Abstract
Behavioral and genetic differences among Wistar-Kyoto (WKY) rats from different vendors and different breeders have long been observed, but generally overlooked. In our prior work, we found that two closely related WKY substrains, the WKY/NCrl and WKY/NHsd rats, differ in a small percentage of their genome which appeared to be highly enriched for autism risk genes. Although both substrains have been used widely in studies of hypertension, attention deficit/hyperactivity disorder (ADHD) and depression, they have not been tested for any autism-related behavioral phenotypes. Furthermore, these two substrains have often been used interchangeably in previous studies; no study has systematically examined the phenotypic differences that could be attributed by their small yet potentially meaningful genetic differences. In this paper we compared these two substrains on a battery of neurobehavioral tests. Although two substrains were similar in locomotor activity, WKY/NCrl rats were significantly different from WKY/NHsd rats in the elevated plus maze test, as well as measures of social interaction and ultrasonic vocalization. These strains were also compared with Sprague Dawley (SD) rats, a common outbred strain, and spontaneous hypertensive rats (SHR), an inbred rat model for ADHD and hypertension, which were derived from the same ancestor strain as the WKY strains. Our behavioral findings suggest that WKY/NCrl rats may be useful as a model autism spectrum disorders due to their lower social interest, lower ultrasonic vocalization and higher anxiety levels when WKY/NHsd rats are used as the control strain. Given the small genetic difference between the two inbred substrains, future studies to identify the exact gene and sequence variants that differ between the two may be useful for identifying the genetic mechanisms underlying these behaviors.
Keywords: WKY, substrain, social interaction, ultrasonic vocalization, inbred rat
1. Introduction
Animal models play a useful role in medical research, and are of particular importance for neurodevelopmental and neuropsychiatric disorders, where the affected tissue (brain) is rarely available for study in humans. Conditions such as depression, autism spectrum disorders (ASDs), and attention deficit/hyperactivity disorder (ADHD) are complex multifactorial disorders having high genetic heritability and complex genetic architecture that involves small effects from hundreds, if not thousands, of genes. Although genetic knockouts and knock-ins mice--or rats recently--are readily available and can provide insights into the molecular mechanisms of genetic disorders, their utility can be limited for these types of disorders in that they cannot fully represent the complex profile of multifactorial genetic causality. In this regard, inbred animals that develop unique phenotypes, due to inbreeding and selection, provide a “naturally” occurring model for complex human genetic disorders. Inbred animals that model neuropsychiatric phenotypes, such as WKY and the Flinders Sensitive Line (FSL) rats as models of depression [1, 2] and SHR rats as a model of ADHD[3], have proven to be extremely useful models to advance our understanding of disease pathophysiology and treatment efficacy.
For ASDs, previous work has focused on inbred mouse strains that have low sociability, such as the BALB/c and BTBR T(+)tf/J strains [4–6],and genetically modified mice [7]. Rats are superior models for studies of ASDs than mice in that they have a richer social behavioral repertoire[8]. Rats can also be easily trained to learn various complex tasks, which is important for characterizing their neurocognitive functions [9]. From the pharmacological point of view, rats are the model of choice for drug testing. However, rat models for ASDs have been mainly limited to prenatal exposure to neurotoxins (such as valproic acid) and experimentally induced hypothyroidism[10, 11]. A handful of gene knockout models for rats have been made available through SAGE Labs in the past couple of years. Inbred rat models for ASDs are lacking.
More than 500 inbred rat lines have been developed in the past decades for numerous human disease and phenotypes. One of the main weaknesses of the common inbred strains used as animal models for human diseases is that they often suffer from a lack of appropriate genetic controls. The often used control strains are, for example, their ancestral outbred lines, or other outbred or inbred lines that do not have the phenotype of interest. Even an inbred control line developed under parallel selection from a common ancestor can often differ from a model line in thousands of genes, including many that are irrelevant to the phenotype of the interest. These differences render the task of identifying causal genes an extremely difficult and time-consuming one as it has historically proven to be.
In our previous study of the genetic heterogeneity of WKY substrains from different sources, we observed two WKY substrains that were extremely similar genetically; The WKY/NHsd and WKY/NCrl substrains only differ in~2.5% of their genome as estimated by a 10K genome-wide SNP array. In contrast, even other lines within the WKY and SHR lineages differed in more than 20–30% of the genome. In addition, we found that their genomic regions tagged by the small numbers of polymorphic SNPs were highly enriched for ASDs candidate genes and pathways involved in brain development and neuronal functions [12]. Although SNP-based analyses can only estimate the possible divergent genomic regions, and cannot reveal the actual causal genetic variants, our analysis suggests that there could be potential differences in genes related to ASDs, thus affecting brain functioning and behavior. Indeed, re-sequencing verified that two known risk genes for ASDs and ADHD in the estimated regions,SLC9A9 and SLC6A3, harbor genetic variants that could be functional [12]. Unfortunately, these two substrains have been used interchangeably in previous research and almost no study, except one examining stress-response[13], has directly compared the two. No study has examined any ASDs-related behaviors between the two WKY substrains. Therefore, in this study, we asked if the genetic differences could be used to predict behavioral differences between these two strains. The unique advantage of studying these two substrains is that if any behaviors differ between them, the genetic cause of these differences may be easily traced back to the small portion of their genomes that differ.
2. Methods
2.1 Animals
32 female rats were used (N=8 for each of the four strains: WKY/NCrl, spontaneous hypertensive rats (SHR) and Sprague Dawley (SD) from Charles River Laboratories, US; WKY/NHsd rats from Harlan Laboratories). Rats were obtained from the vendors at postnatal day23 (p23). Two females from the each litter were requested and littermates were group housed together in a standard cage (26 ×48×21 cm) with paper beddings. Animals were kept at a reversed dark/light cycle (lights on 21:00 to 9:00) with ad lib food and water. Animals were handled daily in the testing room, and were tested for behavioral phenotypes in the following order: elevated plus maze (EP, p29), open field activity (OF, p31), object habituation (OH, p32) and novel object recognition (NOR, p33), three chambered social interaction (p37-40), home cage video recording (p48-49) and ultrasonic vocalization (USV, p103). All procedures were approved by the Institutional Animal Care and Use Committee of SUNY Upstate Medical University and were performed between 10:00–18:00 during animal’s dark cycle. Testing animals were covered from lights at all times unless during the specific procedures as described below.
2.2 Elevated plus maze test (EP)
The EP maze consisted of two open and two closed arms (51×10 cm, LxW) crossing at the center perpendicular to each other at 51cmabove the floor. The closed arms are enclosed by51cmtall opaque walls except the center crossing zone (10×10 cm). The maze is illuminated from an overhead source to reach approximately 400lux. Each rat was placed in the center zone with it head directed toward a closed arm and was allowed to freely explore all arms for 5 min. We used an USB camera controlled by the AnyMaze Software (Stoelting) to video-track the activity. The distance traveled, time in and entries into each of the arms were recorded.
2.3 Open field activity (OF)
We used four square non-transparent open field boxes (51×51×38 cm, WxLxH) for the tests. Each animal was placed in the center of the box and allowed to move freely for 20 min under indirect dim light of approximately 30lux. A remotely controlled USB camera (positioned over the top of the box) and AnyMaze software was used for video-tracking. A preloaded 6×6 geometric grid on the computer screen defined the areas of each box as the corners, borders, and center zones (Figure 2A). We analyzed total distance, average speed, and time spent in different zones of the field.
Figure 2. Open Field and Object Tests.
