Abstract
We investigated perceptual learning in self-motion perception. Blindfolded participants were displaced leftward or rightward by means of a motion platform, and asked to indicate the direction of motion. A total of eleven participants underwent 3360 practice trials, distributed over twelve (Experiment 1) or six days (Experiment 2). We found no improvement in motion discrimination in both experiments. These results are surprising since perceptual learning has been demonstrated for visual, auditory, and somatosensory discrimination. Improvements in the same task were found when visual input was provided (Experiment 3). The multisensory nature of vestibular information is discussed as a possible explanation of the absence of perceptual learning in darkness.
Keywords: self-motion thresholds, whole-body motion, perceptual learning, vestibular thresholds, vestibular learning
Introduction
Perceptual learning is a basic form of learning and refers to the ability to improve perception (Fahle 2004; Herzog and Esfeld 2009). Perceptual learning occurs by the repeated exposure to stimuli. For example, wine experts are able to precisely discriminate properties of a wine such as the year of production whereas for novices red wines taste more or less the same (e.g., Owen and Machamer 1979). Most research in perceptual learning has been carried out in the visual domain (see Fahle and Poggio 2002, for a review). Participants are able to learn to better discriminate contrast (Adini et al. 2002; Adini et al. 2004; Sowden et al. 2002; Yu et al. 2004), line orientation (Fahle and Edelman 1993; Herzog and Fahle 1997; Poggio et al. 1992) or motion direction (Koyama et al. 2004; Kuai et al. 2005; Liu and Vaina 1998).
Perceptual learning has also been demonstrated in auditory, tactile or olfactory perception (e.g., Bao et al. 2004; Sathian and Zangaladze 1998; Wilson and Stevenson 2003). To our knowledge, no study has yet examined perceptual learning in vestibular perception. The vestibular system, located in the inner ear, encodes angular and linear acceleration, including the direction of gravity (Merfeld 2012). Therefore, the vestibular system plays a key role in spatial orientation and self-motion perception.
Besides numerous cortical projections (Lopez et al. 2012) the vestibular system is involved in reflexive behavior such as the vestibulo-spinal reflex (responsible for balance and postural control), vestibulo-autonomic reflex (responsible for regulating blood flow to the brain) and the vestibulo-ocular reflex (VOR; responsible for visual stability during head movements). The VOR has been thoroughly investigated to better understand the vestibular input to oculomotor mechanisms (e.g., Kingma et al. 2001). Interestingly, changes in VOR characteristics such as a lower gain (ratio between compensatory slow phase eye velocity and head angular velocity) have been found in ice skaters (Alpini et al. 2009; Tanguy et al. 2008), ballet dancers (Osterhammel et al. 1968; Tschiassny 1957), and gymnasts (Quarck and Denise 2005). What these experts have in common is that self-motion perception plays an important role for their skills. In these studies, the modulation in VOR gain was interpreted as a result of vestibular habituation due to repeated powerful vestibular stimulation during performance. Conversely, Lee et al. (2004) found higher VOR gain values in pilots compared to controls. In addition, VOR gain values improved in pilots through training. Lee and colleagues (2004) proposed that these modulations are caused by vestibular adaptation and VOR plasticity rather than habituation. However, the exact nature of VOR modulations is not yet clear. All expert groups mentioned above practiced body movements while they were exposed to visual input at the same time. Therefore, VOR modulations may be caused by visual as well as vestibular changes in the reflex arc. Nevertheless, these studies demonstrate the impact of training on visual-vestibular interaction.
The aim of this study was to investigate whether vestibular perception alone can be improved via training of passive self-motion perception1. Using a motion platform, blindfolded participants were displaced leftward or rightward (yaw rotation around the longitudinal body axis or linear translation along the interaural axis; Experiment 1 and Experiment 2) with a velocity that was around their individual threshold of self-motion perception. The participants indicated the direction of self-motion (left-right discrimination). Given that there are improvements in performance in almost any perceptual task, we expected perceptual learning also in the vestibular domain. In addition, participants were trained in self-motion perception in a structured visual environment (Experiment 3).
Self-motion discrimination training may be useful for improving performance in patients suffering from vestibular deficits
Vestibular disorders such as vertigo and dizziness have a one year prevalence of 20% (Lempert and Neuhauser 2009; Neuhauser et al. 2008). People suffering from vestibular disorders often experience problems with balance and self-motion perception, resulting in impaired life quality. Previous studies showed that the threshold for self-motion direction discrimination in patients with vestibular loss is higher when compared to healthy controls (Cutfield et al. 2011; Mallery et al. 2010; Valko et al. 2012) but see also Gianna et al. (1996). These results point to the potential importance of perceptual learning, which could possibly lead to increasing recruitment of extra-vestibular acceleration detection in patients with vestibular loss (Mittelstaedt 1992, 1996). This study therefore serves as a first step in evaluating self-motion discrimination training in healthy participants. If perceptual learning manifests itself in healthy participants, it is by all means conceivable to evaluate this approach as a possible tool in vestibular rehabilitation.
Perceptual learning is typically specific to the trained stimuli and there is usually little or no transfer to untrained stimuli (e.g., Beard et al. 1995; Fine and Jacobs 2002; Karni and Sagi 1991; Schoups et al. 1995). Recently, however, some studies found transfer effects of learning under certain conditions (Aberg et al. 2009; Huang et al. 2008; Jeter et al. 2007; Li et al. 2009; Polat 2009; Xiao et al. 2008). For the main experiment of this study (Experiment 2), we chose a linear leftward-rightward translation for training. To assess whether learning in self-motion perception is specific for the trained motion direction, we assessed several transfer conditions before and after the training, such as forward-backward translation, upward-downward translation, and yaw-rotations. Moreover, we assessed transfer effects within the same motion direction across different durations of motion stimuli (1 s, 2 s, 5 s). In a previous study, we were able to demonstrate that motion-detection thresholds depend on the frequency of motion (Grabherr et al. 2008).
Given that this is the first study on perceptual learning in self-motion perception, we could not rely on previous experience about how to design the training such as the optimal number or the optimal distribution of trials. These factors turned out to play an important role in other domains of perceptual learning (Hussain et al. 2009; Tsodyks and Gilbert 2004; Wright and Sabin 2007). We first designed a pretest (Experiment 1) and then modified parameters for the main experiment (Experiment 2).
