC integrity is not critical to either general cognitive processes, such as attention, or specific highorder functions, such as conflict detection. An alternative, perhaps more prosaic, account is that ACC mediates generation of autonomic bodily responses, integrated with internally or externally generated behavioral demands (13).
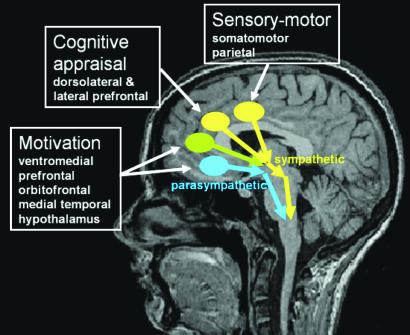
Autonomic arousal is a heuristic for relatively enhanced sympathetic activity (ignoring organ-specific subresponses in both sympathetic and parasympathetic axes). If ACC is a generator of arousal, afferent inputs from adjacent cortices may determine which ACC subregions provide efferent drive to brain-stem autonomic centers. Thus activity in caudal regions of ACC may reflect arousal during pain or physical effort, consequent on input from somatosensory and motor cortices. Dorsal supragenual ACC activity will reflect arousal associated with the cognitive processes supported within to dorsolateral prefrontal cortex, including planning, monitoring, response selection, and anticipation of action. Similarly, genual and subgenual ACC activity may reflect arousal accompanying reward-based emotional/motivational processing within orbitofrontal and medial temporal regions (Fig. 1). However, neuroimaging studies also suggest that human subgenual ACC is functionally dissociable from dorsal ACC. Whereas dorsal ACC activates during effortful tasks, subgenual ACC deactivates during attention-demanding tasks and is more active during baseline resting states (15). Similarly, subgenual neurons show enhanced activity during vegetative states such as sleep (16). Yet functionally and anatomically, subgenual ACC is more strongly linked to autonomic control centers than dorsal ACC (6, 8). These observations suggest that subgenual ACC activity reflects parasympathetic, rather than sympathetic, autonomic drive. Empirical support for this proposition is awaited, but it may account for observed genual extension of ACC activity in states of both sympathetic and parasympathetic arousal, e.g., during hypoglycemia (5).
Fig. 1.
Diagram illustrating a speculative role of ACC subregions for translating regional cortical representations and computations into efferent autonomic bodily responses.
Interoception
The above account outlines the potential role of ACC in generating arousal responses that accompany and perhaps facilitate cognition and behavior. There is also evidence that ACC activity reflects afferent information about the internal homoeostatic state of the body (17) [representing an alternative interpretation of the findings of Teves et al. (5)]. Anatomically, the representation within ACC of feedback from the periphery may be mediated by an offshoot of a dedicated interoceptive pathway. Craig (18) has described a specialized lamina 1 spinothalamocortical tract that conveys interoceptive sensory information to cortex, enabling a detailed dynamic representation of the internal bodily state. In this model, a mapping of the interoceptive state occurs within human orbitofrontal and right insula cortices that represent the neuroanatomical endpoint of the lamina 1 interoceptive system. Craig also proposed a parallel specialization of ACC, for the support and facilitation of motivational behavior, where ACC receives interoceptive information via an ancillary medial lamina 1 pathway (18).
There is empirical evidence that interoceptive representation within right insular and orbitofrontal cortices may be available to conscious awareness (19) [crucially providing a neural substrate for emotional feeling states arising from automatic visceral responses (4, 18, 19)], yet the extent to which ACC activity mediates conscious appraisal or subjective emotional experience is unclear. ACC activity correlates with self-reported ratings of emotion and arousal (20) and primary motivational drives such as thirst (21). Also, consistent with receipt of afferent interoceptive information, ACC activity is enhanced when attention is focused on internal bodily processes, even in the absence of arousal (19). These observations implicate ACC in supporting conscious experience including emotional feeling states (20, 21). However, activity in right insular, not ACC, cortex correlates with conscious awareness of bodily responses (predicting emotional feelings) (19) and also reflects interactions between autonomic feedback and awareness of emotional stimuli (22). The article by Teves et al. (5) is also important in this regard. Enhanced activity in genual ACC and thalamus is associated with a hypoglycemic state that evoked marked changes in autonomic arousal. However, hypoglycemia evoked only weak changes in subjective emotional experience and few subjective symptoms, suggesting that ACC activity relates more to peripheral autonomic response than a conscious experience of emotional feelings.
Insula and Context
In the study by Teves et al. (5), insula activity, in response to the evoked autonomic changes, did not survive threshold significance. This may seem to contradict the argument that insula cortex, especially right anterior insula, provides a detailed representation of internal state (18, 19). One consideration is technical. Transient changes in autonomic arousal may evoke greater insular activity (in functional MRI) than more sustained changes in bodily state (e.g., in positron emission tomography studies). However, this account is not supported by previous observations (17). An alternative is that the cognitive context of the experiment blunted anterior insula responses to the autonomic arousal. In line with arguments of Schachter and Singer (23), the subjects' attribution of autonomic arousal to the experimental process (i.e., hypoglycemia induced by insulin infusion) may represent a satisfactory account, such that a slowonset steady-state autonomic arousal was not signaled as a “mismatch” of anticipated bodily response and hence did not provoke a subjective emotional reaction via right anterior insula activation. The lack of a significant change in anxiety level during hypoglycemia is noted by Teves et al. (5). Despite a relative absence of right insula response, enhanced orbitofrontal activity was observed, consistent with evidence for sensitivity of orbitofrontal neurons to hunger (24, 25), a primary motivational state evoked by hypoglycemia.
