Abstract
Preventing relapse to drinking or escalation to excessive drinking could be aided by identifying factors that predict these behaviors. Animal models, particularly those that utilize operant self-administration techniques, can be useful. In a prior operant study, we noted a good deal of variability in behaviors during training and test sessions. We utilized data obtained from that study of two groups of rats, trained and tested identically except one responded for alcohol and the other for sucrose, to explore for associations related to relapse (reinstatement) or to excessive drinking (maintenance). Data were obtained from sessions conducted under fixed- and progressive-ratio schedules, as well as from extinction and reinstatement sessions. Variables assessed included active and inactive presses, head entries into the dipper trough, and automated recordings of body movements during these sessions, as well as alcohol preference prior to training. First, using multiple regression, we examined whether alcohol preference prior to training associated with any response variable among alcohol-responding rats. Second, using factor analysis, we identified a training variable, body movements, that associated with responses during tests. Using this measure, rats were divided into low and high response groups and compared on active lever presses and head entries across test sessions. Results show that among alcohol-responding rats, alcohol preference predicted head entries during extinction. High body movement rats emitted significantly fewer active lever presses and had fewer head entries across test sessions, particularly during reinstatement, compared to low body movement rats. Results from this exploratory study provide clues for future experimental studies.
Keywords: relapse, sign-tracking, conditioned approach, operant reinforcement
The major challenges in treating alcoholism are to prevent relapse to use after abstinence or a return to excessive drinking. Studies in animals may provide valuable information about risk factors to relapse to drinking because they are conducted under controlled conditions with standardized procedures. Excessive drinking can be modeled in animals using progressive ratio schedules of reinforcement (Richardson & Roberts, 1996). An increasingly popular animal model of relapse is the reinstatement procedure (Le & Shaham, 2002; Shaham, Shalev, Lu, & DeWit, 2003). In this procedure, an animal is well-trained to perform an operant (e.g., lever press) to obtain a drug or alcohol reinforcer. Subsequently, reinforcer delivery is eliminated and operant behavior extinguishes. Behavior can be reinstated by several types of stimuli including exposure to the original reinforcer. The reinstatement model could be used to examine factors that reduce or enhance the probability and degree of relapse.
A great deal of research has been conducted on identifying factors that predict drug self-administration behaviors. For example, many studies demonstrate that there are sex and strain (inbred or selectively bred) differences in self-administration behaviors promoted by various drug reinforcers (Becker & Hu, 2008; Files, Denning, Hyytia, Kiianmaa, & Samson, 1997; Files, Samson, Denning, & Marvin, 1998; George, 1990; Kosten & Ambrosio, 2002; Roth, Cosgrove, & Carroll, 2004; H. H. Samson, Files, Denning, & Marvin, 1998; Vacca et al., 2002). Other research shows that outbred rats that readily self-administer drugs can be identified based on specific behavioral responses to the mild stress of exposure to a novel environment (Marinelli, 2005; Mitchell, Cunningham, & Mark, 2005; Piazza, Deminiere, LeMoal, & Simon, 1990), by impulsive-like behavior (Belin, Mar, Dalley, Robbins, & Everitt, 2008; Dalley et al., 2007; Perry & Carroll, 2008), or by saccharin intake (Carroll, Morgan, Anker, Perry, & Dess, 2008). Further, early life events, such as neonatal isolation or environmental enrichment, affect drug self-administration behaviors in adulthood (T. A. Green, Gehrke, & Bardo, 2002; Kosten & Kehoe, 2007; Kosten, Sanchez, Zhang, & Kehoe, 2004; Thiel, Sanabria, Pentkowski, & Neisewander, 2009). Yet, much of this work has focused on acquisition and maintenance phases of self-administration with much less emphasis on reinstatement or extinction of self-administration behaviors. Further, even less research of this kind has been done using alcohol as the reinforcer. There is one report demonstrating that alcohol-preferring, selectively bred P rats show greater reinstated responding for alcohol compared to the non alcohol-preferring NP rats (Ciccocioppo, Angeletti, & Weiss, 2001) and another report demonstrating that genetically selected Marchigian Sardinian alcohol-preferring rats are more prone to reinstatement (Ciccocioppo et al., 2006).