Method
Participants
A total of 14 participants from the University of Bern took part in this study: four participants in Experiment 1 (2 female, 2 male, age range from 23 to 29), seven participants in Experiment 2 (5 female, 2 male, age range from 21 to 28, none of the participants took part in Experiment 1), and five participants in Experiment 3 (2 female, 3 male, age range from 24 to 29, 2 participants took part in Experiment 1). All participants completed a questionnaire to assess health and possible vestibular disorders such as dizziness, vertigo, or hearing problems. None of them indicated any history of vestibular disorders. All participants gave informed consent prior to the study.
Apparatus and motion stimuli
A 6-degree of freedom electric motion platform served to generate motion stimuli (6DOF2000E, MOOG Inc., East Aurora, NY; see Fig. 1 in Hartmann et al. 2012, for an image of the apparatus). We used single cycle sinusoidal acceleration motion profiles (Grabherr et al. 2008). Peak velocity, acceleration, and displacement (all proportional) of the motion stimuli were adjusted according to participants’ thresholds. The duration of the motion stimulus in the training phase was constant for all participants (2 s). Previous studies showed that self-motion velocity thresholds depend on the duration of the motion. In particular, thresholds increase for motion durations of 2 s and longer while they are similar for short motion durations (Benson et al. 1989; Grabherr et al. 2008; Soyka et al. 2011). These findings suggest that there is more room for improvement for longer motion durations. We did not choose motion durations longer than 2 s in order to keep the trial duration (and consequently the duration of a training session) in a reasonable range. A PC with a MATLAB-based custom-made software was used to control the motion platform and recording of participants’ responses.
General procedure
Participants were seated in a chair that was mounted on the motion platform. Seat belts were fastened around participants’ shoulders, torso and hips. Their head was restrained with fixation straps. An adjustable foot rest served to position the feet. To minimize a possibly confounding influence of visual or auditory cues on self-motion perception, participants were blindfolded (Experiment 1 and Experiment 2) and exposed to white noise presented via in-ear headphones during both the threshold assessment and the learning phase.
Each motion stimulus was preceded by a low-pitched tone (2000 ms before motion onset). A high-pitched tone was played 500 ms after motion offset in order to indicate that the motion had stopped and to prompt the participants to respond. Participants were asked to press either the button in their left or right hand, according to the instructions provided before each recording session. In case the participants were uncertain about the direction of self-motion, they were instructed to make their best guess. When the motion had stopped and the response was given, the next trial was triggered by the experimenter with a delay of approximately 3 s. Feedback was provided to speed up learning (Herzog and Fahle 1997). In the training phase, participants received an error tone after each incorrect response. In addition to the trial feedback, participants were informed about the percentage of correct responses after each block.
In order to assess learning and transfer effects, self-motion velocity thresholds were recorded before and after the training phase. Thresholds were determined by means of a two-alternative 3-down 1-up adaptive staircase procedure following a PEST algorithm (Leek 2001; Taylor and Creelman 1967). In this procedure, three correct responses in a row are needed in order for the velocity level to be reduced. The velocity level is increased after each incorrect response. This procedure converges at a threshold value of 79.4% correctly detected trials (Leek 2001). The initial peak velocity at the beginning of the procedure was well above threshold (starting values were 0.06 m/s for linear motion and 8°/s for angular motion). The procedure stopped when three maximum reversals and four minimum reversals were reached. A maximum reversal is defined by three correct responses in a row after an incorrect response. A minimum reversal means an incorrect response following at least three correct responses. The last minimum reversal was reached on average after 43 trials. In order to increase the reliability of the threshold measurements, the threshold of each of the six different motion conditions was assessed twice in separate recording sessions. Within the recording sessions, the order of the different motion conditions was random. The threshold values were then defined as the mean peak velocity of the last minimum and the last maximum reversal of the two sessions. The threshold value also served as a point of reference for determining the peak velocity for the motion stimulus during training. We aimed for a velocity level that produces around 65–75% correct responses at the beginning of the training. Since the thresholds that we obtained produced a higher number of correct responses (79.4%), the values used for the training was about 15% lower than the threshold value (see Herzog and Fahle 1997, for a similar approach).
Data analysis
We calculated d prime (d′ = zhit rate – zfalse alarm rate) for each block as the performance measure. Given that we used a two-alternative forced choice task, hit rate was defined as the number of correct rightward detections divided by the total number of rightward trials, and false alarm rate was defined as number of incorrect rightward responses divided by the total number of leftward trials (Aberg and Herzog 2012). Learning effects during the training phase were assessed by means of the individual regression slopes of d′ as a function of blocks. Improvement in performance as a result of training leads to a positive regression slope. Regression slopes were tested against zero by means of a one-sample t-test (with the exception of Experiment 1 where no statistical significance tests were performed). Note that when performance in the first block was outside the intended range of 65–75% correct responses, the velocity level was slightly adjusted for the following blocks. These initial blocks were not included in the data analysis.
In order to assess transfer effects, we calculated the threshold ratio (threshold post training / threshold pre training) for each motion condition and participant. A ratio of 1 indicates no change through training, and a ratio smaller than 1 indicates an increase in sensitivity. The threshold ratios were tested against 1 by means of one-sample t-tests (with the exception of Experiment 1 where no statistical significance tests were performed).
Experiment 1
The aim of Experiment 1 was twofold. We wanted to assess the shape of the learning curve (continuous progress or saturation) and whether there is a difference between perceptual learning of translations (based on otolith input) and perceptual learning of rotations (based on semicircular canal input). While the interpretation of angular motion is straightforward, the interpretation of linear motion is more difficult because otolith signals per se cannot distinguish between linear acceleration and a tilt of the gravity vector (Einstein’s equivalence principle), thus leading to ambiguous sensory information.
