Abstract
Background
Plasmacytoid dendritic cells have been implicated in the pathogenesis of systemic sclerosis through mechanisms beyond the previously suggested production of type I interferon.
Methods
We isolated plasmacytoid dendritic cells from healthy persons and from patients with systemic sclerosis who had distinct clinical phenotypes. We then performed proteome-wide analysis and validated these observations in five large cohorts of patients with systemic sclerosis. Next, we compared the results with those in patients with systemic lupus erythematosus, ankylosing spondylitis, and hepatic fibrosis. We correlated plasma levels of CXCL4 protein with features of systemic sclerosis and studied the direct effects of CXCL4 in vitro and in vivo.
Results
Proteome-wide analysis and validation showed that CXCL4 is the predominant protein secreted by plasmacytoid dendritic cells in systemic sclerosis, both in circulation and in skin. The mean (±SD) level of CXCL4 in patients with systemic sclerosis was 25,624±2652 pg per milliliter, which was significantly higher than the level in controls (92.5±77.9 pg per milliliter) and than the level in patients with systemic lupus erythematosus (1346±1011 pg per milliliter), ankylosing spondylitis (1368±1162 pg per milliliter), or liver fibrosis (1668±1263 pg per milliliter). CXCL4 levels correlated with skin and lung fibrosis and with pulmonary arterial hypertension. Among chemokines, only CXCL4 predicted the risk and progression of systemic sclerosis. In vitro, CXCL4 downregulated expression of transcription factor FLI1, induced markers of endothelial-cell activation, and potentiated responses of toll-like receptors. In vivo, CXCL4 induced the influx of inflammatory cells and skin transcriptome changes, as in systemic sclerosis.
Conclusions
Levels of CXCL4 were elevated in patients with systemic sclerosis and correlated with the presence and progression of complications, such as lung fibrosis and pulmonary arterial hypertension. (Funded by the Dutch Arthritis Association and others.)
Systemic sclerosis (also called scleroderma) is a complex heterogeneous fibrosing autoimmune disorder with an unknown pathogenesis. The way in which its three major pathologic hallmarks — extensive fibrosis, vasculopathy, and immune dysfunction — are interconnected is unknown. Mechanistic understanding is limited, in part, by a lack of animal models and by clinically heterogeneous patient populations.1 This disorder is classified into two major subtypes on the basis of the extent of cutaneous fibrosis: limited cutaneous and diffuse cutaneous systemic sclerosis.2 Pulmonary fibrosis and pulmonary arterial hypertension are the two most serious complications — currently the major causes of death among patients with this disorder. Thus, in addition to clarifying pathogenic mechanisms, the identification of biomarkers for the presence and progression of clinical complications of systemic sclerosis has potential use in the assessment of disease activity.
On the basis of key observations by LeRoy3 that collagen production was increased in fibroblasts that were isolated from scleroderma skin and cultured in vitro, much of the research on systemic sclerosis has focused on altered fibroblast biology. More recent studies, however, indicate that immune cells are important in pathogenesis.4,5 Indeed, genetic association studies have revealed that the most highly associated susceptibility markers include the genes encoding immune signaling molecules T-bet,6 STAT4,7,8 and IRF58,9 and the T-cell–receptor zeta chain.8 STAT4 and IRF5 are both implicated in the secretion of type I interferon, a cytokine that has been shown to be present in both cutaneous and peripheral-blood mononuclear cells.10 Plasmacytoid dendritic cells are the major source of type I interferon, and as such have been implicated in multiple autoimmune conditions that have a type I interferon signature, including systemic lupus erythematosus,11 Sjögren’s syndrome,12 and rheumatoid arthritis.13 Although two studies have shown that serum samples obtained from patients with systemic sclerosis showed type I interferon– inducing activity, the role of plasmacytoid dendritic cells in systemic sclerosis has not been fully explored.14,15 The aim of our study was to identify a possible role for plasmacytoid dendritic cells in the pathogenesis of systemic sclerosis that is associated with the clinical phenotype.