During the course of conducting a study on maintenance and reinstatement of alcohol self-administration (Kosten, 2011), we noticed a good deal of variability among rats in several variables such as in the number of trials needed to reach criteria for extinction as well as in response levels. This study included two groups of rats, those self-administering an alcohol solution and those self-administering a sucrose solution. We collected data on several measures, in addition to lever presses, including automated recordings of body movement episodes and head entries into the dipper access area as well as prior alcohol preference in a two-bottle choice procedure. The purpose of the present study was to utilize these data to probe for potential factors that might predict relapse or excessive drinking behaviors.
The present study is exploratory in nature; we gathered data on several assessments made during the conduct of maintenance and reinstatement of self-administration sessions. These phases included training sessions, in which the reinforcer was available for delivery under a fixed-ratio 2 schedule of reinforcement, and test sessions. Tests were conducted under a progressive-ratio schedule of reinforcement as well as sessions in which the reinforcer was not available (e.g., extinction) or was available briefly (e.g., reinforcer-induced reinstatement). Two specific questions were addressed: 1) does alcohol preference prior to training predict responding for alcohol in an operant procedure and; 2) do any of the measures obtained during operant training sessions predict responses under maintenance, extinction, or reinstatement test conditions?
Method
Subjects
The 21 subjects employed in this study were drawn from a larger study investigating the effects of pharmacologically manipulating the P2rx4 gene on alcohol self-administration and reinstatement behaviors reported upon previously (Kosten, 2011). Briefly, subjects were male, adult rats (Sprague-Dawley; Harlan Sprague-Dawley Inc., Indianapolis, IN) housed individually in polypropylene cages with food and water available ad libitum except as noted. Cages were kept in a temperature- and humidity-controlled vivarium maintained on a reverse 12:12 light/dark cycle (lights on at 19:00). The Baylor College of Medicine Institutional Animal Care and Use Committee approved the protocol and the study followed the “Principles of Laboratory Animal Care” (NIH publication No.85-23, revised 1996). Initially, rats were maintained on a modified “drinking in the dark” procedure that consisted of multiple scheduled access to alcohol solution (5% w/v) concurrently with water (Bell & McBride, 2009) as described previously (Kosten, 2011). After 6-wks of alcohol exposure, rats were returned to ad libitum water access and began training for operant self-administration. Of the 21 subjects, 10 were ultimately trained to lever press for an alcohol/sucrose solution (10% ethanol/2% sucrose) and 11 were trained to lever press for sucrose solution (3%). These concentrations of the two solutions were chosen because they support equivalent levels of operant responding under the conditions used in this study (Czachowski, Samson, & Dening, 1999; H. Samson, Slawecki, Sharpt, & Chappell, 1998; Vosler, Bombace, & Kosten, 2001).
Operant self-administration training
Sessions were conducted in standard operant chambers housed within sound-attenuating cubicles with fans, a house light on one side of the chamber, and two levers on the opposite wall (Coulbourn Instruments, Whitehall, PA). A triple cue light was positioned above each lever in between which was an access area that allowed the protrusion of a dipper (0.1 ml capacity). The dipper was immersed in a solution reservoir and it could be activated to protrude through the access area for 3-sec by lever presses. Protrusion of the dipper was accompanied by illumination of a light within the access area and the triple cue lights. This access area was also equipped with infrared sensors that allowed tabulation of head entries into the area. Numbers of whole body movement episodes were recorded through the use of an infrared detector mounted on the ceiling of the operant chamber. A movement episode was defined as a continuous output for the duration that movements occur within any interval of less than 400-ms. That is, if the detector unit continues to detect movement, with any breaks in movement detection being less than 400 milliseconds, the activity will be considered a single movement episode. Data tabulation and programming of stimulus parameters were performed using a Coulbourn Instruments based hardware and software (Graphic State Notation) system.
The 30-min training sessions began with the illumination of the house light. Details on the initial training were described previously (Kosten, 2011). The final response requirements were that only one of the two levers was active and the animal was required to depress this lever twice (i.e., fixed ratio 2 or FR2) to receive delivery of the solution. Briefly, rats were trained initially under an FR1 schedule of reinforcement until they met criteria to move up to the FR2 schedule of reinforcement (<20% variability of active lever presses emitted over 2 days and < 5 inactive lever presses). The animal then had to meet the same criteria under the FR2 schedule to move into test phases (e.g., maintenance and reinstatement). Some rats entered the maintenance test phase first and other rats entered the reinstatement test phase first.