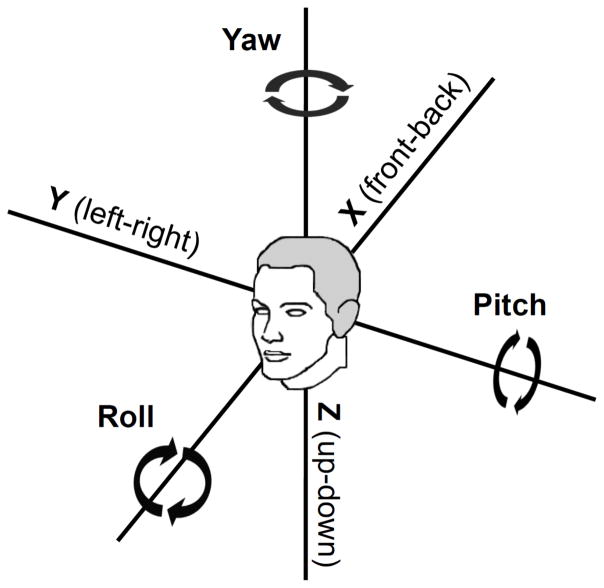
Participants performed a total of 12 training sessions. Each session lasted around 40 minutes and consisted of four blocks of 70 trials. In each session, 140 leftward and 140 rightward motion stimuli were presented in a random order, resulting in a total number of 3360 trials. The blocks were separated by a short break of 2 min. The training took place from Monday to Friday of two consecutive weeks and on Monday and Tuesday of the third week. Two participants were trained with leftward-rightward linear translation stimuli, and the other two with leftward-rightward yaw rotation stimuli. For the former group, the following thresholds were assessed before and after the training: linear leftward-rightward motion (y axis; with 1 s, 2 s, and 5 s duration), linear upward-downward motion (z axis; 2 s), linear forward-backward translation (x axis; 2 s), yaw rotation to the left and to the right (2 s). For the latter group, the following thresholds were assessed: yaw rotation to the left and to the right (with 1 s, 2 s, and 5 s duration), roll and pitch rotation (2 s), and linear leftward-rightward motion (y axis; 2 s). See Fig. 1 for an overview of the different motions.
Fig. 1.
Coordinate system with the linear (x, y, z) and angular (yaw, pitch, roll) motion directions.
Results and discussion of Experiment 1
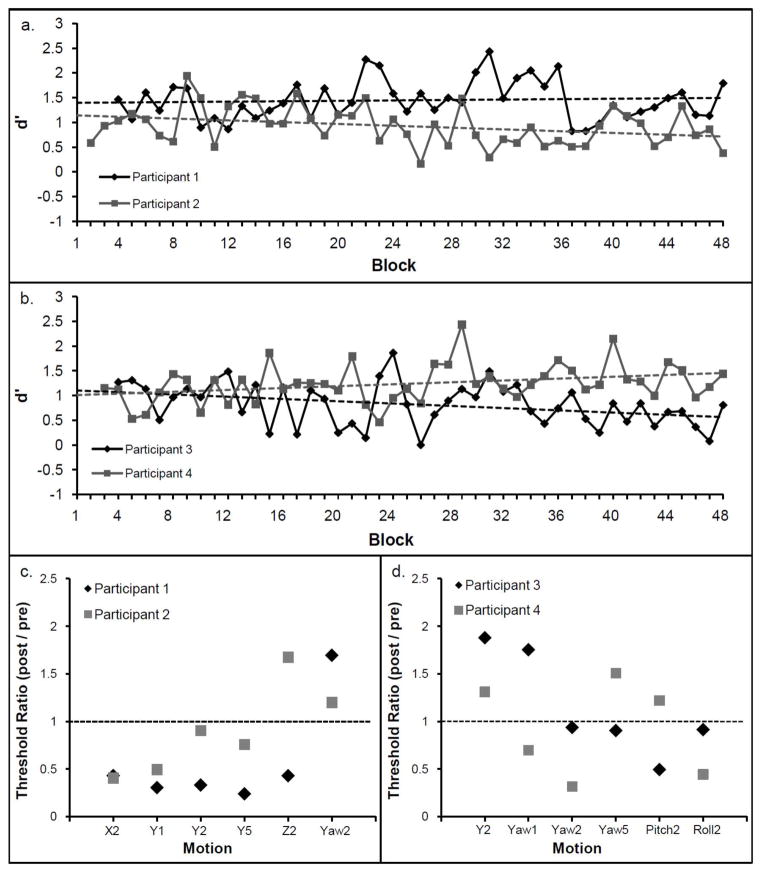
Fig. 2a shows the regression slopes of the participants who were trained on linear self-motion, and Fig. 2b those of the participants who were trained on angular self-motion. The small positive regression coefficients and the two negative regression slopes show no consistent learning effect for translation or for rotation. The mean d′ in the first two sessions (Block 1–8) was 1.05 for all four participants (SEM = 0.07; ≈ 70.3% correct responses) whereas the mean d′ in the last two sessions (Block 40–48) was 1.00 (SEM = 0.08; ≈ 68.4% correct responses). The absence of learning was also confirmed by the threshold ratios. There was no selective or systematic reduction in self-motion perception threshold after the training (see Fig. 2c and d).
Fig. 2.
Performance (d prime; d′) for each of the 48 blocks for participants trained with leftward-rightward linear motion (a) and leftward-rightward angular motion (b). The dotted line shows the linear fit for each participant. Threshold ratios for each motion are displayed in (c) and (d). The horizontal dotted line in (c) and (d) represent no change between pre and post training threshold. Y = linear leftward-rightward motion, x = linear forward-backward motion, z = linear upward-downward motion. The digits indicate the duration of the motion in s.
Given the large number of training sessions (12) and the large number of total trials (3360), the absence of learning was surprising. However, visual perceptual learning does not purely depend on the number of total trials, but also on the distribution of the trials per training sessions. It has been shown that a minimal number of trials within a daily session is necessary for improvements between sessions (Aberg et al. 2009; Hauptmann et al. 2005; Wright and Sabin 2007). Aberg et al. (2009) found no learning in a Chevron task with 160 trials per session whereas learning took place with 400 trials per session. The number of daily trials in Experiment 1 was 280. One possible reason for the absence of learning could therefore be that the number of daily trials was not above the critical minimal number of trials needed for perceptual learning. In Experiment 2, we increased the number of daily trials from 280 to 560.
Only Participant 1 who trained linear self-motion showed a slight tendency to improve threshold (see Fig. 2c). In Experiment 2, all participants trained linear leftward-rightward translation. This condition allows for investigating transfer effects within otolith-based motion perception and possible transfer effects to canal-based motion perception.
Experiment 2
Seven participants were tested in Experiment 2. None of them participated in Experiment 1. They were trained on linear leftward-rightward self-motion (2 s duration). In contrast to Experiment 1, participants performed eight blocks of 70 trials per day during six days. In each session, 280 leftward and 280 rightward motion stimuli were presented in a random order, resulting in a total of 3360 trials (same as in Experiment 1). A daily session lasted about 80 minutes, and each block was separated by a short break of 2 min. After Block 4, a longer break was provided in which participants came out of the chair for 4–8 min in order to counteract any potential discomfort and fatigue effects.