Methods
Study Patients
In our study, we evaluated 779 patients with systemic sclerosis — 462 with the limited cutaneous subtype (limited disease) and 317 with the diffuse cutaneous subtype (diffuse disease). Throughout the study, the patient cohort from the Boston University School of Medicine was the identification cohort for studies of plasmacytoid dendritic cells and included 20 healthy donors and 53 patients with systemic sclerosis; the latter included 16 patients with limited disease, 18 with late diffuse disease (duration, >3 years), and 19 with early diffuse disease (duration, <2 years). In addition, for the chemokine analysis, plasma was obtained from an additional 22 healthy donors, 15 patients with limited disease, and 31 patients with diffuse disease. The replication cohorts comprised patients from the University of Nijmegen, the Netherlands (148 patients), Lund, Sweden (197), Milan (120), Verona, Italy (18), Ghent, Belgium (79), and Houston (50). Samples from an additional 68 patients from Milan were included to compare CXCL4 levels in patients with early systemic sclerosis with levels in patients in various phases of preclinical systemic sclerosis, including those with only Raynaud’s phenomenon with or without specific antinuclear antibodies, anti–topoisomerase or anti–centromere antibodies, or capillary nailfold lesions resembling systemic sclerosis.
For the studies of the CXCL4 expression in plasmacytoid dendritic cells, skin sections were obtained from 3 patients with early diffuse systemic sclerosis from Boston University and 6 patients with late diffuse disease from the University of Dusseldorf, Germany. All patients met the preliminary criteria of the American College of Rheumatology for the classification of systemic sclerosis (Table 1).16 The clinical phenotype of the patients is described further in the Supplementary Appendix, available with the full text of this article at NEJM.org. All samples were obtained after patients provided written informed consent and after approval of the study by the institutional review board at each participating center.
Table 1.
Disease Characteristics of 779 Patients with Systemic Sclerosis.*
| Characteristic | Limited Cutaneous Systemic Sclerosis (N = 462) |
Diffuse Cutaneous Systemic Sclerosis (N = 317) |
|---|---|---|
| Female sex — no. (%) | 378 (82) | 218 (69) |
| Age at onset — yr | 42.4±12.3 | 43.8±11.2 |
| Disease duration — yr | 9.4±8.0 | 5.8±6.2 |
| Positive test for antinuclear antibodies— % | 97 | 86 |
| Modified Rodnan skin-thickness score† | 5.4±2.4 | 15.9±8.2 |
| Pulmonary arterial hypertension — % | 39 | 23 |
| Lung fibrosis — % | 26 | 48 |
| Current therapies — % | ||
| Mycophenolate mofetil | 0 | 41 |
| Cyclophosphamide | 0 | 23 |
| Prednisolone | 25 | 30 |
| Hydroxychloroquine | 18 | 11 |
| Anti–interleukin-3 antibody | 0 | 2 |
| Methotrexate | 1 | 5 |
| Tacrolimus | 12 | 0 |
Plus–minus values are means ±SD.
P = 0.03 for the comparison between patients with diffuse disease and those with limited disease. There were no other significant between-group differences. Scores on the modified Rodnan skin-thickness scale are calculated by clinical palpation of 17 body areas, with the thickening of each area scored as 0 (normal), 1 (mild), 2 (moderate), or 3 (severe).
To compare CXCL4 levels in samples obtained from patients with systemic sclerosis with levels in samples from healthy donors, we obtained plasma samples from 257 age- and sex-matched healthy persons from the Nijmegen Biomedical Study.17 To compare CXCL4 levels in patients with systemic sclerosis with levels in patients with other clinical conditions, we determined the levels of CXCL4 in stored samples from 109 patients with systemic lupus erythematosus who fulfilled the American College of Rheumatology criteria18,19 (Table S1 in the Supplementary Appendix), from 93 patients with ankylosing spondylitis20 (Table S2 in the Supplementary Appendix), and from patients with various stages of liver fibrosis (see the Supplementary Appendix for details).
Cell-Based Studies and Measurement of Inflammatory Mediators
All the techniques that we used in this study are described in detail in the Supplementary Appendix. Briefly, we performed proteome-wide analysis of supernatant from plasmacytoid dendritic cells, using surface-enhanced laser desorption/ionization–time-of-flight (SELDI-TOF) mass spectrometry. Quantification of secreted cytokines was performed with the use of an enzyme-linked immunosorbent assay or Luminex immunoassay. We used primary endothelial cells and peripheral plasmacytoid dendritic cells to study the effect of CXCL4 in vitro. To assess CXCL4 function in vivo, C57BL/6 mice were exposed to subcutaneous CXCL4 for further RNA and histologic analysis.