Variables for analyses
Data reported upon in the present study were obtained from two types of sessions (training and test). The training session was the FR2 session on which the animal met criteria to move to test sessions. Test sessions included: 1) those conducted under a progressive ratio (PR) schedule performed as detailed previously (Kosten, 2011; Walker & Koob, 2007); 2) the first session conducted under extinction conditions (no solution present in reservoir with all other stimulus parameters remaining constant); and 3) a combined drug and cue-induced reinstatement session as described previously (Kosten, 2011). All sessions except the PR session were 30-min in length. The PR session was 3-hr in length.
Six dependent variables were used in this exploratory analysis study. These include total numbers of presses on active and inactive levers, whole body movement episodes, and head entries into the dipper access area across each session. In addition, number of trials required to meet extinction criteria (≤ 5 active lever presses emitted over 2 consecutive days) were tabulated and alcohol preference (% alcohol intake of total intake) on the last drinking-in-the-dark session was calculated. First, alcohol preference data from the alcohol responding rats were used to determine if it associated with any responses measured during the test sessions. Second, data obtained on the FR2 training session were used to explore for significant correlations with responses obtained under the PR, extinction, and reinstatement test sessions.
Data analysis
Alcohol preference (% alcohol intake/total intake) on the last drinking-in-the-dark session was used as the regressor in a multiple (simple) regression model using the data from the 10 alcohol-responding rats. The dependent variables included trials to meet extinction criteria, and numbers of active and inactive lever presses, head entries into the dipper trough, and body movement episodes on the first of each of the three test session types (PR, extinction, and reinstatement). Variables in which the probability of the adjusted R2 was less than 0.05 are considered significant.
Examination of correlation matrices suggested that the variable of body movement episodes during the FR2 training session was associated with several test factors. We confirmed our impression using factor analysis. This was the only variable to show significant (>0.70) loading on the second factor of a principal factors analysis (eigenvalue=4.62; 17.11% total variance). A multiple regression model was then run using this variable as the regressor with the data from all 21 rats and the same set of dependent variables detailed above and the same statistical test.
We then rank ordered rats based on these movement episode data and chose those from the upper quartile of the range to be assigned to the “High” movement episode group and those from the lower quartile to be assigned to the “Low” movement episode group (n=6 ea). These groups were compared on active and inactive lever press responses and on head entries into the dipper trough across the three tests. Separate 2 × 3 ANOVAs were run for each dependent measure (active and inactive lever presses; head entries) in which movement episode group was the between groups factor with repeated measures on the three test types. The p value was set at 0.05.
Results
Alcohol preference
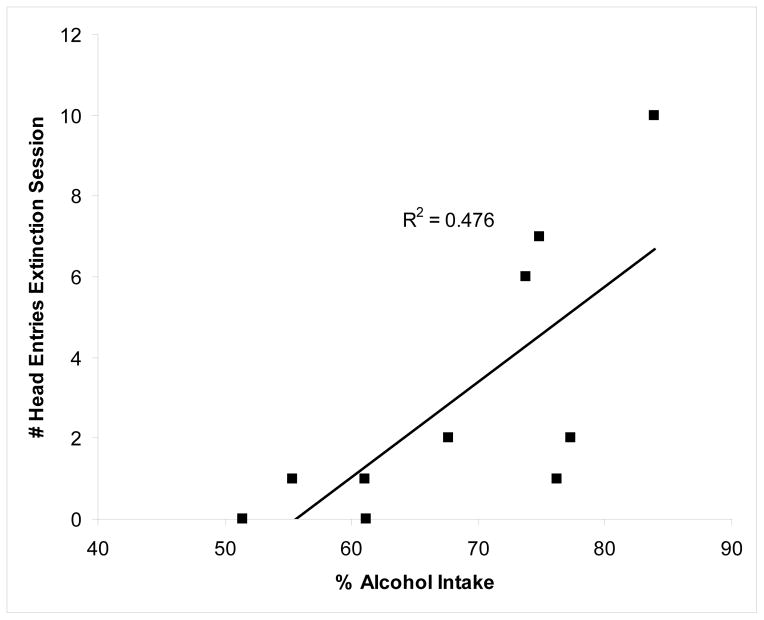
Alcohol preference (% alcohol intake of total intake on the last drinking-in-the-dark session) showed a significant and positive correlation with head entries during Extinction for the alcohol responding group, F(1,8)=9.19; P<0.02), in the multiple regression analysis. The adjusted R2 was 0.476. No other variable showed a significant association with alcohol preference, P’s>0.10.