The following thresholds were assessed before and after the training in order to assess learning and transfer effects: linear leftward-rightward motion (y axis; with 1 s, 2 s, and 5 s duration), upward-downward translation (z axis; 2 s), linear forward-backward translation (x axis; 2 s), and yaw rotation to the left and right (2 s).
Results and discussion of Experiment 2
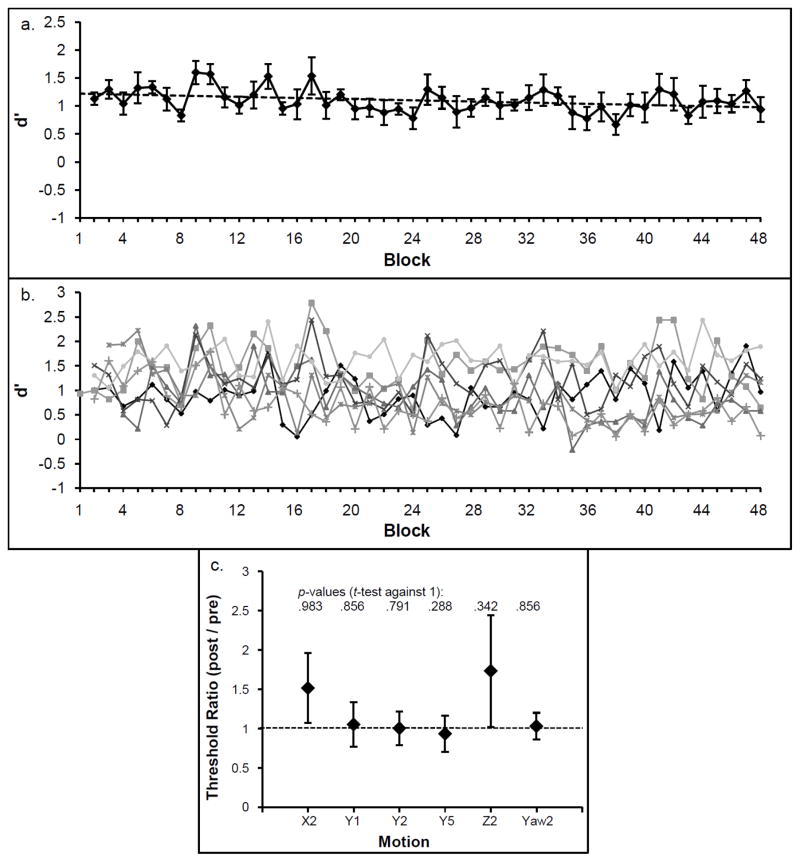
Fig. 3a shows the mean d′ for each block. The linear regression slope for d′ is negative, showing no overall learning effects and individual regression slopes did not differ significantly from zero, [t(6) = −1.03, p = .344, mean regression slope: −.005 (SEM = .011)]. The mean d′ in the first session (Block 1–8) was 1.18 (SEM = 0.07; ≈ 69.5% correct responses) and the mean d′ in the last session (Block 40–48) was 1.08 (SEM = 0.08; ≈ 69.3% correct responses). The individual slopes show that the absence of learning was a general pattern (Fig. 3b).
Fig. 3.
Mean d prime (d′) is shown for each of the 48 blocks (a). The dotted line represents the linear fit. Individual d′ values are displayed in (b). The mean threshold ratios are displayed in (c). The horizontal dotted line represents no change between pre and post training threshold. P values show that none of the motion thresholds differed significantly from 1. Y = linear leftward-rightward motion, x = linear forward-backward motion, z = linear upward-downward motion. The digits indicate the duration of the motion in s. Error bars depict +/− 1 SEM.
Absence of learning was confirmed when thresholds before and after training were compared. None of the threshold ratios differed significantly from 1. Threshold ratio and corresponding p values are displayed in Fig. 3c.
Previous research has shown that learning is more effective for participants who show a higher threshold before the training phase when compared to participants with a lower threshold (Fahle 1997; Fahle and Edelman 1993). One could therefore argue that a potential reason for the absence of learning in our study was that participants were by chance good performers, i.e., had a low initial threshold. However, this possible explanation can be ruled out. First, the mean leftward-rightward linear threshold from our sample (.013 m/s) was identical to the mean threshold for the same motion condition that we obtained for another sample of 13 participants (also .013 m/s; unpublished data). This suggests that the thresholds of the sample in this study were within a normal range. Second, there was no positive correlation between the initial threshold value and learning gain (expressed as regression slope). Surprisingly, this correlation even turned out to be negative, r = −.76, p = .047. Therefore, participants with a low initial threshold tended to show a decrease in performance. Note that, in the first session, percentage of correct responses ranged from 65% to 77%, showing that participants performed the task in the targeted level.
Since this was the first study on perceptual learning in self-motion perception, we could not rely on specific parameters that have been established in previous studies. However, we used a design that was based on previous studies in the visual domain where perceptual learning has been demonstrated compellingly. For example, the presentation of stimuli with a constant intensity during training is an established method in perceptual learning (e.g., Herzog and Fahle 1998; Yu et al. 2004). Here, we used a fixed set of trials whereby the velocity was individually adjusted based on participants’ thresholds. The individual adjustment guaranteed that the difficulty at the beginning of the training was in an optimal range for perceptual learning (Fine and Jacobs 2002). Another important factor for learning is feedback. Herzog and Fahle (1997) found that feedback leads to a larger improvement in perceptual learning when compared to no feedback or manipulated feedback. Here, we provided trial by trial as well as block feedback. Furthermore, the number of trials is a critical factor for learning. On the one hand, a minimal number of trials within sessions is necessary for improvements between sessions (Aberg et al. 2009; Hauptmann et al. 2005; Wright and Sabin 2007). On the other hand, too many trials have been shown to prevent learning (Censor and Sagi 2009). The amount of 560 daily trials used in Experiment 2 was above the critical minimal number of trials found in the visual and auditory domain (160 trials: Aberg et al. 2009; 360 trials: Wright and Sabin 2007) and below the amount of trials that has been found to prevent learning (800 trials: Censor et al. 2006). Attention to the stimulus is also an important factor for perceptual learning (Ahissar and Hochstein 2002; but see also Watanabe et al. 2001). In the present study, each motion stimulus was preceded by a tone with a fixed delay to the onset of the motion, allowing participants to focus their attention to the subsequent motion stimulus.
Even though all these essential aspects of perceptual learning were considered, no improvements in performance were found. Our results suggest that self-motion perception in the dark cannot be improved via training. However, training has been shown to modulate vestibular functions, for example in pilots (Lee et al. 2004). One possibility is that vestibular functions improve only in the presence of a concurrent visual input. Here, we tested this hypothesis.