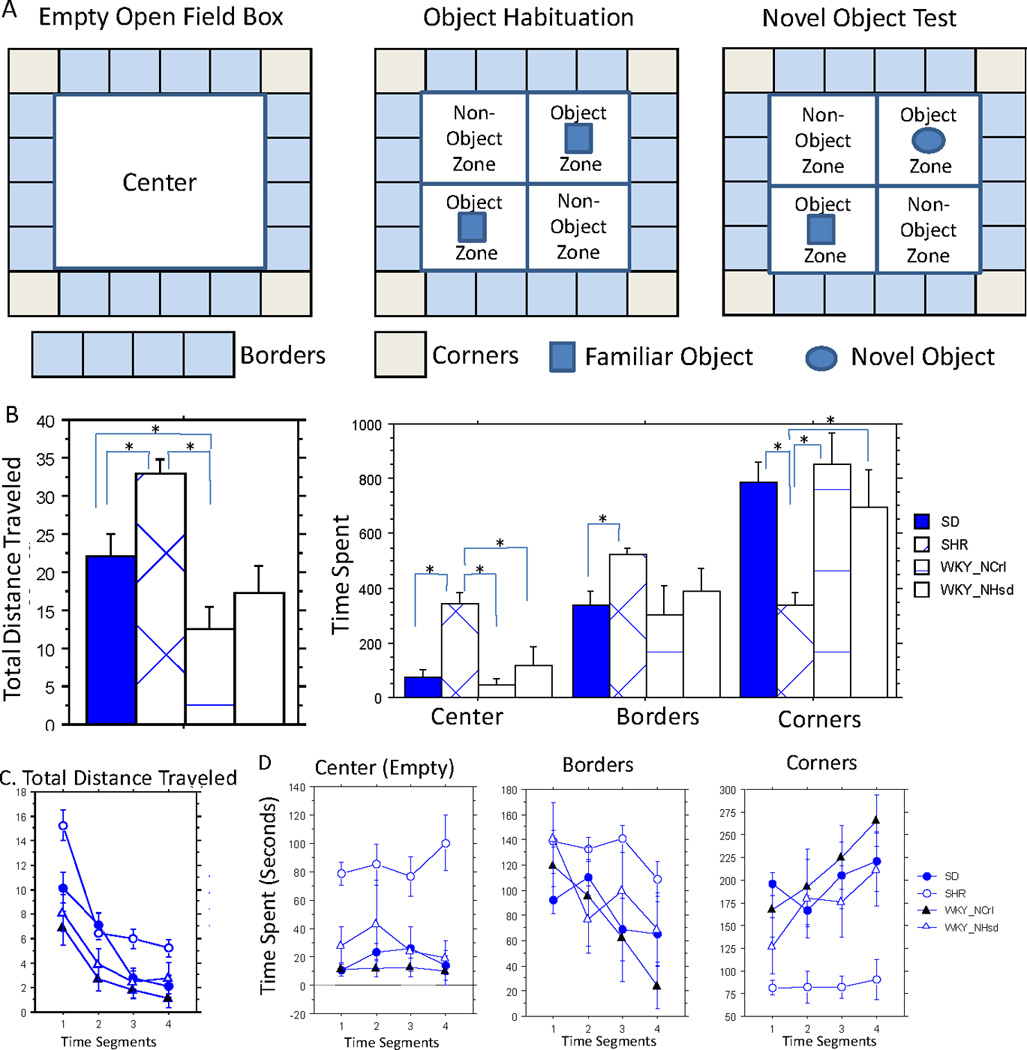
A. The experimental design for the open field box used in open field, object habituation and novel object tests. B. Open field (OF) 20min total results. Left. The total distance traveled in total 20min test. Right. Time spent in each area of the open field box. C. OF segmented results showing the distance traveled in each of the 5 min segments. D. OF segmented results showing the time spent in each area of the OF box over the time segments. Significant post-hoc comparisons were denoted with "*".
2.4 Object Habituation (OH) and Novel Object Tests (NO)
The day after the open field test, the animals were placed back into the same open field chamber with the presence of two identical objects (2.5cmplastic cubes) and recorded for four 5-min trials (with 1.5 hr between trials) to habituate the animals to the objects. The locations of the objects within the OF boxes are illustrated in Figure 2A. For the same 6×6 geometric grid, the center area of the open field box was divided into four zones: two object interaction zones and two non-object interaction zones (Figure 2A). On the second day after the habituation, animals were placed back into the same chamber with the replacement of one habituated object with a novel object (round metal nuts with similar diameter as the cubes). Each animal was tested for one 10 min trial. We measure the distance traveled and the amount of time that animals spent within each zone, as well as the number of entries into the zones.
2.5 Three-Chambered Social Interaction Test
The test was performed in two custom-made three-chambered boxes (114×51×51 cm, LxWxH) over four days (p37-40) to evaluate sociability, habituation to the same social object, social memory and preference for social novelty. The boxes were divided into three equal chambers at length by two retractable doors. On day one, the subject animal was placed in the center chamber for a 10-min habituation with both doors closed. Then a small empty wire cage (12×18×12 cm, WxLxH) was placed in one side(empty cage chamber); simultaneously, a conspecific female rat that had no previous contact with the subject was placed in another small wire cage, which was placed in the other side chamber (rat cage chamber). Retractable doors between the chambers were then raised to allow the test rat to freely explore all three chambers for 10 min. This test evaluated sociability, i.e. the preference for a social object (the conspecific rat), or an empty wire cage. The habituation and sociability tests were repeated for three more days. On day four, immediately following the completion of the habituation and sociability tests, the empty wired cage was replaced by another wired cage containing a novel conspecific female rat that had no previous contact with the test rat. This was to evaluate the test animal’s preference for a novel vs. familiar social object. The social novelty test lasted for 10min for each animal. All tests were performed in the dark using an infrared camera mounted on top of the test apparatus. Videos were analyzed using AnyMaze software. Results included the number of entries into and the time spent in each chamber.
2.6 Home Cage Activity and Social Behavior
Rats were housed with two littermates per cage; two infrared video cameras recorded the side view of the cages in the dark for the analysis of their home cage activities. Each camera recorded four cages together and the videos were recorded for 6 hours from 10am-4pm (dark cycle). Eight cages were recorded in one day and the other eight cages were recorded the second day. Videos were coded by an observer (different from the experimenter and raters)blind to the genotype of the rats for completeness and clarity. Three 20min video segments (one hour total) that had good quality at the same time frame for both days were extracted for scoring. A sample video extracted from the unused portions of the recording was used to train two independent raters to achieve high inter-rater agreement (correlation coefficient >.9, p<.05). Raters were trained to score five different behavioral categories with AnyMaze software. The scoring began with one rat in the cage for 5 min. It then switched to the other rat for 5 min, then back and forth for 5 repeats. Results were summed as the one hour total scores for the cage, representing an average score from both rats in that cage. The rated behavioral categories included: time during which animals were 1) in fighting, 2) self-grooming, 3) inactivity (sleeping or laying with no body part movement except respiration), 4) rearing, 5) in physical contact with one another. Although we record all fighting episodes and do not differentiate play fighting from real fighting, almost all the fightings that we observed were playing fighting and no injury were observed. The physical contact time included time in fighting; therefore, we also examined the non-fighting physical contact time separately by removing the fighting time from the total physical contact time. It represented a variety of other non-fighting social behavior with direct physical contact, such as reciprocal grooming, huddling, eating and sleeping together
2.7 Ultrasonic Vocalization (USV)
Female adult rats emit USVs mainly during interactions with male rats. In order to record USVs from a single female rat, we placed each test female in a cage with soiled bedding from male rats that had no prior contact with the test females. USVs were recorded for 10 min using a USV detector (MED Associates) mounted directly above the cage. Both the cage and the USV detector were enclosed within a sound-proof chamber. Vocalizations were recorded using MED USV application with manufacturer default settings with two preset bandwidths of 50–70kHz and 20–50kHz, a threshold of 25dB and a minimal gap of 0.06s between USVs. Sonograms were visualized using the MED USV Viewer and the quantitative data reflecting the onset and durations of vocalizations were exported as text file for statistical analysis.