Movement episodes
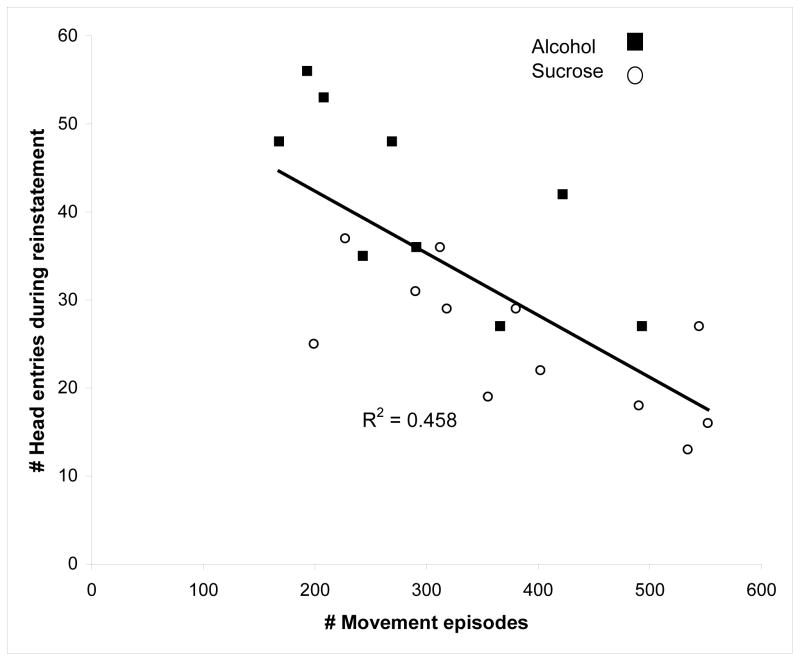
The results of the multiple regression analysis in which number of movement episodes during the FR2 session was used as the regressor revealed two significant negative correlations. This variable was significantly correlated with active lever presses during the Reinstatement session, F(1,19)= 6.86; P<0.02, with an adjusted R2 of 0.226. It also correlated significantly with head entries during the same test session, F(1,19)=17.87; P<0.0005, with an adjusted R2 of 0.458. The association of movement episodes during FR2 session with head entries during the Reinstatement session is depicted in the scatterplot in Fig. 2.
Figure 2.
Correlation of body movement episodes exhibited during the FR2 training session with head entries during the Reinstatement session is shown for all rats. Alcohol-responding rats are depicted by the closed squares and the sucrose-responding rats are depicted by the open circles. The association is significant and shows an adjusted R2 of 0.4576.
High vs. Low Movement Episode Groups
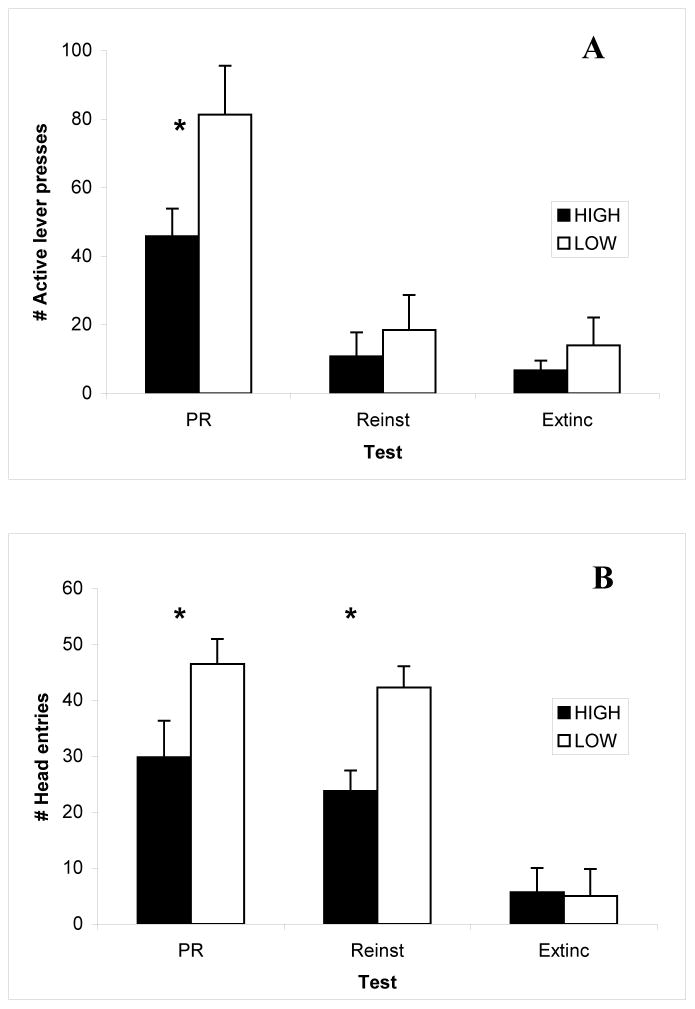
Rats with movement episodes in the upper quartile during the FR2 session were assigned to the “High” movement episode group and those in the lower quartile to the “Low” movement episode group (n=6 ea) and compared on three sets of measures, active and inactive lever presses, and head entries, across the three test sessions, PR, Extinction, and Reinstatement. Levels of inactive lever presses were minimal, averaging from 0 to less than 2 over these sessions, and did not differ between groups, P’s >0.05 (data not shown). Active lever press responding differed by Test session, F(2,20)=27.23; P<0.0001, as seen in the top panel of Fig 3. As expected, these numbers were higher during the session in which the reinforcer was available for delivery (PR session) compared to the sessions in which it was not available (Reinstatement and Extinction). Low movement episode rats emitted significantly more active lever presses compared to High movement episode rats across tests as supported by the significant Group effect, F(1,10)=15.66; P<0.005. These data are shown in the top panel of Fig. 3. Post-hoc analyses revealed significant Group differences during the PR session, P<0.05.
Figure 3.
Mean (± S.E.M.) numbers of lever presses on the active lever (Panel A) and head entries (Panel B) are presented for Low movement episode rats (open bars) and High movement episode rats (solid bars) during the three test sessions (PR- progressive ratio; Reinst – Reinstatement; and Extinc – Extinction). Low movement episode rats emit more active lever presses and show greater numbers of head entries than high movement episode rats across sessions. Significant post-hoc effects on specific test sessions are depicted by asterisks (*).