Experiment 3
Five students participated in Experiment 3. The design was identical to Experiment 2 with the following exceptions. First, participants performed the training without the blindfold, i.e., they were exposed to a visually rich environment. To this end, we placed an image of a landscape (250 × 118 cm) on the wall in front of the participants (with a distance of 200 cm). We also placed four objects between the participants and the wall (wooden sticks that were hanging from the ceiling, two to the left and two to the right of the participants in arbitrary positions). Second, participants performed only four instead of six sessions, resulting in a total of 2240 trials (this change was made because participants in Experiment 3 reached a level of around 80% correct responses in the fourth session).
The following thresholds were assessed before and after the training in order to assess learning and transfer effects: linear leftward-rightward motion (y axis; 2 s) and linear upward-downward motion (z axis; 2 s). The thresholds were assessed in the visual condition and in the blindfolded condition.
Results and discussion of Experiment 3
Fig. 4a shows the mean d′ for each block. The linear regression slope for d′ is positive, showing a learning effect. Individual regression slopes differed significantly from zero, [t(4) = 3.60, p = .023, mean regression slope: .04 (SEM = .01)]. The mean d′ in the first session (Block 1–8) was 0.85 (SEM = 0.08; ≈ 65.3% correct responses) and the mean d′ in the last session (Block 25–32) was 1.78 (SEM = 0.29; ≈ 80.0% correct responses).
Fig. 4.
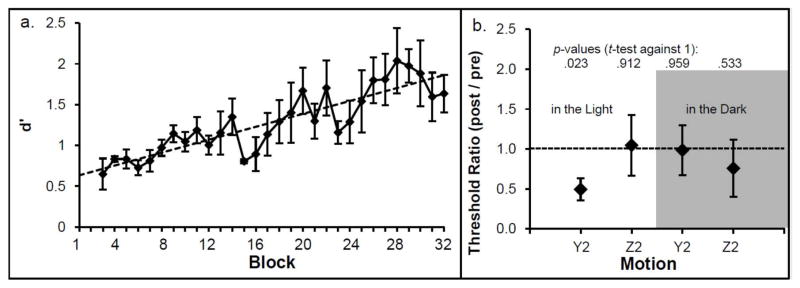
Mean d prime (d′) is shown for each of the 32 blocks (a). The dotted line represents the linear fit. The mean threshold ratios are displayed in (b). The horizontal dotted line represents no change between pre and post training threshold. P values show that only the trained motion thresholds (Y2) differed significantly from 1. Y = linear leftward-rightward motion, z = linear upward-downward motion. The digits indicate the duration of the motion in s. The dark frame indicates that thresholds were measured in the dark (blindfolded). Error bars depict +/− 1 SEM.
Learning was confirmed when thresholds before and after training were compared. The threshold ratio for the trained motion (linear leftward-rightward) differed significantly from 1, t(4) = −3.61, p = .023, whereas the threshold ratio for all other motion conditions did not; see Fig. 4b.
The results of Experiment 3 show that improving linear leftward-rightward self-motion detection via training is possible. Future research needs to be carried out to further investigate the nature of the visual cues and how they interact with the vestibular information to provide learning. Most importantly, the results of Experiment 3 confirm that the absence of learning in Experiment 1 and 2 cannot be explained by the parameters we chose and highlight the importance of visual input in improving self-motion perception.
Discussion
The aim of this study was to demonstrate perceptual learning for self-motion perception. A total of eleven blindfolded healthy participants underwent training with 3360 leftward or rightward self-motion stimuli (Experiment 1 and Experiment 2). None of the participants showed a reliable improvement in performance (the mean learning gain was even slightly negative). These results are surprising given that large increases of performance through training were found in other sensory domains with experimental protocols that were very similar to the one we used here. However, reliable improvement in self-motion perception was found when participants performed the same task while exposed to a structured visual environment (Experiment 3).
Why does vestibular learning not occur without visual input? A peculiarity of self-motion perception is that it is usually accompanied by visual information (we rarely move in complete darkness or with eyes closed). Merfeld et al. (2010) compared leftward-rightward roll tilt thresholds in the dark (vestibular only condition) with the same motion thresholds measured when the lights were on (vestibular and visual condition). They found that thresholds measured with lights on were lower than in the dark2, showing that visual information contributes to self-motion perception. Merfeld et al.’s (2010) results also demonstrate that vestibular thresholds do not represent peak performance. Rather, thresholds are lowest when visual and vestibular cues are both available. In Experiment 1 and Experiment 2, no visual self-motion information was provided. Therefore, training to perceive inertial self-motion cues in isolation might not lead to improvements. When visual input was provided (Experiment 3), perception of self-motion discrimination improved, suggesting that visual input is a crucial factor for spatial orientation learning. Another possible cause for the absence of learning in the dark could be that self-motion perception while wearing a blindfold is an unfamiliar experience. However, in other domains, more pronounced learning effects have been shown for unfamiliar (such as Gabor gratings; see Dosher and Lu 1998) than for familiar stimuli (such as cardinally oriented lines; see Fine and Jacobs 2002 for a review). Nevertheless, the fundamental multimodal character of spatial orientation differentiates the vestibular system from other sensory modalities (Merfeld 2012). Areas involved in self-motion perception, such as the insula, are highly multimodal (e.g., Fasold et al. 2002; Lopez et al. 2012). No single brain region seems to contain neurons that receive exclusively vestibular afferent signals (Lopez and Blanke 2011).
The multimodal nature of vestibular information manifests itself at the level of the VOR. Eye movement recordings for athletes and pilots for whom self-motion perception plays a crucial role show alterations in VOR parameters (Ahn 2003; Alpini et al. 2009; Lee et al. 2004; Quarck and Denise 2005), suggesting a malleability of visuo-vestibular interactions as a result of training. However, it is an open question to what extent the VOR and self-motion perception are governed by the same mechanisms (Merfeld et al. 2005b, a). The VOR has been shown to differ from self-motion perception with respect to the “velocity storage” mechanism, a central mechanism that modulates the afferent vestibular signals in order to extend the duration of reflexive eye movements beyond the duration of the physical stimulus (Raphan et al. 1979). It is possible that different processes underlie VOR and self-motion perception (Grabherr et al. 2008; Sinha et al. 2008; Soyka et al. 2012). Our results suggest that, at a minimum, different processes may be involved in VOR changes that can occur after repeated self-motion in the dark (see for example Clément et al. 2008) and self-motion perception in the dark (that did not change via perceptual training). However, we found vision-dependent learning of self-motion perception, suggesting that self-motion perception in the light might be related to changes in the VOR gain (since both can be changed via training). In line with this, there is also evidence suggesting that self-motion perception may be controlled by the same velocity storage network that also controls reflexive eye movements (Bertolini et al. 2011; Bertolini et al. 2012; Okada et al. 1999).