Last, Teves et al. (5) importantly described a global reduction in overall cerebral blood flow during hypoglycemia and autonomic arousal. This observation serves as a reminder that in functional neuroimaging experiments, our indices of neural activity may depend directly or indirectly on the subject's physiological state. Regional cerebral blood flow or blood oxygenation-dependent signal must be disambiguated from confounding biological responses to be useful indices of neuronal activity. Although a sustained drop in circulating glucose level may represent an extreme challenge on cerebral metabolism and neurovascular coupling, task-induced changes in global activity (reflecting systemic arousal, hypoglycemia, or respiratory modulation) may confound identification of regional effect-related responses. Moreover, entrainment of peripheral autonomic and respiratory responses can occur with task demands on a trial-by-trial basis and differentially impact on the signal and signal-to-noise ratio across different neurovascular territories. Thus, there remains a continuing need for inferences gained from neuroimaging experiments to be validated in animal and clinical studies.
See companion article on page 6217 in issue 16 of volume 101.
References
- 1.Spyer, K. M. (1999) in Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, eds. Mathias, C. J. & Bannister, R. (Oxford Univ. Press, Oxford, U.K.), pp. 45-55.
- 2.MacLean, P. D. (1973) A Triune Concept of Brain and Behaviour, eds. Boag, T. J. & Campbell, D. (University of Toronto Press, Toronto).
- 3.Damasio, A. R. (1994) Descartes' Error: Emotion, Reason and the Human Brain (Grosset Putnam, New York).
- 4.Damasio, A. R. (1999) The Feeling of What Happens: Body and Emotion in the Making of Consciousness (Harcourt Brace, New York).
- 5.Teves, D., Videen, T. O., Cryer, P. E. & Powers, W. J. (2004) Proc. Natl. Acad. Sci. USA 101, 6217-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaada, B. R. (1951) Acta Physiol. Scand. 24, Suppl. 83, 1-285. [PubMed] [Google Scholar]
- 7.Pool, J. L. & Ransohoff, J. (1949) J. Neurophysiol. 12, 385-392. [DOI] [PubMed] [Google Scholar]
- 8.Barbas, H, Saha, S., Rempel-Clower, N & Ghashghaei, T. (2003) BMC Neuroscience 4, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerns, J. G., Cohen J. D., MacDonald, A. W., Cho, R. Y., Stenger, V. A. & Carter, C. S. (2004) Science 303, 1023-1026. [DOI] [PubMed] [Google Scholar]
- 10.Paus, T., Koski, L., Caramanos, Z. & Westbury, C. (1998) NeuroReport 9, R37-R47. [DOI] [PubMed] [Google Scholar]
- 11.Allman, J. M., Hakeem, A., Erwin, J. M., Nimchinsky, E. & Hof, P. (2001) Ann. N. Y. Acad. Sci. 935, 107-117. [PubMed] [Google Scholar]
- 12.Zahn, T. P., Grafman, J. & Tranel, D. (1999) Neuropsychologia 37, 1227-1241. [DOI] [PubMed] [Google Scholar]
- 13.Critchley, H. D., Mathias, C. J., Josephs, O., O'Doherty, J., Zanini, S., Dewar, B. K., Cipolotti, L., Shallice, T. & Dolan, R. J. (2003) Brain 126, 2139-2152. [DOI] [PubMed] [Google Scholar]
- 14.Nagaratnam, N., Nagaratnam, K., Ng, K. & Diu, P. (2004) J. Clin. Neurosci. 11, 25-30. [DOI] [PubMed] [Google Scholar]
- 15.Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A. & Shulman, G. L. (2001) Proc. Natl. Acad. Sci. USA 98, 676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolls, E. T., Inoue, K. & Browning, A. (2003) J. Neurophysiol. 90, 134-142. [DOI] [PubMed] [Google Scholar]
- 17.Critchley, H. D., Mathias, C. J. & Dolan, R. J. (2001) Nat. Neurosci. 2, 207-212. [DOI] [PubMed] [Google Scholar]
- 18.Craig, A. D. (2002) Nat. Rev. Neurosci. 3, 655-666. [DOI] [PubMed] [Google Scholar]
- 19.Critchley, H. D., Wiens, S., Rotshtein, P., Öhman, A. & Dolan, R. J. (2004) Nat. Neurosci. 7, 189-195. [DOI] [PubMed] [Google Scholar]
- 20.Lane, R. D., Chua, P. M. & Dolan, R. J. (1999) Neuropsychologia 37, 989-997. [DOI] [PubMed] [Google Scholar]
- 21.Egan, G., Silk, T., Zamarripa, F., Williams, J., Federico, P., Cunnington, R., Carabott, L., Blair-West, J., Shade, R., McKinley, M., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 15241-15246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Critchley, H. D., Mathias, C. J. & Dolan, R. J. (2002) Neuron 3, 653-663. [DOI] [PubMed] [Google Scholar]
- 23.Schachter, S. & Singer, J. E. (1962) Psychol. Rev. 69, 379-399. [DOI] [PubMed] [Google Scholar]
- 24.Critchley, H. D. & Rolls, E. T. (1996) J. Neurophysiol. 75, 1673-1686. [DOI] [PubMed] [Google Scholar]
- 25.Gottfried, J. A., O'Doherty, J. & Dolan, R. J. (2003) Science 301, 1104-1107. [DOI] [PubMed] [Google Scholar]