The numbers of head entries also differed by Test session, F(2,20)= 35.92; P<0.0001, as seen in the bottom panel of Fig. 3. For this measure, the numbers were high during both the PR and Reinstatement sessions and low during the Extinction session. Again, Low movement episode rats exhibited a greater number of head entries compared to High movement episode rats as supported by the significant Group effect, F(1,10)= 6.80; P<0.05. There was a trend towards significance for the Group X Test interaction effect, F(2,20)= 3.25; P<0.06. This may reflect that there was little difference between groups during the Extinction session whereas the post-hoc analyses revealed significant group differences during both the PR and Reinstatement sessions, P’s<0.05.
Discussion
In this exploratory study, we probed for variables associated with behaviors measured during operant self-administration sessions and found some strong, specific, and meaningful correlations that could be valuable in predicting relapse to excessive drinking in alcoholics. First, greater alcohol preference predicts greater head entries during Extinction in alcohol-trained rats. No other measure obtained during any of the sessions related to alcohol preference. Second, greater movement episodes during an FR2 training session associated with fewer head entries and active lever presses during the Reinstatement session in both alcohol- and sucrose-trained rats. Further, Low movement episode rats exhibited more head entries and higher levels of lever pressing across test session types compared to High movement episode rats.
Alcohol preference
We anticipated that alcohol preference in the alcohol-trained group would relate to one of the measures obtained during operant sessions (A. S. Green & Grahame, 2008). Indeed, rat lines selectively bred for alcohol preference in two-bottle drinking tests, such as P (alcohol-preferring), AA (Alko, accepting), or sP (Sardinian alcohol-preferring), exhibited greater lever presses and alcohol intake relative to their respective comparison groups (NP, ANA, and sNP) in studies using operant self-administration of alcohol (Files et al., 1997; Ritz, George, & Meisch, 1989a, 1989b; H. H. Samson et al., 1998; Vacca et al., 2002). Unlike these former studies that compared rat lines selectively bred based on divergent responses to alcohol, in the present study with outbred rats, there was no relation between alcohol preference and active lever presses during the maintenance session or under conditions of no reinforcer availability (Reinstatement or Extinction). Interestingly, alcohol preference significantly associated with head entries during the Extinction session such that greater alcohol preference predicted more head entries (see Fig. 1). This model accounted for more than 47% of the variance and suggests that rats with greater preference for alcohol will exhibit greater alcohol-seeking behavior but only when the alcohol is not present.