Statistical Analysis
We used Student’s t-test or the Mann–Whitney U test for the analysis of quantitative traits, as appropriate, and the Kaplan–Meier method to estimate survival in patients with high CXCL4 levels at baseline, as compared with those with low CXCL4 levels, with survival time set at 24 months. After verifying that the proportionality of hazards was not violated, we used Cox regression analysis to estimate the effect size of CXCL4 categorization on the basis of plasma levels on the time to event; results are presented as hazard ratios along with their asymptotic 95% confidence intervals. Listwise deletion was used in cases with missing data.
To assess the most effective cutoff value for CXCL4, we used DeLong’s method to compute a receiver-operating-characteristic (ROC) curve. The area (±SE) under the ROC curve was 0.987±0.009 (95% confidence interval [CI], 0.952 to 0.996; P<0.001). At the cutoff value for CXCL4 of 11,589 pg per milliliter, the sensitivity was 100%, and the specificity was 94%. At the cutoff value of 9789 pg per milliliter, the sensitivity was 100%, and the specificity was 93%. For a practical cutoff for clinical practice, we chose 10 ng per milli liter for CXCL4, to be conservative with respect to sensitivity. P values of less than 0.05 (all two-sided) were considered to indicate statistical significance and were adjusted with the use of a Bonferroni correction.
Results
CXCL4 Secretion by Plasmacytoid Dendritic Cells
To assess the production of type I interferon by plasmacytoid dendritic cells, we isolated BDCA4+ cells from 20 healthy donors and compared results in their plasmacytoid dendritic cells with those in cells obtained from 54 patients with systemic sclerosis: 17 with limited disease and 37 with diffuse disease (Fig. S1A in the Supplementary Appendix). Patients with diffuse disease were further stratified according to disease duration, with 18 patients in the early-disease group and 19 in the late-disease group. A chromogenic reaction was reproducibly present in the media from the plasmacytoid dendritic cells obtained from patients with early systemic sclerosis. Measurement of multiple inflammatory mediators in these supernatants showed almost no detectable production of type I interferon or other mediators, with differential expression of only interleukin-8 and interleukin-6 (Fig. S1B through S1G in the Supplementary Appendix).