2.8 Statistical Analysis
The measurements from each test were exported from the recording software and imported to STATA 12 for statistical analysis. We used several different statistical models to examine strain/substrain differences. For measurements of time (or duration) and distance traveled, which are normally distributed continuous variables, we used Gaussian linear regression. For the number of entries (or number of USV calls), which are count variables, we used a Poisson model. For repeated trials, or data presented in time segments (longitudinal data), we used random-effects Gaussian linear or Poisson regression models. For home cage behavioral scores, we used correlation coefficients to estimate inter-rater reliability. In addition, raters were structured as a panel variable and we used multilevel mixed-effects linear regression to estimate the effects of strains. Individual strains/substrains were compared by post estimation using Wald tests.
3. Results
3.1 Elevated Plus Maze (EP) Test
The four strains did not differ in time spent in the center (starting) zone, but significantly differed in the time spent in both the open (F(3, 28) = 12.12, p<0.0001) and closed arms (F(3, 28) = 7.62, p=0.0007) (Figure 1A). The SHR spent significantly more time in the open arms and less time in the closed arms than SD and WKY/NCrl strains. However, they did not differ from WKY/NHsd rats for these measures. In contrast, WKY/NCrl rats were similar to SD rats and spent less time in the open arms and more time in the closed arms than the SHR and WKY/NHsd rats. The SHR and WKY/NHsd rats spent similarly more time exploring over the edge of the open arms (probing zones) than the WKY/NCrl and SD rats(F(3, 28) = 3.19, p<0.039). Figure 1C shows that the SHR and WKY/NHsd rats had similar ratios of time spent in the open vs. closed arms; their ratios were significantly higher than those of the WKY/NCrl and SD rats (F(3, 28) = 4.27, p<0.013).
Figure 1. Elevated Plus Maze Results.
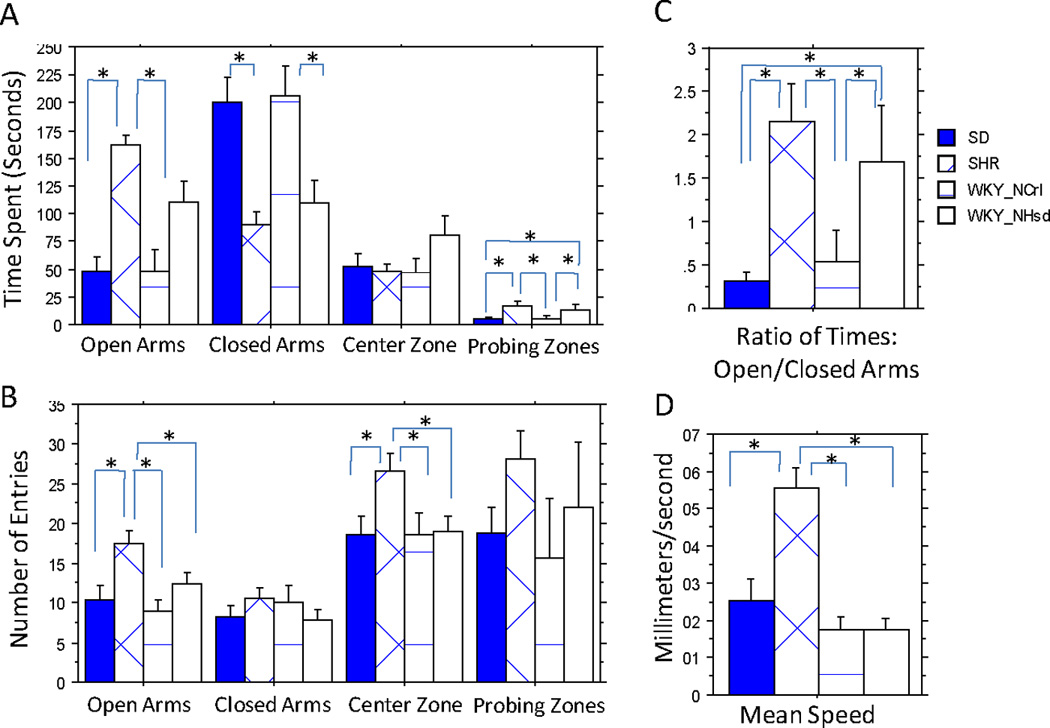
Elevated Plus Maze. A. Time spent in each zone. SHR and WKY/NHsd rats were similar in spending more time in the open arms and probing over the edges of the open arms (probing zones), and less time in the closed arms than both the SD and WKY/NCrl rats. No difference was observed for the center zone. B. Numbers of entries into each zone. SHR had the most numbers of entries to the open arms and center. All other three strains were similar. C. Ratios of time spent in open vs closed arms. SHR and WKY/NHsd rats were similar and had significantly higher ratios than both the SD and WKY/NCrl. D. Average speed recorded on the elevated plus maze apparatus. SHR has significantly higher speed than all three other strains which were similar. Significant post-hoc comparisons were denoted with "*".
Although SHR and WKY/NHsd rat groups were similar for anxiety-like measures, the SHR rats had the highest mean speed (F(3, 28) = 15.02, p<0.0001, Figure 1D) and number of entries to the open arms (F(3, 26) = 5.75, p<0.004) and center zone (F(3, 26) = 3.1, p<0.044) (Figure 1B). In contrast, the WKY/NHsd rats did not differ from the WKY/NCrl and SD strains in speed and number of entries to the zones.
3.2 Open Field Test (OF)
In the open field test, there was a significant strain difference in total distance traveled (F(3, 27) = 9.81, p=0.0002). SHRs traveled more than the other three strains over the 20 minutes; WKY/NCrl rats traveled significantly less than SHR and SD rats, but did not differ significantly from WKY/NHsd rats (Figure 2B Left). We found a significant strain effect on the time that the animals spent in the center (F(3, 27) = 9.94, p=0.0001) and the corners of the field (F(3, 27) = 5.82, p=0.003). SHRs spent more time in the center and less time in the corner than the other strains. The other three strains did not differ significantly from one another (Figure 2B Right).
We examined the OF across the 20 min session in four 5-min segments. All strains gradually decreased activity levels over segments as measured by the distance traveled (Figure 2C). There were significant effects for strain (X(3) = 53.51, p<0.0001) and segments (X2(3) = 123.83, p<0.0001), but no significant interaction between the two. The time the animal spent in the center, borders and corners of the field was plotted over time, showing the habituation profile (Figure 2D). There was a significant decrease in the times spent at borders by WKY/NCrl rats (X2(3) = 10.07, p<0.018) over the segments. There was a significant effect of strain on the time spent in the center open area (X2(3) = 76.79, p<0.0001), borders (X2(3) = 46.55, p<0.0001), and corners (X2(3) = 90.20, p<0.0001) due to the distinct behavior of the SHR. The other three strains were not significantly different from each other.
3.3 Object Habituation (OH) and Novel Object Tests (NO)
During the OH and NO tests, the animals' activity in the total open area, as well as the borders and corners of the field are presented in Figure 3A to compare with those of the OF test in the empty arena of the open field box (Figure 2D). There was a significant strain difference for the time spent in the total open area (X2(3) = 30.74, p<0.0001), borders (X2(3) = 46.38, p<0.0001) and corners (X2(3) = 46.04, p<0.0001). SHRs still spent the most amount of time in the center and the least amount of time in the corners than other strains. Different from the empty arena, WKY/NHsd rats spent relatively more time in the center and the borders, and less time in the corners than the WKY/NCrl rats, but still to a lesser degree than the SHR rats. SD rats were similar to the WKY/NCrl rats with the least amount of time in the center. SD rats were similar to the SHRs in spending the most amount of time in the borders, and similar to the WKY/NHsd rats in spending a medium amount of time in the corners. We found no effect of trials on these measurements.
Figure 3. Object Habituation and Novel Object Tests.