In light of the present results, the idea of using self-motion perception training in darkness is not promising. The finding that blindfolded healthy participants with a higher initial threshold profited least from the training is in contrast to the idea that self-motion perception training is beneficial for persons with a deficit in vestibular processing. Experiment 3 showed that improvements in self-motion perception in the light had a specific effect on the threshold of the trained condition (leftward-rightward motion in the light) and did not influence thresholds in the dark (“pure” vestibular threshold). This suggests that the vestibular component of the visual-vestibular processing network remained unchanged. It has been shown in other domains that learning in a crossmodal condition does not necessarily transfer to the unimodal conditions (Alais and Cass 2010; but see Beer and Watanabe 2009). Nevertheless, self-motion perception training could be helpful when visual input is provided. To what extent such training could be helpful for patients with vestibular deficits needs to be established in future work.
To conclude, we report an unexpected absence of learning in self-motion perception in the dark. Improvements in the same paradigm were found when visual input was provided. We speculate that the multimodal character of self-motion perception or the lack of a vestibular-specific neuronal network could be a reason for the absence of learning in an isolated vestibular condition.
Acknowledgments
This study was funded by the Swiss National Science Foundation (Pro*Doc grant PDFMP1_127238 and Sinergia grant CRSII1-125135/1). We thank Cora Bobst, Wilhelm Klatt, and Antje Stahnke for assistance in data collection.
Footnotes
The perception of passive whole-body motion is based on visual, auditory, vestibular, somatosensory, proprioceptive, and viscerosceptive signals. In the present study, participants were displaced in darkness and exposed to white noise. Therefore, we regard vestibular signals as the main source of self-motion information (see also Benson et al. 1986; Bertolini et al. 2012; Kingma 2005; Valko et al. 2012).
This was true when the duration of the motion was between 1–10 s.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aberg KC, Herzog MH. Different types of feedback change decision criterion and sensitivity differently in perceptual learning. J Vis. 2012;12(3):12.3.3. doi: 10.1167/12.3.3. [pii] [DOI] [PubMed] [Google Scholar]
- Aberg KC, Tartaglia EM, Herzog MH. Perceptual learning with Chevrons requires a minimal number of trials, transfers to untrained directions, but does not require sleep. Vision Res. 2009;49 (16):2087–2094. doi: 10.1016/j.visres.2009.05.020. S0042-6989(09)00260-0 [pii] [DOI] [PubMed] [Google Scholar]
- Adini Y, Sagi D, Tsodyks M. Context-enabled learning in the human visual system. Nature. 2002;415(6873):790–793. doi: 10.1038/415790a415790a. [pii] [DOI] [PubMed] [Google Scholar]
- Adini Y, Wilkonsky A, Haspel R, Tsodyks M, Sagi D. Perceptual learning in contrast discrimination: the effect of contrast uncertainty. J Vis. 2004;4(12):993–1005. doi: 10.1167/4.12.2. doi:10:1167/4.12.2/4/12/2/ [pii] [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. The role of attention in learning simple visual tasks. In: Fahle M, Poggi T, editors. Perceptual learning. MIT Press; Cambridge, MA: 2002. pp. 253–271. [Google Scholar]
- Ahn SC. Short-term vestibular responses to repeated rotations in pilots. Aviat Space Environ Med. 2003;74 (3):285–287. [PubMed] [Google Scholar]
- Alais D, Cass J. Multisensory perceptual learning of temporal order: audiovisual learning transfers to vision but not audition. PLoS One. 2010;5 (6):e11283. doi: 10.1371/journal.pone.0011283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpini D, Botta M, Mattei V, Tornese D. Figure ice skating induces vestibulo-ocular adaptation specific to required athletic skills. Sport Sciences for Health. 2009;5 (3):129–134. [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7 (9):974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- Beard BL, Levi DM, Reich LN. Perceptual learning in parafoveal vision. Vision Res. 1995;35(12):1679–1690. doi: 10.1016/0042-6989(94)00267-p. 0042-6989(94)00267-P [pii] [DOI] [PubMed] [Google Scholar]
- Beer AL, Watanabe T. Specificity of auditory-guided visual perceptual learning suggests crossmodal plasticity in early visual cortex. Exp Brain Res. 2009;198 (2):353–361. doi: 10.1007/s00221-009-1769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AJ, Hutt EC, Brown SF. Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med. 1989;60 (3):205–213. [PubMed] [Google Scholar]
- Benson AJ, Spencer MB, Stott JR. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med. 1986;57 (11):1088–1096. [PubMed] [Google Scholar]
- Bertolini G, Ramat S, Bockisch CJ, Marti S, Straumann D, Palla A. Is Vestibular self-motion perception controlled by the velocity storage? Insights from patients with chronic degeneration of the vestibulo-cerebellum. PLoS One. 2012;7 (6):e36763. doi: 10.1371/journal.pone.0036763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini G, Ramat S, Laurens J, Bockisch CJ, Marti S, Straumann D, Palla A. Velocity storage contribution to vestibular self-motion perception in healthy human subjects. J Neurophysiol. 2011;105 (1):209–223. doi: 10.1152/jn.00154.2010. jn.00154.2010 [pii] [DOI] [PubMed] [Google Scholar]
- Censor N, Karni A, Sagi D. A link between perceptual learning, adaptation and sleep. Vision Res. 2006;46 (23):4071–4074. doi: 10.1016/j.visres.2006.07.022. S0042-6989(06)00333-6 [pii] [DOI] [PubMed] [Google Scholar]
- Censor N, Sagi D. Explaining training induced performance increments and decrements within a unified framework of perceptual learning. Learning & Perception. 2009;1 (1):3–17. [Google Scholar]
- Clément G, Tilikete C, Courjon JH. Retention of habituation of vestibulo-ocular reflex and sensation of rotation in humans. Exp Brain Res. 2008;190 (3):307–315. doi: 10.1007/s00221-008-1471-0. [DOI] [PubMed] [Google Scholar]