Figure 1.
The correlation between alcohol preference (% alcohol intake/total intake) prior to initiating self-administration training and head entries during the Extinction session are shown in this scatterplot for the alcohol-responding rats. The association is significant and shows an adjusted R2 of 0.4763.
Body movement episodes
Greater body movement episodes during the FR2 training session predicted fewer active lever presses and head entries during the Reinstatement session. We believe this relationship is meaningful and has utility for understanding risk factors in relapse. Numbers of body movement episodes exhibited is a relatively consistent trait. These numbers correlated extremely well across training and test sessions (data not shown) suggesting that there was specificity in this measure. The relevance of the body movement episodes variable was further tested by dividing rats into two groups – those with a high number of body movement episodes and those with a low number of body movement episodes. Low movement episode rats emitted greater numbers of active lever presses during the PR session and showed greater head entries during the PR and Reinstatement sessions compared to the High movement episode rats (see Fig. 3). These results may suggest that Low movement episode rats were more focused on reinforcer delivery than High movement episode rats or that the reinforcer was more effective for the former group.
The Low and High movement episodes distinction obtained in this study differs from that used previously (Marinelli, 2005; Mitchell et al., 2005; Piazza et al., 1990). In these prior studies, low responding (LR) and high responding (HR) rats were determined by assessing ambulatory activity in a large apparatus during the animal’s first exposure to it. In contrast, body movement episodes assessed in this study include both ambulatory and other body movements and were obtained in a smaller-sized operant chamber after several exposures to this environment. Thus, unlike the studies with LR and HR rats, the measure of body movements in the current study would not reflect the likely stress response to being placed within a novel environment. In addition, the measure of movement episodes employed in the present study reflects numbers of bouts of continuous movements, not numbers of specific movement types.
Relevance of active lever presses
Active lever presses could be considered sign-tracking whereas head entries could be considered conditioned approach or goal-tracking (Breland & Breland, 1961; Krank, 2003; Messing, Kleven, & Sparber, 1986; Tomie, 1996). That is, the lever paired with delivery of the reinforcer in a reliable manner can come to elicit approach towards it even if it has no consequences for reinforcer delivery or if these responses interfere with obtaining the reinforcer. This is considered sign-tracking. Goal-tracking, in this case, is approaching the area where the dipper is located and can be measured as head entries. However, the present study of operant self-administration was not designed as a sign-tracking study. That is, the typical method for a sign-tracking study involves non-contingent presentations of a stimulus such as a lever protrusion followed by food or other reinforcer delivery in an autoshaping procedure. In the present study, the Reinstatement session did follow presentation of 15 noncontingent alcohol deliveries under similar stimulus conditions as training. This may explain why there were a greater number of head entries during this session. Further, this session was run after several extinction sessions, enough for the rat to show minimal levels of lever presses. Interestingly, lever press responses are typically used to track and define extinction. Data from the present study suggest that while sign-tracking (lever presses) may be extinguished, goal-tracking (head entries) is not.
Relevance of body movement episodes
There are distinctions that can be made between Extinction and Reinstatement sessions in body movement episodes and its potential to address goal- and sign-tracking behaviors. For example, comparing numbers of active lever presses under the different session types (top panel of Fig. 3) to the numbers of head entries (bottom panel of Fig. 3) shows that lever press responses are quite low during the Reinstatement session and head entries are quite high. This is not what is seen during the PR session when alcohol or sucrose is available for delivery. In PR sessions, both types of responses are high. This may suggest that the variable of movement episodes predicts both goal- and sign-tracking behaviors under conditions in which the reinforcer is available but only predicts goal-tracking under conditions when the reinforcer is not available but the cues and context to which its delivery was associated are present. Teasing apart such behavioral indicators may aid in the development of therapeutic agents that could target either early intervention abstinence issues or later issues, such as protracted withdrawal and relapse prevention.