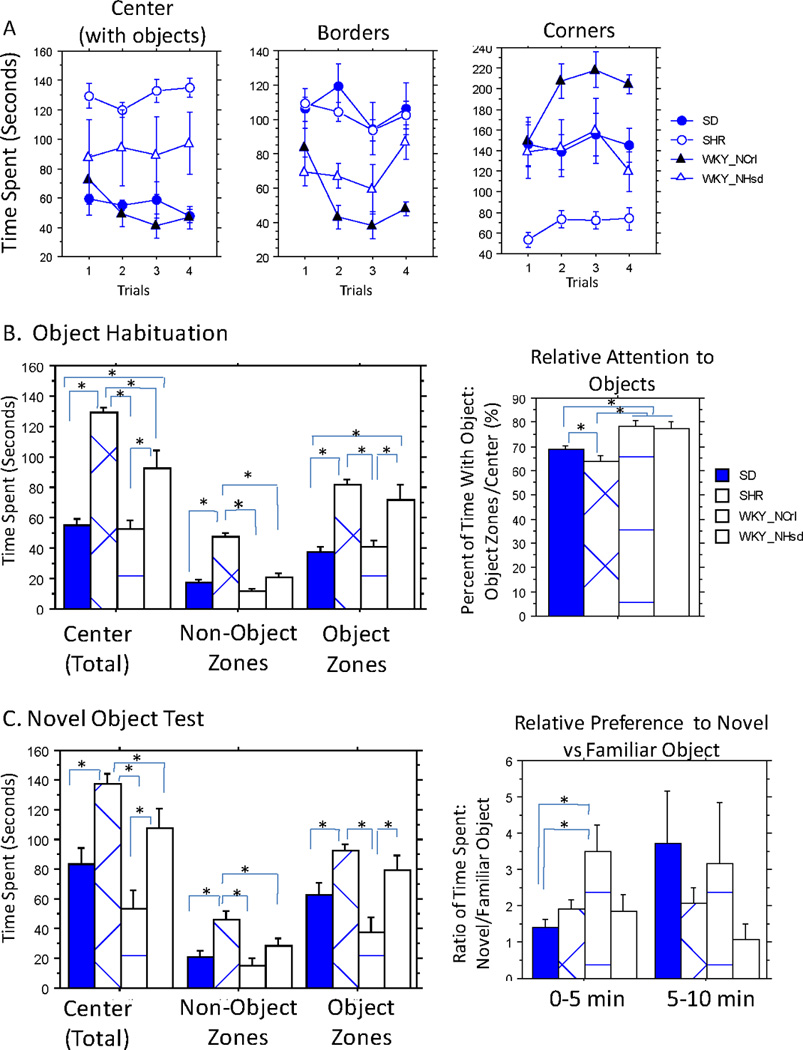
A. Segmented data showing the time spent in each area of the open field box over four trials of object habituation. B. Averaged data over four trials of object habituation. Left. Time spent in the total center open area, and the separated object and non-object zones. Right. The percentage of total center time spent in the object specific zones. Notice that the two WKY substrains were similar, and their differences C. Novel object test results. Left. Time spent in the total center open area, and the separated object and non-object zones. Right. The Ratio of time spent in novel object vs the old familiar object zones in each 5min segments. Significant post-hoc comparisons were denoted with "*".
We further examined strain differences in the object and non-object zones of the center area. The time spent in the separate object and non-object zones of the center area also did not show any significant change with repeated trials during OH, therefore we analyzed the combined results from four trials (Figure 3B). Strain differences are highly significant for time spent in the total center area (F(3, 27) = 11.59, p<0.0001), or in either object(F(3, 27) = 6.37, p=0.002) or non-object-specific zones (F(3, 27) = 33.02, p<0.0001, Figure 3B Left). Furthermore, strain differences in the percentage of time in the object-specific zones were highly significant (F(3, 27) = 10.61, p=0.0001, Figure 3B Right). Despite the fact that SHR spent the most time in the center, they spent the least percentage of their time in object specific zones (63.8 ± 6.4% of the total time in the center) than three other strains, indicating more time in the non-object zones. Although two WKY were significantly different in terms of total center time, they spent similar percentages of time in the object specific zones (WKY/NHsd77.3±7.6% and WKY/NCrl 78.5±5.7%, Figure 3B Right), which were significantly higher than both SD (68.8±4.1%) and SHR. There was no difference in the relative preference to either of the objects (not shown). When an novel object was introduced to replace one of the old objects, animals showed significant strain differences in preference to the novel vs the old objects. Although WKY/NCrl rats still spent the least amount of time in the center and object zones (Figure 3C Left), they showed significantly higher preference to the novel object vs the old one than all three other strains, particularly in the first 5 min of exposure to the novel object (F(3,25) = 4.36, p=0.013, Figure 3C Right). They maintained a similarly higher level of preference to the novel vs the old objects (>3 fold) during the later 5 min, however, the strain differences were no longer significant.
3.4 Three-chambered social interaction test (TC)
The three-chambered social interaction test consisted of a once/day social object habituation/sociability test towards the same con-specific stranger rat over four days, and one trial of social novelty test at the fourth day following the sociability test. This test only included three strains: SD and the two WKY substrains. During the social object habituation/sociability test, both SD and WKY/NHsd rats spent significantly more time in the rat chamber and less time in the middle chamber relative to the WKY/NCrl group. These times increased over days for the rat chamber and decreased for the middle chamber, suggesting an increasing familiarity associated social interest (Figure 4A). The social familiarity-associated changes were significant for SD rats (rat chamber: F(3, 28)= 3.14, p =0.04; Middle Chamber: F(3, 28)= 2.69, p =0.07) and WKY/NHsd rats (rat chamber: F(3, 23)= 3.52, p =0.03; Middle Chamber: F(3, 28)= 3.13, p =0.04), but not for WKY/NCrl rats. In contrast, WKY/NCrl rats spent significantly less time in the rat chamber (strain effect: X2(2) = 20.98, p<0.0001) and more time in the middle chamber (strain effect: X2(2) = 22.52, p<0.0001). These differences were more pronounced in the 3rd and 4th trials. The WKY/NHsd and SD rats were not statistically different in these measures. All animals spent very little time in the empty cage chamber over the four trials.
Figure 4. Three –Chambered social interaction test.
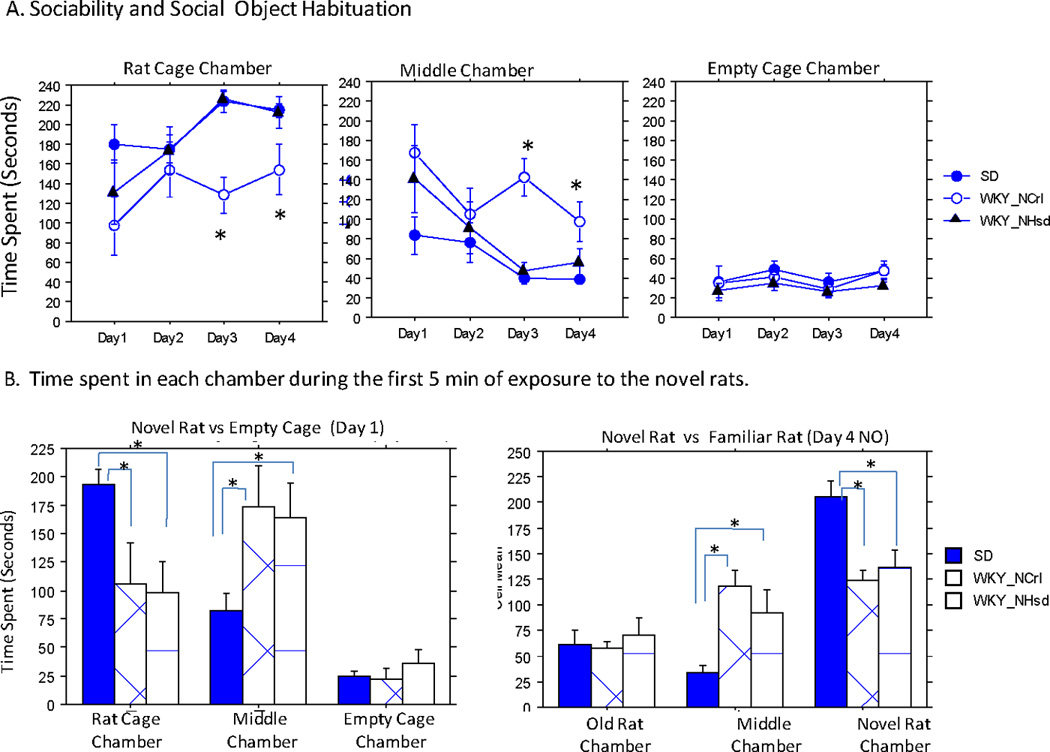
A. Sociability and social memory test. Animals were exposed to the same conspecific rat over four repeated trials. Total times spent in each chamber during each trial of sociability test were normalized to total seconds/5 min segments and plotted over four trials to show sociability and social memory/object habituation.