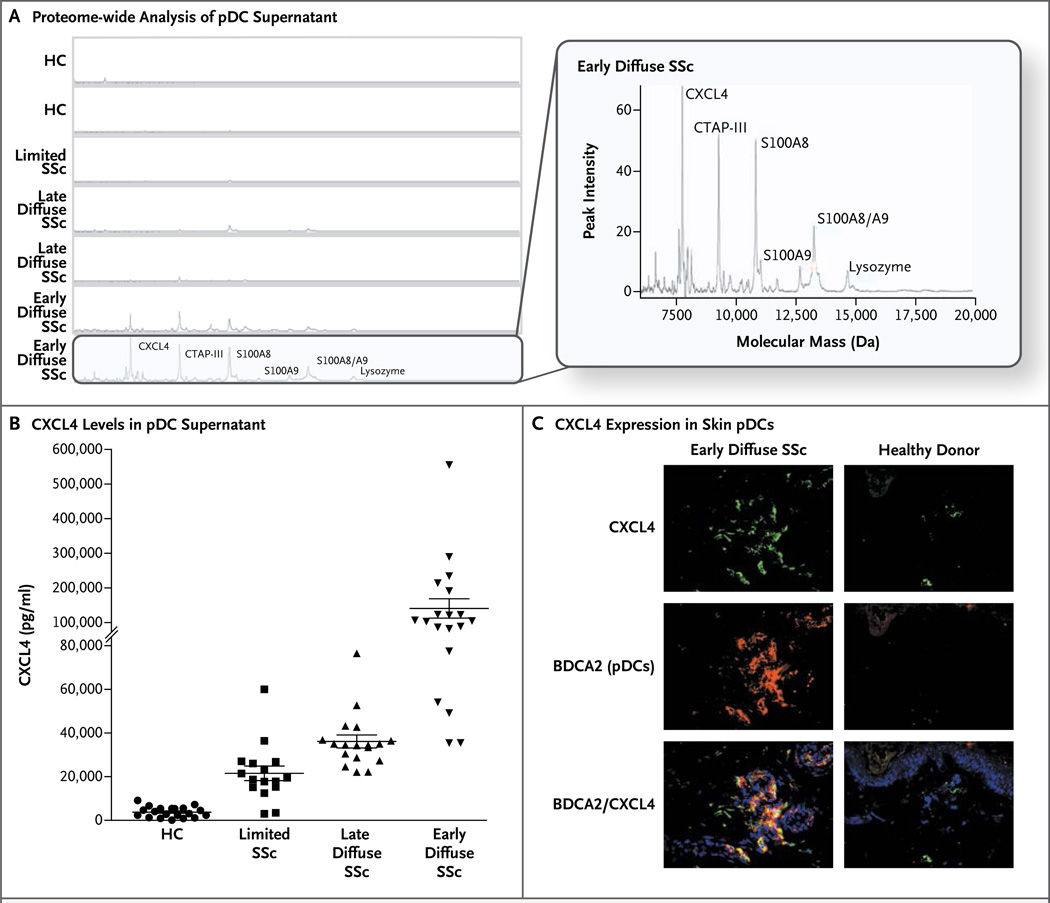
Proteome-wide measurements from eight independent experiments robustly showed that only plasmacytoid dendritic cells from patients with early systemic sclerosis secreted a unique pattern of peaks that were identified as CXCL4, CTAP-III, S100A8/9, and lysozyme (Fig. 1A, and Fig. S1H in the Supplementary Appendix). Confirmatory assessments showed markedly increased levels of CXCL4 in patients with systemic sclerosis (Fig. 1B). Increased levels of CXCL4 messenger RNA (mRNA) were observed only in purified plasmacytoid dendritic cells, and increased CXCL4 protein expression was observed in circulating plasmacytoid dendritic cells (Fig. S1I and S1J) and in plasmacytoid dendritic cells in skin from patients with systemic sclerosis (Fig. 1C, and Fig S1K in the Supplementary Appendix).
Figure 1. Identification of CXCL4 as the Major Protein Product of Plasmacytoid Dendritic Cells in Systemic Sclerosis.
Panel A shows the results of proteome-wide analysis of supernatants from plasmacytoid dendritic cells (pDC) obtained from patients with various subtypes of systemic sclerosis (SSc) and from healthy controls (HC). Highlighted in the larger view is the analysis of samples obtained from patients with early diffuse systemic sclerosis, showing peaks for CXCL4, connective tissue-activating peptide III (CTAP-III), S100A8/A9 (MRP8/14), and lysozyme. Panel B shows the level of CXCL4 in supernatants from plasmacytoid dendritic cells from the different groups that were investigated — 20 healthy donors, 16 patients with limited disease, 19 with late diffuse disease, and 18 with early diffuse disease — on CXCL4-specific enzyme-linked immunosorbent assay. The horizontal lines indicate means, and I bars standard deviations. Panel C shows frozen skin sections from a representative patient with early diffuse disease (at left) and from a healthy control (at right), which were stained for the plasmacytoid dendritic-cell marker BDCA2 (red), CXCL4 (green), and DAPI-labeled nuclei (blue). BDCA2+ cells expressing CXCL4 are seen as orange (merged, lower panels).