Study limitations
There are some limitations in the present study. The sample sizes for the two reinforcer-type groups were small (n’s=10–11) for a correlational study and so we combined the two groups to achieve greater statistical power. This assumes that one type of orally-delivered reinforcer is like another. Clearly, this is not always the case. Yet, both alcohol- and sucrose-trained groups showed similar associations between body movements and head entries during Reinstatement. This can be seen in Fig. 2 in which the individual data points in the scatter-plot are depicted by group. Further, there was no difference in the proportion of rats from the alcohol- and sucrose-trained groups that were assigned to either Low or High movement episodes groups. This study is also limited in that it did not assess the acquisition phase of self-administration, the phase most often studied in research on risk factors for addiction. Acquisition studies require a systematic training procedure. Training during the initial FR1 phase was tailored for each rat in the present study. Finally, a limitation of this study is that construct and predictive validity of the reinstatement model of relapse has not been confirmed (Katz & Higgins, 2003; Koob, Lloyd, & Mason, 2009). Nonetheless, this study points to the utility of conducting exploratory analyses using multiple dependent variables in a manner similar to analyses of data obtained from clinical trial studies. Of course, such studies necessitate the use of multiple regression analyses, as in the present study, or other technique in order to adjust for the influence of potential correlations among the predictor variables. Overall, this approach may be useful in unifying preclinical and clinical research findings.
Experimental strategy
The data from the present study can be useful for preclinical studies aimed at studying potential pharmacological treatments for addiction. For example, the measure of body movement episodes could be used to screen for rats that show greater reinstatement in order to test potential treatment agents. This would help avoid floor effects since levels of reinstated responding for alcohol are typically not very high. Further, this may improve the model as it would more closely align with clinical studies that enroll participants prone to relapse. Head entries can be interpreted as a measure of conditioned approach. Thus, it would be useful to see if this association between body movement episodes and behavior during reinstatement generalizes to other reinforcers besides alcohol and sucrose. Finally, understanding the neurobiological bases of the commonalities between levels of body movement episodes and head entries during Reinstatement would provide valuable information for understanding risk factors in addiction.
Yet, aspects of this study go beyond the particular findings and relate to the strategy employed. It consists of several parts. One is to use additional dependent measures such as head entries and body movement episodes. These behaviors can be valuable because, unlike lever pressing, numbers of body movement episodes and head entries were not used as criteria for extinction and thus they are somewhat independent of the lever press response. The second part is the use of exploratory data analysis. Calculating correlations among measures and exploring the significant correspondences (positive or negative) can uncover unexpected findings. Moreover, changes in one variable but not another may assist data interpretation. A third aspect is to study behavior across multiple conditions, such as maintenance, extinction, and reinstatement. As methods for handling large data sets continue to develop, the use of multiple dependent measures will permit the discovery of orderly relations that would otherwise be missed. Finally, the suggested strategy will also help increase the return on resources invested in conducting the studies and may be useful in unifying Preclinical trial studies.
Acknowledgments
This research was supported by NIAAA grant U01-AA013476. The funding source had no role other than financial support.
We greatly acknowledge the technical assistance provided by A. Aruffo, L. Heggeness, W. Huang, M. Kosten, P. O’Malley, M. Rengasamy, J. Taylor, W. Webb, and X. Xiang.
Footnotes
Both authors contributed in a significant way to the manuscript and have read and approved the final version.
The authors have no conflicts of interest to disclose.
References
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers in Neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1652–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, McBride WJ. Drinking in the dark - Multiple scheduled access (DID-MSA) 2009 from http://www.scripps.edu/cnad/inia/modelratdrinkingindark.pdf.