B. Social novelty was assessed during the first 5 min of the exposure to the novel conspecific rats on both day 1 and day 4 tests. Time spent in each chamber were plotted to show the strain difference in their relative preference to the novel rats vs either the empty cage (day 1, Left) or a familiar rat (day 4, Right). Significant post-hoc comparisons were denoted with "*".
We assessed the animals’ responses to a novel social object during the first five minutes of the first trial of habituation (vs. the empty cage) and during the social novelty (novel vs. familiar rat). The time that animals spent in each chamber during these two exposures to the novel rats are plotted in Figure 4B. We found a similar strain difference in their response to the novel rat (vs. the empty cage or a familiar rat) and time spent in the middle chambers in both tests. In both tests, the two WKY strains were similar and spent less time in the novel rat chambers (strain differences for day 1: F(2, 20)= 3.80, p=0.04; day 4: F(2, 20) = 9.65, p=0.001) and more time in the middle chamber (strain differences for day 1: F(2, 20)= 3.25, p=0.06; day 4: F(2, 20)= 8.46, p=0.002) than the SD rats.
3.5 Home Cage Observation (HC)
Compared with SD rats, both WKY strains showed a similarly low preference to the novel rats in the three chamber test. Yet only the WKY/NCrl rats lacked interest in the familiar rats. Because of this finding, we sought to determine if WKY/NCrl rats also lacked social interest in their familiar littermates in their home cage compared with the WKY/NHsd rats. Therefore, we video recorded home cage behavior during the rat’s dark cycle to score the behaviors of two littermates that were group-housed within the same cage. The home cage videos were scored for five different behavioral categories(Figure 5) by two independent raters who were blinded to the experimental groups. We found no significant difference between the raters for all five scores and the correlations between the two raters were high: Social-Fighting, r=0.96, p<0.0001; Self-Grooming, r=0.70, p=0.003; Inactivity, r=0.99, p<0.0001; other physical contact (excluding fighting), r=0.95, p<0.0001; Rearing, r=0.91, p<0.0001. We found significant strain effects for these measures. Overall, the two WKY strains were highly similar in four measures except the non-fighting physical contact time. WKY/NCrl rats spent significantly less time in direct physical contact with their littermates (excluding the fighting time) than the WKY/NHsd rats (X2(1) = 5.89, p=0.015 for non-fighting social contact, Figure 5). It is noteworthy that WKY/NHsd rats also spent significantly less time in physical contact with their littermates when compared with the SHR and SD rats and that SHRs were not different from SDs in non-fighting physical contact time (Figure 5).
Figure 5. Home Cage Observations.
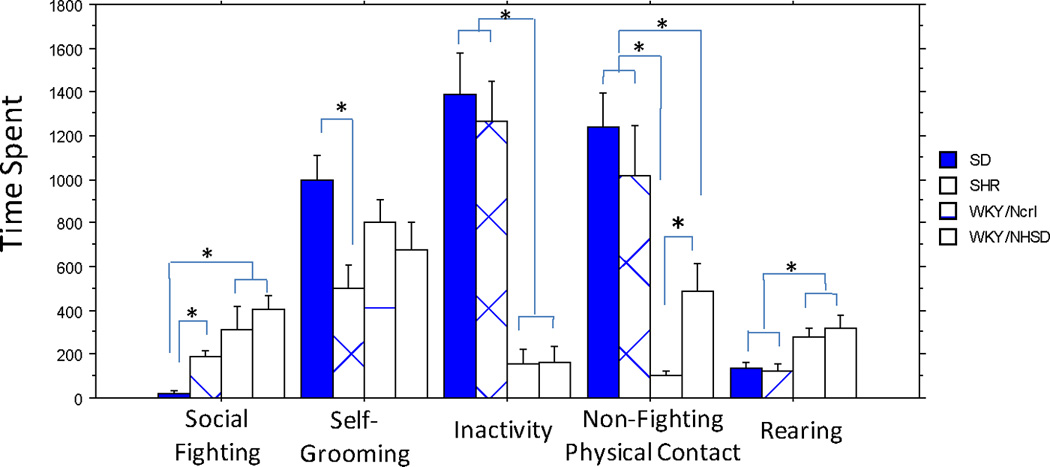
Total time spent in each behavior category were plotted to show strain difference. Note that WKY/NCrl rats spend significantly less time in direct contact with the littermates (when not fighting) than all other three strains including WKY/NHsd, although the two WKY substrains were highly similar in all four other measurements. SHR is similar to SD rats in non-fighting physical contact, rearing and inactivity levels. However, SHR spent more time in fighting and less time in self-grooming than SD. Significant post-hoc comparisons were denoted with "*".
Surprisingly, both WKY substrains were similarly more active in their home cages (less time in inactivity) and spent more time in rearing and fighting with their littermates than both SHRs and SDs. SHRs also spent significantly more time in fighting than the SDs, although SHRs were not different from SDs in total inactive time and rearing. The two WKY substrains were not different from either SHR or SD in the time spent in self-grooming. However, SHRs spent significantly less time engaged in self-grooming than the SD rats.
3.6 Ultrasonic Vocalization (USV)
Only one USV call was detected within the lower frequency bandwidth, consistent with the notion that high frequency calls (50kHz) are associated with appetitive states and that low frequency calls (22kHz) are associated with aversive states [14]. We focused our analysis on the high frequency bandwidth. The total number of USV calls emitted during the 10min recording was significantly higher for SD rats than for the other strains (Strain F(3, 27) =2.98, p=0.049; Figure 6A). WKY/NCrl rats emitted the least numbers of calls, but were not significantly different from the SHR and WKY/NHsd strains. We plotted the numbers of USV calls per minute over the 10 mins (Figure 6B).
Figure 6. Ultrasonic Vocalization.