- Cutfield NJ, Cousins S, Seemungal BM, Gresty MA, Bronstein AM. Vestibular perceptual thresholds to angular rotation in acute unilateral vestibular paresis and with galvanic stimulation. Ann N Y Acad Sci. 2011;1233 (1):256–262. doi: 10.1111/j.1749-6632.2011.06159.x. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. PNAS. 1998;95 (23):13988. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M. Specificity of learning curvature, orientation, and vernier discriminations. Vision Res. 1997;37(14):1885–1895. doi: 10.1016/s0042-6989(96)00308-2. S0042-6989(96)00308-2 [pii] [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: a case for early selection. J Vis. 2004;4(10):879–890. doi: 10.1167/4.10.4. doi:10:1167/4.10.4/4/10/4/ [pii] [DOI] [PubMed] [Google Scholar]
- Fahle M, Edelman S. Long-term learning in vernier acuity: effects of stimulus orientation, range and of feedback. Vision Res. 1993;33 (3):397–412. doi: 10.1016/0042-6989(93)90094-d. [DOI] [PubMed] [Google Scholar]
- Fahle M, Poggio T. Perceptual learning. MIT Press; Cambridge, MA: 2002. [Google Scholar]
- Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, Wenzel R. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002;17 (3):1384–1393. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- Fine I, Jacobs RA. Comparing perceptual learning tasks: a review. J Vis. 2002;2(2):190–203. doi: 10.1167/2.2.5. doi:10:1167/2.2.52/2/5 [pii] [DOI] [PubMed] [Google Scholar]
- Gianna C, Heimbrand S, Gresty M. Thresholds for detection of motion direction during passive lateral whole-body acceleration in normal subjects and patients with bilateral loss of labyrinthine function. Brain Res Bull. 1996;40 (5–6):443–447. doi: 10.1016/0361-9230(96)00140-2. [DOI] [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res. 2008;186 (4):677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Grabherr L, Mast FW. Moving along the mental number line: Interactions between whole-body motion and numerical cognition. J Exp Psychol Hum PerceptPerform. 2012;38 (6):1416–1427. doi: 10.1037/a0026706. [DOI] [PubMed] [Google Scholar]
- Hauptmann B, Reinhart E, Brandt SA, Karni A. The predictive value of the leveling off of within session performance for procedural memory consolidation. Brain Res Cogn Brain Res. 2005;24 (2):181–189. doi: 10.1016/j.cogbrainres.2005.01.012. S0926-6410(05)00019-4 [pii] [DOI] [PubMed] [Google Scholar]
- Herzog MH, Esfeld M. How the mind constitutes itself through perceptual learning. Learning & Perception. 2009;1 (1):147–154. doi: 10.1556/lp.1.2009.1.11. [DOI] [Google Scholar]
- Herzog MH, Fahle M. The role of feedback in learning a vernier discrimination task. Vision Res. 1997;37(15):2133–2141. doi: 10.1016/s0042-6989(97)00043-6. S0042698997000436 [pii] [DOI] [PubMed] [Google Scholar]
- Herzog MH, Fahle M. Modeling perceptual learning: difficulties and how they can be overcome. Biol Cybern. 1998;78 (2):107–117. doi: 10.1007/s004220050418. [DOI] [PubMed] [Google Scholar]
- Huang CB, Zhou Y, Lu ZL. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. PNAS. 2008;105 (10):4068–4073. doi: 10.1073/pnas.0800824105. 0800824105 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z, Sekuler AB, Bennett PJ. How much practice is needed to produce perceptual learning? Vision Res. 2009;49 (21):2624–2634. doi: 10.1016/j.visres.2009.08.022. S0042-6989(09)00381-2 [pii] [DOI] [PubMed] [Google Scholar]
- Jeter PE, Dosher BA, Liu SH. Transfer (vs. specificity) following different amounts of perceptual learning in tasks differing in stimulus orientation and position. J Vis. 2007;7 (9):84–84. [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. PNAS. 1991;88 (11):4966. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma H. Thresholds for perception of direction of linear acceleration as a possible evaluation of the otolith function. BMC Ear Nose Throat Disord. 2005;5 (1):5. doi: 10.1186/1472-6815-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma H, Kavelaars J, van Tienen N, Caris R. Evaluation of the Statolith Function by Measurement of Ocular Counterrolling? Oto-Rhino-Laryngologia Nova. 2001;11 (2):68–79. [Google Scholar]
- Koyama S, Harner A, Watanabe T. Task-dependent changes of the psychophysical motion-tuning functions in the course of perceptual learning. Perception. 2004;33 (9):1139–1147. doi: 10.1068/p5195. [DOI] [PubMed] [Google Scholar]
- Kuai S-G, Zhang J-Y, Klein SA, Levi DM, Yu C. The essential role of stimulus temporal patterning in enabling perceptual learning. Nat Neurosci. 2005;8 (11):1497–1499. doi: 10.1038/nn1546. [DOI] [PubMed] [Google Scholar]
- Lee MY, Kim MS, Park BR. Adaptation of the horizontal vestibuloocular reflex in pilots. Laryngoscope. 2004;114 (5):897–902. doi: 10.1097/00005537-200405000-00021. 00005537-200405000-00021 [pii] [DOI] [PubMed] [Google Scholar]
- Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys. 2001;63 (8):1279–1292. doi: 10.3758/bf03194543. [DOI] [PubMed] [Google Scholar]
- Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol. 2009;256 (3):333–338. doi: 10.1007/s00415-009-0149-2. [DOI] [PubMed] [Google Scholar]
- Li R, Polat U, Makous W, Bavelier D. Enhancing the contrast sensitivity function through action video game training. Nat Neurosci. 2009;12 (5):549–551. doi: 10.1038/nn.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Vaina LM. Simultaneous learning of motion discrimination in two directions. Brain Res Cogn Brain Res. 1998;6(4):347–349. doi: 10.1016/s0926-6410(98)00008-1. S0926-6410(98)00008-1 [pii] [DOI] [PubMed] [Google Scholar]
- Lopez C, Blanke O. The thalamocortical vestibular system in animals and humans. Brain Res Rev. 2011;67 (1–2):119–146. doi: 10.1016/j.brainresrev.2010.12.002. S0165-0173(11)00002-6 [pii] [DOI] [PubMed] [Google Scholar]
- Lopez C, Blanke O, Mast FW. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neurosci. 2012;212:159–179. doi: 10.1016/j.neuroscience.2012.03.028. S0306-4522(12)00289-8 [pii] [DOI] [PubMed] [Google Scholar]