CXCL4 in the Circulation
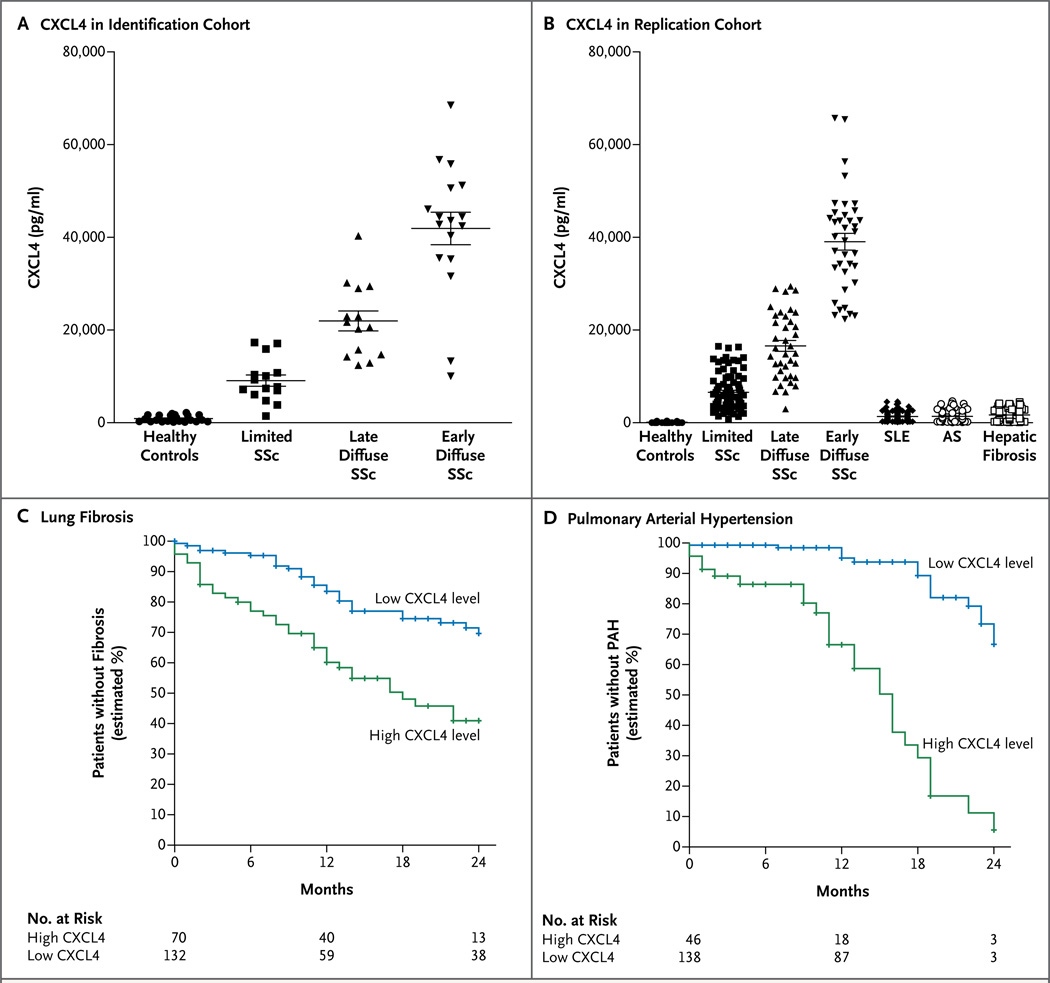
In the identification cohort, levels of circulating CXCL4 were markedly higher in patients with systemic sclerosis than in healthy controls and were particularly high in patients with early diffuse disease (P<0.001 for both comparisons) (Fig. 2A). Increased CXCL4 levels were successfully validated in the replication cohort (which included 86 patients with systemic sclerosis with limited cutaneous disease, 38 with late diffuse disease, and 37 with early diffuse disease) and were compared with 129 age- and sex-matched healthy donors and 109 patients with systemic lupus erythematosus, 93 with ankylosing spondylitis, and 93 with liver fibrosis (Fig. 2B).
Figure 2. Increased Levels of Circulating CXCL4 in Systemic Sclerosis and the Association with Lung Fibrosis and Pulmonary Arterial Hypertension.
High levels of CXCL4 were observed in the circulation of patients with limited systemic sclerosis (SSc), late diffuse SSc, and early diffuse SSc in the identification cohort at Boston University (Panel A) and in 161 patients with corresponding subtypes of systemic sclerosis in two independent (Dutch and Swedish) replication cohorts, as compared with 129 age- and sex-matched healthy controls, 109 patients with systemic lupus erythematosus (SLE), 93 with ankylosing spondylitis (AS), and 93 with hepatic fibrosis (Panel B). The horizontal lines indicate means, and I bars standard deviations. Shown are the estimated times until the development of lung fibrosis (Panel C) and pulmonary arterial hypertension (PAH) (Panel D) within the first 24 months after the diagnosis of systemic sclerosis among patients with a high circulating CXCL4 level (≥10 ng per milliliter) and among those with a low CXCL4 level (<10 ng per milliliter).