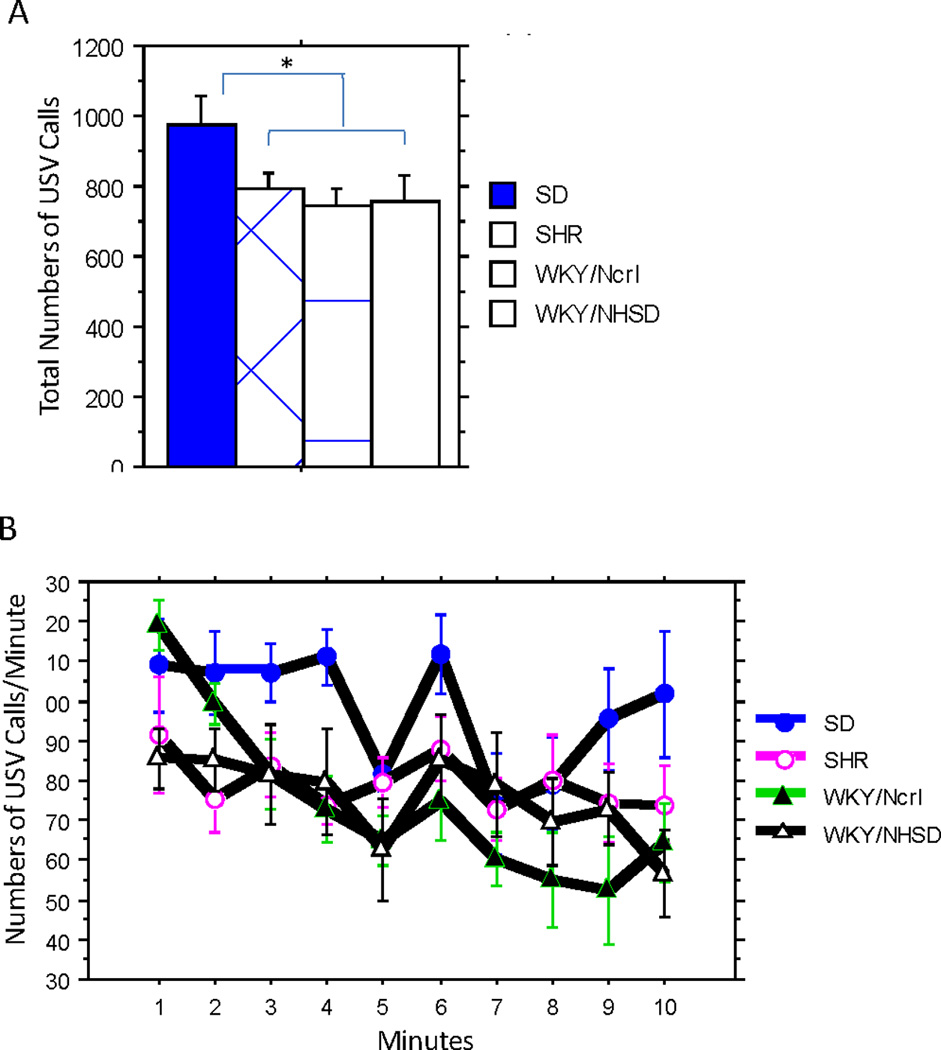
USV of individual female rats exposed to male bedding. A. Total number of calls in 10 minutes of exposure. SD rats emit significant more calls than all three inbred strains. Significant post-hoc comparisons were denoted with "*". B. Numbers of calls per minute were plotted over time. WKY/NCrl rats demonstrated a quick decline in the first 5 min and maintained the lowest number of calls during the second 5 min segment. WKY/NHsd and SHR were not different.
Upon visual inspection of the call profile, we noticed that in addition to the total number of calls, WKY/NCrl rats showed a different time course in USVs during the 10minexposure to the male bedding. We therefore used the random-effects Poisson regression model to evaluate USV changes over time between different strains. We found significant effects of strain (X2(3) = 10.45, p=0.015), time (X2(9) = 151.88, p<.0001), as well as their interaction (X2(27) = 289.52, p<.0001). The strain difference was primarily due to WKY/NCrl rats, which demonstrated a steep decrease in USV calls in the first 5 min and maintained the lowest levels of calls during the last 5 min. WKY/NCrl rats were significantly different from WKY/NHsd rats(X2(1) = 7.56, p=0.006), as well as the SD and SHR rats. WKY/NHsd rats were not significantly different from the SD and SHR rats.
4. Discussion
An ideal animal model not only mimics the fundamental behavioral characteristics of the disorder (face validity), but also conforms to a theoretical rationale (construct validity), and predicts behavior, genetics, and neurobiology (predictive validity) [3]. Previously using SNP-based array analysis, we reported that WKY/NCrl rats (US source) differ from WKY/NHsd rats in only ~2.5% of their genomes and that these regions tagged by the polymorphic SNPs are enriched with known autism risk genes[12]. In the current study, we sought to test if the genetic differences could be used to predict the behavioral differences between these two strains, i.e, if the two strains would also differ in behaviors relevant to ASDs. Therefore, we compared the two substrains on autism related behavioral phenotypes such as social interaction and USVs. We also compared them in a battery of neurobehavioral tests evaluating their difference in known phenotypes of the WKY strains such as hypoactivity and anxiety. To our knowledge, this is the first study that has directly and comprehensively compared these two commonly used rat strains that had been often believed to be equivalent and interchangeable. We also compared these strains with SHRs, a widely-used rat model for ADHD that shares a common ancestor with the WKY strains, and an outbred strain, Sprague Dawley, commonly used as a normal control strain. Overall, our results showed that the two WKY strains were highly similar in a number of measures, such as general motor activity levels and low interest in novel social objects, when compared with SHR and SD rats. This finding is consistent with their overall genetic similarity. However we also found several behavioral differences between the two substrains that maybe of particular relevance to ASDs, namely the lower social interest to the familiar animals, lower USV calls and higher levels of anxiety for WKY/NCrl rats.
The lower social interest towards familiar animals (or their odor) in the WKY/NCrl rats was consistently seen across several different behavioral paradigms. In their home cage, WKY/NCrl rats spent significantly less time in direct physical contact with their littermates, although they participated in play fighting as much as the WKY/NHsd rats. In the three-chambered sociability tests, while WKY/NHsd rats were highly similar to SD rats, spending increasing time examining the same conspecific rat during repeated exposures, WKY/NCrl rats avoided the rat and stayed in the center chamber where they had been habituated.
USV is one of the most important communication means for rodents. USVs are often accompanied by other social signals, for example separations and reunions of pups with their mother and littermates [15, 16], play, social investigation between unfamiliar females and sexual behavior[17–19]. The frequencies of USV calls are indicators of their affective states [14]. The number and intensity of the calls are associated with social drives. For example, male social mice (for example B6 and FVB strains) emitted more calls when with an estrus female and made more intensified calls when their female partner is removed from the cage; however BTBR mice, a model for autism, emitted fewer calls and were less affected by the removal of the female partner[20], suggesting a lack of social interest to call for females. Our USV results also suggest that WKY/NCrl females have deficits in communication and a lower interest in males, supported by evidence of their quick decrease in USV calls during the initial exposure (in minutes) and the lowest level of calls maintained during the later phase.
Although no studies have directly examined the two WKY substrains in social behavior, one report showed that WKY pups emitted lower rates of USVs during separation from their mothers when compared with both Wistar rats and Flinders Sensitive Line (FSL) rats, a model for depression[21]. The low rate of USVs was interpreted as a model of depression. We do not, however, know which substrain of WKY rats were used in that study. Both substrains were used equally previously in similar studies of depressive behavior. No studies have examined USVs in adult WKYs or in appetitive states. Our study is the first to show that WKY rats, particularly the WKY/NCrl substrain, emits fewer USVs in a non-stressed social environment, suggesting deficits in communication and social drive, rather than stress-responsive or depression related withdrawal behavior. Future studies of USVs in juvenile play settings will be particularly useful to determine if low rates of USVs are a suitable model for the social and communication problems of ASDs.
Another significant behavioral difference between the two WKY strains was in anxiety-like behaviors. The WKY/NCrl strain was more anxious than the WKY/NHsd strain in terms of avoiding open spaces in both the elevated plus maze and open field tests. WKY/NCrl rats were similar to SD rats for these measures and all three strains were more anxious than the SHR rats. The difference between the two WKYs in the avoidance of the center/open area was more obvious when objects were introduced into the open field boxes. WKY/NHsd rats overcame their center avoidance and examined the objects in the center zones. WKY/NCrl and SD rats remained away from the center open area to a similar degree. However, SD rats showed increased movement in the border zones while WKY/NCrl rats remained in the corners. These results suggest a different coping response to the presence of objects. Although WKY/NCrl rats spent less time in the center, the two WKY strains were similar in terms of their relative attention to the object vs. the non-object center area. In contrast, the SHR was the least attentive to the objects and spend more time in simply running around, consistent with the SHR’s hyperactivity and inattentiveness. Although the WKY/NCrl rats spent the least amount of time in the center of the field, particularly the object zones, they showed higher level of relative preference to the novel vs. the familiar objects, which maybe reminiscent of the intense interest in non-social objects in some autistic children[22–24]. WKY strains are known to be more stress-responsive and have been proposed to be an animal model for depression. Only one previous study directly compared these two WKY substrains[13]. In that study, both substrains developed more stress-induced stomach ulcers than two outbred strains. The WKY/NCrl rats developed more ulcers than the WKY/NHsd rats. This is consistent with our observation that WKY/NCrl rats were more anxious than the WKY/NHsd rats.