- Mallery R, Olomu O, Uchanski R, Militchin V, Hullar T. Human discrimination of rotational velocities. Exp Brain Res. 2010;204 (1):11–20. doi: 10.1007/s00221-010-2288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM. Spatial orientation and the vestibular system. In: Wolfe JM, Kluender KR, Levi DM, editors. Sensation & Perception. 3. Sinauer Associates, Inc; Massachusetts: 2012. [Google Scholar]
- Merfeld DM, Karmali F, Nicoucar K, Lin K. Perceptual roll tilt thresholds demonstrate visual-vestibular fusion. J Vestib Res. 2010;20(3, 4) [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during translation and tilt. J Neurophysiol. 2005a;94 (1):186–198. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined Tilt&Translation. J Neurophysiol. 2005b;94 (1):199–205. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H. Somatic versus vestibular gravity reception in man. Ann N Y Acad Sci. 1992;656:124–139. doi: 10.1111/j.1749-6632.1992.tb25204.x. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H. Somatic graviception. Biol Psychol. 1996;42 (1–2):53–74. doi: 10.1016/0301-0511(95)05146-5. [DOI] [PubMed] [Google Scholar]
- Neuhauser HK, Radtke A, von Brevern M, Lezius F, Feldmann M, Lempert T. Burden of dizziness and vertigo in the community. Arch intern med. 2008;168 (19):2118. doi: 10.1001/archinte.168.19.2118. [DOI] [PubMed] [Google Scholar]
- Okada T, Grunfeld E, Shallo-Hoffmann J, Bronstein A. Vestibular perception of angular velocity in normal subjects and in patients with congenital nystagmus. Brain. 1999;122 (7):1293–1303. doi: 10.1093/brain/122.7.1293. [DOI] [PubMed] [Google Scholar]
- Osterhammel P, Terkildsen K, Zilstorff K. Vestibular habituation in ballet dancers. Acta Otolaryngol. 1968;66 (3):221–228. doi: 10.3109/00016486809126289. [DOI] [PubMed] [Google Scholar]
- Owen DH, Machamer PK. Bias-free improvement in wine discrimination. Perception. 1979;8 (2):199. doi: 10.1068/p080199. [DOI] [PubMed] [Google Scholar]
- Poggio T, Fahle M, Edelman S. Fast perceptual learning in visual hyperacuity. Science. 1992;256 (5059):1018–1021. doi: 10.1126/science.1589770. [DOI] [PubMed] [Google Scholar]
- Polat U. Making perceptual learning practical to improve visual functions. Vision Res. 2009;49 (21):2566–2573. doi: 10.1016/j.visres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Quarck G, Denise P. Caractéristiques du reflexe vestibulo-oculaire chez les gymnastes. Science et motricité. 2005;(2):101–112. [Google Scholar]
- Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR) Exp Brain Res. 1979;35 (2):229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A. Perceptual learning in tactile hyperacuity: Complete intermanual transfer but limited retention. Exp Brain Res. 1998;118 (1):131–134. doi: 10.1007/s002210050263. [DOI] [PubMed] [Google Scholar]
- Schoups AA, Vogels R, Orban GA. Human perceptual learning in identifying the oblique orientation: retinotopy, orientation specificity and monocularity. J Physiol. 1995;483 (Pt 3):797–810. doi: 10.1113/jphysiol.1995.sp020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Zaher N, Shaikh AG, Lasker AG, Zee DS, Tarnutzer AA. Perception of self motion during and after passive rotation of the body around an earth-vertical axis. Progr Brain Res. 2008;171:277–281. doi: 10.1016/S0079-6123(08)00639-0. S0079-6123(08)00639-0 [pii] [DOI] [PubMed] [Google Scholar]
- Sowden PT, Rose D, Davies IR. Perceptual learning of luminance contrast detection: specific for spatial frequency and retinal location but not orientation. Vision Res. 2002;42(10):1249–1258. doi: 10.1016/s0042-6989(02)00019-6. S0042698902000196 [pii] [DOI] [PubMed] [Google Scholar]
- Soyka F, Giordano P, Barnett-Cowan M, Bülthoff H. Modeling direction discrimination thresholds for yaw rotations around an earth-vertical axis for arbitrary motion profiles. Exp Brain Res. 2012;220 (1):89–99. doi: 10.1007/s00221-012-3120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka F, Robuffo Giordano P, Beykirch K, Bulthoff HH. Predicting direction detection thresholds for arbitrary translational acceleration profiles in the horizontal plane. Exp Brain Res. 2011;209 (1):95–107. doi: 10.1007/s00221-010-2523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguy S, Quarck G, Etard O, Gauthier A, Denise P. Vestibulo-ocular reflex and motion sickness in figure skaters. Eur J Appli Physiol. 2008;104 (6):1031–1037. doi: 10.1007/s00421-008-0859-7. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Creelman CD. Pest - Efficient Estimates on Probability Functions. J Acoust Soc Am. 1967;41 (4p1):782. [Google Scholar]
- Tschiassny K. Studies concerning vestibular factors in the ballet dancer, the pigeon, and the blind person. Trans Am Acad Ophthalmol Otolaryngol. 1957;61 (4):503–506. [PubMed] [Google Scholar]
- Tsodyks M, Gilbert C. Neural networks and perceptual learning. Nature. 2004;431 (7010):775–781. doi: 10.1038/nature03013. nature03013 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko Y, Lewis RF, Priesol AJ, Merfeld DM. Vestibular labyrinth contributions to human whole-body motion discrimination. J Neurosci. 2012 doi: 10.1523/JNEUROSCI.2157-12.2012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Náñez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413 (6858):844–847. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003;26 (5):243–247. doi: 10.1016/S0166-2236(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Wright BA, Sabin AT. Perceptual learning: how much daily training is enough? Exp Brain Res. 2007;180 (4):727–736. doi: 10.1007/s00221-007-0898-z. [DOI] [PubMed] [Google Scholar]
- Xiao L-Q, Zhang J-Y, Wang R, Klein SA, Levi DM, Yu C. Complete Transfer of Perceptual Learning across Retinal Locations Enabled by Double Training. Curr Biol. 2008;18 (24):1922–1926. doi: 10.1016/j.cub.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Klein SA, Levi DM. Perceptual learning in contrast discrimination and the (minimal) role of context. J Vis. 2004;4(3):169–182. doi: 10.1167/4.3.4. doi:10:1167/4.3.44/3/4 [pii] [DOI] [PubMed] [Google Scholar]