In addition, in the replication cohort, there were clear differences between the two groups in previously suggested biomarkers for systemic sclerosis, such as CCL5,21 von Willebrand factor,22 CCL18,23 CCL2,24 and CXCL1024(Fig. S2B in the Supplementary Appendix). In an exploratory analysis, we found that CXCL4 levels gradually and significantly increased per group in the following order: patients with primary Raynaud’s phenomenon, those with Raynaud’s phenomenon and positive antinuclear antibodies, and those with very early systemic sclerosis (Raynaud’s phenomenon plus anti–topoisomerase or anti–centromere antibodies and the presence of nailfold angiopathy), whereas the levels of CCL2, CXCL10, CCL5, von Willebrand factor, and CCL18 did not increase (Fig. S2C in the Supplementary Appendix).
We next assessed the association between CXCL4 levels and the clinical phenotype. CXCL4 levels correlated with the extent of skin fibrosis in the limited cutaneous phenotype (R2 = 0.59, P<0.001) and the diffuse cutaneous phenotype (R2 = 0.74, P<0.001). Patients with high levels of circulating CXCL4 (≥10 ng per milliliter), as compared with those with low CXCL4 levels, had significantly earlier evidence of lung fibrosis, as measured by a relative decline of more than 30% in the forced vital capacity (hazard ratio, 3.67; 95% CI, 2.31 to 4.31; P<0.001) or by the presence of bilateral fibrosis on high-resolution computed tomography (CT) (hazard ratio, 2.60; 95% CI, 1.61 to 5.26; P<0.001) (Fig. 2C). Furthermore, patients with systemic sclerosis who had evidence of pulmonary arterial hypertension had markedly increased circulating CXCL4 levels, as compared with those without such evidence (19,078±629 vs. 5023±329 pg per milliliter, P<0.001). High CXCL4 levels were associated with the earlier development of pulmonary arterial hypertension, as determined on right-heart catheterization (hazard ratio, 8.33; 95% CI, 4.43 to 15.72; P<0.001) (Fig. 2D).
We also investigated whether CXCL4 could serve as a biomarker in a prospective cohort of 79 patients who were followed for 18 months. Patients who had a high baseline level of CXCL4, as compared with other biomarkers for systemic sclerosis, had a significantly faster decline in diffusion capacity of the lung for carbon monoxide (P = 0.002), higher prevalence of high-resolution CT–confirmed lung fibrosis (22% vs. 8%, P<0.001), and faster progression of skin fibrosis (P<0.001) (Fig. S2D in the Supplementary Appendix). None of the other chemokines that were measured correlated with progression. Table S3 in the Supplementary Appendix provides a summary of CXCL4 plasma levels in all the study cohorts.