Anxiety, stress and depression can all influence social behavior. For example, in the three-chambered sociability test, animals may not interact with conspecific rats simply due to anxiety-related avoidance if the test rat senses itself to be subordinate to the conspecific rat[25]. However, we believe that the lower social interest demonstrated by the WKY/NCrl rats was not due to their higher anxiety levels because the home cage scores and the USV experiments, both of which were in the most natural and non-stressful environments, consistently showed less social drive in the WKY/NCrl rats. Depression can also lead to decreased social drive and increased social withdrawal. We cannot exclude the influence of depression on social behavior in the home cage or on USVs when exposed to male beddings. However, Pare et al. [13] reported that the two substrains were not different in the forced swim test, a test for depression-like behavior based on behavioral despair theory [26]. No studies have compared the two substrains on other measures of depression such as social defeat and sucrose consumption.
On the other hand, autism shares a great deal of symptom overlap with depression and anxiety. The comorbid rate is higher for children with ASDs than for typically developing children [27–29], especially for higher functioning patients. For autism patients, anxiety contributes to avoidance of social contact, and promotes further isolation [30]. Among youths with comorbid ASD and anxiety, social phobia is the most prevalent type of anxiety disorder [31]. Anxiety and psychological distress are also linked to functional gastrointestinal (GI) disorders such as irritable bowel syndrome (IBS) that are often co-morbid with ASDs[32]. The higher likelihood of stress induced ulcers in WKY/NCrl rats is consistent with these reports of autism-related GI sensitivities. Depression also commonly co-occurs with ASDs. Although autistic children show difficulty understanding and expressing emotions and feelings, they still experience negative feelings such as sadness and loss of interest. Depression occurs in about 30% of adolescents and adults with Asperger syndrome[33]. Recent genetic studies suggest that ASDs share significant overlap in etiology with depression and anxiety disorders. For example, oxytocin and its molecular pathways have been implicated in both autism, depression and anxiety disorders, and are key regulators for social and emotional behaviors such as social memory, cooperation, trust, empathy, social buffering against stress, bonding, maternal care and separation anxiety, etc.[34–36]. Another example is calcium channel genes, for example CaV1.2, which were associated with multiple psychiatric disorders including autism and depression[37–39]. Interestingly, some antidepressants are effective for autistic symptoms[40]. It is certainly possible that an autistic model could express co-morbid depression and anxiety, given their shared genetic overlap. A developmental and integrated approach to understand how co-morbid conditions such as anxiety and depression develops, and how they affect the core manifestations of ASDs, is important.
Finally, we noted that the two WKY substrains did not differ in terms of general locomotor activity. Both were similarly hypoactive compared to the SHR in the elevated plus maze and open field tests, consistent with many previous reports of the SHR being hyperactive when compared with WKYs[41]. This indicates that hypo-activity in the open field or other testing chambers is likely to be an inherited and strain-specific trait for WKY rats when compared with SHR. However, we found that the SHR was not hyperactive in the home cage during dark cycle. In fact, we found that SHR were similarly to SD rats and were hypoactive when compared with both WKY strains in home cage during dark cycles. Hlavacova et al have observed that SHR were less active than Lewis rats in the home cage during dark cycles [42]. Furthermore, by recording 24-hr profile, Adriani et al observed that although SHR were more active than WKYs during some time period of light phases, they were actually less active than WKYs during the first 6 hours of onset of dark phases[43]. Our observations were consistent with Adriani's findings and suggest that the two WKY strains are equivalent in regards to their use as a control for the SHR in modeling hyperactivity, however, not in their home cage during dark cycle.
We have summarized our behavioral findings in Table 1 with ranking 1–4 (with 4 meaning displaying the most of the traits and 1 being the least) to describe the degree of the similarity and differences among the four strains with special emphasis on the differences between the two WKY substrains (highlighted in red). Overall, the WKY/NCrl substrain appears to meet several core symptom deficits for ASDs when compared with the WKY/NHsd substrain as their genetic control. Although we observed known traits that are likely inherited and strain-specific, novel traits, particularly those of relevance to ASDs, require cautious interpretation. Because we only examined females in the current study, we do not know if males would also demonstrate similar traits. For females particularly in the USV experimental paradigm, we do not know if the animals differed in their estrous phases at the time of the testing, which may have affected their vocalization in response to the male odor/bedding. Future replication studies are needed to validate the findings in male animals and females with known estrous status.
Table 1.
Summary of strain specific traits.
| Tests/Traits | SD | SHR | WKY/NCrl | WKY/NHsd |
|---|---|---|---|---|
| EP/Anxiety to the open space | 4 | 1 | 4 | 2 |
| EP/Activity level | 1 | 4 | 1 | 1 |
| OF/Activity level | 1 | 4 | 1 | 1 |
| OF/Anxiety to the open space & preference to corners | 4 | 1 | 4 | 4 |
| OH/Avoidance to the open space or avoid the close proximity to objects | 4 | 1 | 4 | 2 |
| OH/Patrol the borders or indirect exploration of the objects in distance | 4 | 4 | 1 | 2 |
| OH/Preference to corners | 2 | 1 | 4 | 2 |
| OH, NO/Relative preference to the object zones vs the non-object zones in the center open area of the open field | 3 | 2 | 4 | 4 |
| NO/Relative preference to the novel vs the familiar objects | 3 | 2 | 4 | 1 |
| TC/Preference to novel rats | 4 | 1 | 1 | |
| TC/Preference to the middle chambers | 1 | 4 | 4 | |
| TC/Preference to the familiar rats | 4 | 1 | 4 | |
| TC/Avoidance to the familiar rats | 1 | 4 | 1 | |
| HC/Activity level(rearing, fighting, etc) | 1 | 2 | 4 | 4 |
| HC/Physical contact | 4 | 4 | 1 | 2 |
| HC/Self-grooming | 4 | 1 | 3 | 3 |
| USV/Number of Calls | 4 | 3 | 1 | 2 |
| USV/Habituation to the novel environmental stimulus | 1 | 1 | 4 | 1 |
EP, elevated plus maze test; OF, open field activity; OH, object habituation; NO, novel object test; TC, three-chambered social interaction test; HC, home cage observation; USV, ultrasonic vocalization
Note: Four strains were ranked from 1 to 4 according to their behavioral differences with 4 meaning displaying the most of the traits and 1 being the least. Significant differences between the two WKY substrains were highlighted in red.
Although our previous SNP-based estimates provided some insights as to the causes of the phenotypical differences, recent rat whole-genome sequencing data will help to pinpoint the exact genetic variations that may be responsible [44]. With better understanding of their strain-specific behavioral phenotype and more accurate mapping of their small genetic divergence, this pair of inbred rats may provide a unique opportunity to study the genetics of autistic traits and its co-morbid conditions such as anxiety and depression.
Highlights.
WKY/NCrl and WKY/NHsd are the genomically closest substrains within the WKY lineage.
WKY/NCrl and WKY/NHsd substrains are highly similar in locomotor activity.
WKY/NCrl substrain has significantly lower social interest and ultrasonic vocalizations and higher anxiety measures when compared with WKY/NHsd substrain.
WKY/NCrl may be a model for autism with WKY/NHsd as the control
Acknowledgments
We thank Dr. Valerie Bolivar for helpful comments on the manuscript. This study was supported by NIH Grant R01MH066877to Dr. Stephen Faraone and a NARSAD Young Investigator Award to Dr. Yanli Zhang-James.
Abbreviations
- ADHD
Attention deficit/hyperactivity disorder
- ASDs
Autism spectrum disorders
- EP
Elevated plus maze test
- FSL
Flinders Sensitive Line
- NO
Novel Object Tests
- OF
Open field activity
- OH
Object Habituation
- SD
Sprague Dawley rats
- SHR
Spontanenous hypertensive rats
- TC
Three-Chambered Social Interaction Test
- USV
Ultrasonic Vocalization
- WKY
Wistar-Kyoto rats
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yanli Zhang-James, Email: Zhangy@upstate.edu.