- Breland K, Breland M. The misbehavior of organisms. American Psychologist. 1961;16:681–683. [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behavioural Pharmacology. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Weiss F. Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcoholism: Clinical and Experimental Research. 2001;25:1414–1419. doi: 10.1097/00000374-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, et al. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addiction Biology. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH, Dening CE. Independent ethanol-and sucrose-maintained responding on a multiple schedule of reinforcement. Alcohol: Clinical and Experimental Research. 1999;23:398–403. [PubMed] [Google Scholar]
- Dalley JW, Fryer T, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Files FJ, Denning CE, Hyytia P, Kiianmaa K, Samson HH. Ethanol-reinforced responding by AA and ANA rats following the sucrose-substitution initiation procedure. Alcoholism: Clinical and Experimental Research. 1997;21:749–753. [PubMed] [Google Scholar]
- Files FJ, Samson HH, Denning CE, Marvin S. Comparison of alcohol-preferring and non-preferring selectively bred rat lines. II. Operant self-administration in a continuous-access situation. Alcoholism: Clinical and Experimental Research. 1998;22:2147–2158. [PubMed] [Google Scholar]
- George FR. Genetic approaches to studying drug abuse: Correlates of drug self-administration. Alcohol. 1990;7:207–211. doi: 10.1016/0741-8329(90)90006-x. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology. 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta Stone approach. Nature Reviews Drug Discovery. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA. Pharmacologically targeting the P2rx4 gene on maintenance and reinstatement of alcohol self-administration in rats. Pharmacology Biochemistry and Behavior. 2011;98:533–538. doi: 10.1016/j.pbb.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: Insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Kehoe P. Early life stress and vulnerability to addiction: translational studies with neonatal isolation of rat pups. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. San Diego, CA: Elsevier; 2007. pp. 105–126. [Google Scholar]
- Kosten TA, Sanchez H, Zhang XY, Kehoe P. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behavioural Brain Research. 2004;151:137–149. doi: 10.1016/j.bbr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Krank MD. Pavlovian conditioning with ethanol: sign-tracking (autoshaping), conditioned incentive, and ethanol self-administration. Alcoholism: Clinical and Experimental Research. 2003;27:1592–1598. doi: 10.1097/01.ALC.0000092060.09228.DE. [DOI] [PubMed] [Google Scholar]
- Le AD, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacology & Therapeutics. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Marinelli M. The many facets of the locomotor response to a novel environment test: theoretical comment on Mitchell, Cunningham, and Mark (2005) Behavioral Neuroscience. 2005;119:1144–1151. doi: 10.1037/0735-7044.119.4.1144. [DOI] [PubMed] [Google Scholar]
- Messing RB, Kleven MS, Sparber SB. Delaying reinforcement in an autoshaping task generates adjunctive and superstitious behaviors. Behavioural Processes. 1986;13:327–338. doi: 10.1016/0376-6357(86)90028-8. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Cunningham CL, Mark GP. Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behavioral Neuroscience. 2005;119:464–472. doi: 10.1037/0735-7044.119.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, LeMoal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behavioural Pharmacology. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Ritz MC, George FR, Meisch RA. Ethanol self-administration in ALKO rats: I. Effects of selection and concentration. Alcohol. 1989a;6:227–233. doi: 10.1016/0741-8329(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Ritz MC, George FR, Meisch RA. Ethanol self-administration in ALKO rats: II. Effects of selections and fixed-ratio size. Alcohol. 1989b;6:235–239. doi: 10.1016/0741-8329(89)90024-4. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neuroscience and Biobehavioral Reviews. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Samson H, Slawecki C, Sharpt A, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcoholism: Clinical and Experimental Research. 1998;22:1783–1787. [PubMed] [Google Scholar]
- Samson HH, Files FJ, Denning C, Marvin S. Comparison of alcohol-preferring and nonpreferring selectively bred rat lines. I. Ethanol initiation and limited access operant self-administration. Alcoholism: Clinical and Experimental Research. 1998;22:2133–2146. [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, DeWit H. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. International Journal of Neuropsychopharmacology. 2009;12:1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A. Locating reward cue at response manipulandum (CAM) induces symptoms of drug abuse. Neuroscience and Biobehavioral Reviews. 1996;20:505–535. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Vacca G, Serra S, Brunetti G, Carai MA, Samson HH, Gessa GL, et al. Operant self-administration of ethanol in Sardinian alcohol-preferring rats. Alcoholism: Clinical and Experimental Research. 2002;26:1678–1685. doi: 10.1097/01.ALC.0000036285.62071.DA. [DOI] [PubMed] [Google Scholar]
- Vosler PS, Bombace JC, Kosten TA. A discriminative, two-lever test of dizocilpine’s ability to reinstate ethanol-seeking behavior. Life Sciences. 2001;69:591–598. doi: 10.1016/s0024-3205(01)01150-x. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The g-aminobutyric acid-b receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcoholism: Clinical and Experimental Research. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]