Li Yang, Email: yangli_pkuimh@bjmu.edu.cn.
Frank A. Middleton, Email: middletf@upstate.edu.
Lina Yang, Email: yanglin@upstate.edu.
Jameson Patak, Email: patakj@upstate.edu.
Stephen V Faraone, Email: sfaraone@childpsychresearch.org.
References
- 1.Overstreet DH. The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev. 1993;17:51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- 2.Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 3.Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- 8.Wohr M, Scattoni ML. Behavioural methods used in rodent models of autism spectrum disorders: current standards and new developments. Behav Brain Res. 2013;251:5–17. doi: 10.1016/j.bbr.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 9.Abbott A. Laboratory animals: the Renaissance rat. Nature. 2004;428:464–466. doi: 10.1038/428464a. [DOI] [PubMed] [Google Scholar]
- 10.Bambini-Junior V, Rodrigues L, Behr GA, Moreira JC, Riesgo R, Gottfried C. Animal model of autism induced by prenatal exposure to valproate: behavioral changes and liver parameters. Brain Res. 2011;1408:8–16. doi: 10.1016/j.brainres.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Sadamatsu M, Kanai H, Xu X, Liu Y, Kato N. Review of animal models for autism: implication of thyroid hormone. Congenital anomalies. 2006;46:1–9. doi: 10.1111/j.1741-4520.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang-James Y, Middleton FA, Faraone SV. Genetic architecture of Wistar Kyoto rats (WKY) and Spontaneous hypertensive Rats (SHR) substrains from difference sources. Physiological genomics. 2013 doi: 10.1152/physiolgenomics.00002.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pare WP, Kluczynski J. Differences in the stress response of Wistar-Kyoto (WKY) rats from different vendors. Physiol Behav. 1997;62:643–648. doi: 10.1016/s0031-9384(97)00191-1. [DOI] [PubMed] [Google Scholar]
- 14.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychological bulletin. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 15.Hofer MA, Shair H. Ultrasonic vocalization during social interaction and isolation in 2-weeek-old rats. Developmental psychobiology. 1978;11:495–504. doi: 10.1002/dev.420110513. [DOI] [PubMed] [Google Scholar]
- 16.Sewell GD. Ultrasonic communication in rodents. Nature. 1970;227:410. doi: 10.1038/227410a0. [DOI] [PubMed] [Google Scholar]
- 17.Schwarting R. Rodent ultrasonic communication and its relevance for models of neuropsychiatric disorders. e-Neuroforum. 2010;1:71–80. [Google Scholar]
- 18.Nyby J. Ultrasonic vocalizations during sex behavior of male house mice (Mus musculus): a description. Behav Neural Biol. 1983;39:128–134. doi: 10.1016/s0163-1047(83)90722-7. [DOI] [PubMed] [Google Scholar]
- 19.Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus) J Comp Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- 20.Yang M, Loureiro D, Kalikhman D, Crawley JN. Male mice emit distinct ultrasonic vocalizations when the female leaves the social interaction arena. Front Behav Neurosci. 2013;7 doi: 10.3389/fnbeh.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braw Y, Malkesman O, Merenlender A, Bercovich A, Dagan M, Overstreet DH, et al. Withdrawal emotionalregulation in infant rats from genetic animal models of depression. Behav Brain Res. 2008;193:94–100. doi: 10.1016/j.bbr.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Anthony LG, Kenworthy L, Yerys BE, Jankowski KF, James JD, Harms MB, et al. Interests in high-functioning autism are more intense, interfering, and idiosyncratic than those in neurotypical development. Development and psychopathology. 2013;25:643–652. doi: 10.1017/S0954579413000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasson NJ, Touchstone EW. Visual Attention to Competing Social and Object Images by Preschool Children with Autism Spectrum Disorder. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1910-z. [DOI] [PubMed] [Google Scholar]
- 24.Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KS, Bodfish JW. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism research : official journal of the International Society for Autism Research. 2008;1:31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell and tissue research. 2013;354:107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- 26.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 27.Green SA, Ben-Sasson A. Anxiety disorders and sensory over-responsivity in children with autism spectrum disorders: is there a causal relationship? J Autism Dev Disord. 2010;40:1495–1504. doi: 10.1007/s10803-010-1007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadow KD, DeVincent CJ, Pomeroy J, Azizian A. Psychiatric symptoms in preschool children with PDD and clinic and comparison samples. J Autism Dev Disord. 2004;34:379–393. doi: 10.1023/b:jadd.0000037415.21458.93. [DOI] [PubMed] [Google Scholar]
- 29.Muris P, Steerneman P, Merckelbach H, Holdrinet I, Meesters C. Comorbid anxiety symptoms in children with pervasive developmental disorders. Journal of anxiety disorders. 1998;12:387–393. doi: 10.1016/s0887-6185(98)00022-x. [DOI] [PubMed] [Google Scholar]
- 30.Myles BS, Barnhill GP, Hagiwara T, Griswold DE, Simpson RL. A synthesis of studies on the intellectual, academic, social, emotional and sensory characteristics of children and youth with Asperger syndrome. Education and Training in Mental Retardation and Developmental Disabilities. 2001;36:304–311. [Google Scholar]
- 31.Ung D, Wood JJ, Ehrenreich-May J, Arnold EB, Fuji C, Renno P, et al. Clinical characteristics of high-functioning youth with autism spectrum disorder and anxiety. Neuropsychiatry. 2013;3 doi: 10.2217/npy.13.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coury DL, Ashwood P, Fasano A, Fuchs G, Geraghty M, Kaul A, et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. 2012;130(Suppl 2):S160–S168. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- 33.Ghaziuddin M, Ghaziuddin N, Greden J. Depression in persons with autism: implications for research and clinical care. J Autism Dev Disord. 2002;32:299–306. doi: 10.1023/a:1016330802348. [DOI] [PubMed] [Google Scholar]
- 34.Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 35.Saito Y, Suga M, Tochigi M, Abe O, Yahata N, Kawakubo Y, et al. Neural correlate of autistic-like traits and a common allele in the oxytocin receptor gene. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith AS, Wang Z. Hypothalamic Oxytocin Mediates Social Buffering of the Stress Response. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao P, Soong TW. CaV1.2 channelopathies: from arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflugers Archiv : European journal of physiology. 2010;460:353–359. doi: 10.1007/s00424-009-0753-0. [DOI] [PubMed] [Google Scholar]
- 38.Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Detera-Wadleigh SD, Akula N. A systems approach to the biology of mood disorders through network analysis of candidate genes. Pharmacopsychiatry. 2011;44(Suppl 1):S35–S42. doi: 10.1055/s-0031-1275275. [DOI] [PubMed] [Google Scholar]
- 40.Hurwitz R, Blackmore R, Hazell P, Williams K, Woolfenden S. Tricyclic antidepressants for autism spectrum disorders (ASD) in children and adolescents. Cochrane Database Syst Rev. 2012;3:CD008372. doi: 10.1002/14651858.CD008372.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meneses A, Perez-Garcia G, Ponce-Lopez T, Tellez R, Gallegos-Cari A, Castillo C. Spontaneously hypertensive rat (SHR) as an animal model for ADHD: a short overview. Reviews in the neurosciences. 2011;22:365–371. doi: 10.1515/RNS.2011.024. [DOI] [PubMed] [Google Scholar]
- 42.Hlavacova N, Bakos J, Jezova D. Differences in home cage behavior and endocrine parametres in rats of four strains. Endocrine regulations. 2006;40:113–118. [PubMed] [Google Scholar]
- 43.Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: evidence for impulsive and non-impulsive subpopulations. Neurosci Biobehav Rev. 2003;27:639–651. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Atanur SS, Diaz AG, Maratou K, Sarkis A, Rotival M, Game L, et al. Genome Sequencing Reveals Loci under Artificial Selection that Underlie Disease Phenotypes in the Laboratory Rat. Cell. 2013;154:691–703. doi: 10.1016/j.cell.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