In Vitro Studies
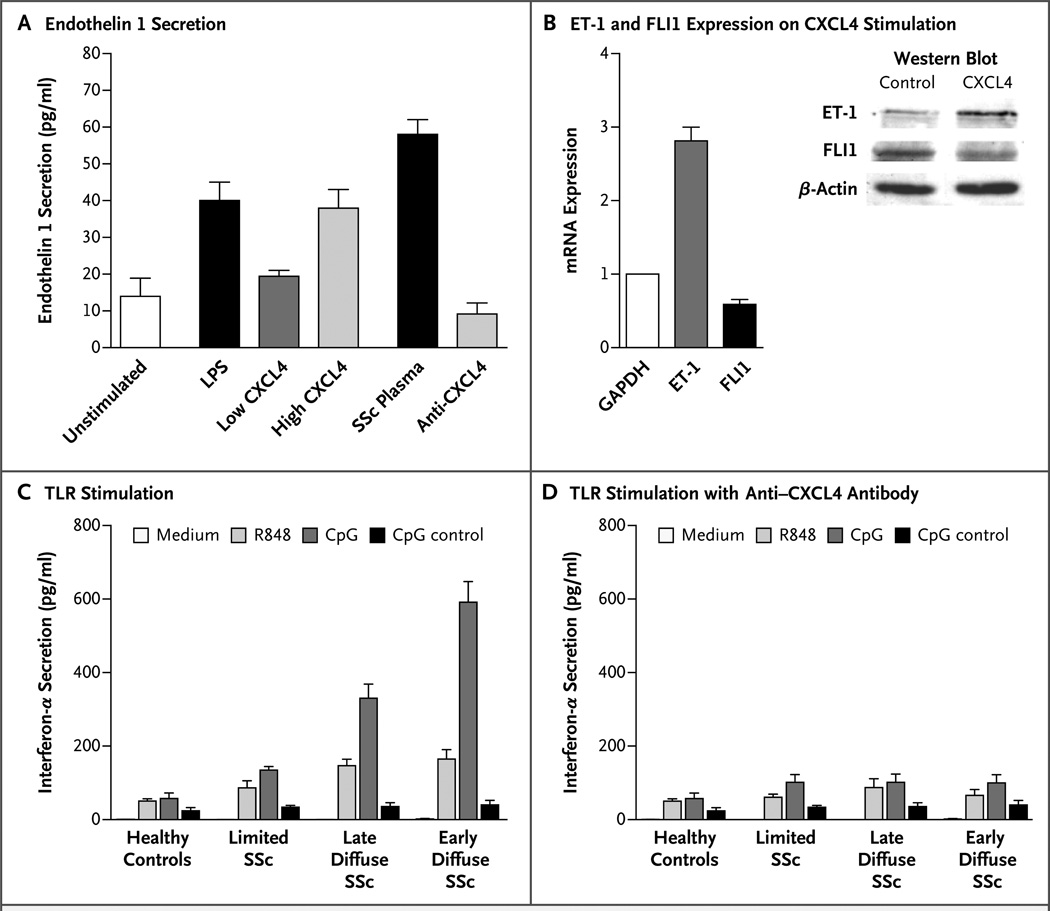
We examined CXCL4 in vitro using plasma obtained from patients with systemic sclerosis and commercially available CXCL4. Both the CXCL4 and plasma induced the secretion of endothelin 1and down-regulation of the transcription factor FLI1 in human umbilical-vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HDMECs). The addition of anti–CXCL4 antibody attenuated these responses (Fig. 3A and 3B). In addition, CXCL4 inhibited the effect of vascular endothelial growth factor on the proliferation of HDMECs (P<0.001), a finding that we speculate may underlie the rarefaction of vessels in patients with systemic sclerosis (data not shown).
Figure 3. Changes in Endothelial Cells and Augmented Responses in Toll-Like Receptors Induced by CXCL4.
The addition of lipopolysaccharide (LPS), low and high levels of CXCL4, and plasma obtained from a patient with diffuse systemic sclerosis (SSc) induce the secretion of endothelin 1 by human umbilical-vein endothelial cells, reactions that were attenuated by the addition of a neutralizing antibody against CXCL4 (Panel A). In five independent experiments, CXCL4 was shown to reduce the expression of transcription factor FLI1 (P<0.001) and induce the expression of endothelin 1 (ET-1) (P =0.003), both on the RNA and protein level, in human dermal microvascular endothelial cells (Panel B). The expression level for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was set at 1. On stimulation with ligands of toll-like receptors (TLR) (including R848, CpG, and CpG control), plasmacytoid dendritic cells from patients with systemic sclerosis secreted more interferon α (a type I interferon) than did controls (Panel C). This reaction was fully reversed by the addition of anti-CXCL4 antibody (Panel D). The culture medium was RPMI-1640 with 10% fetal-calf serum. All values are expressed as means; T bars represent standard deviations.
Plasmacytoid dendritic cells and their activation by toll-like receptors are thought to play a role in systemic sclerosis. On stimulation by ligands of toll-like receptors, plasmacytoid dendritic cells from patients with systemic sclerosis secreted more type I interferon than did those from controls, a difference that was fully reversed by the addition of anti–CXCL4 antibody or heparinase (Fig. 3C and 3D). In contrast, anti–CXCL12 antibody (SDF-1) did not have this effect (data not shown).
CXCL4 and Inflammatory Changes in Skin
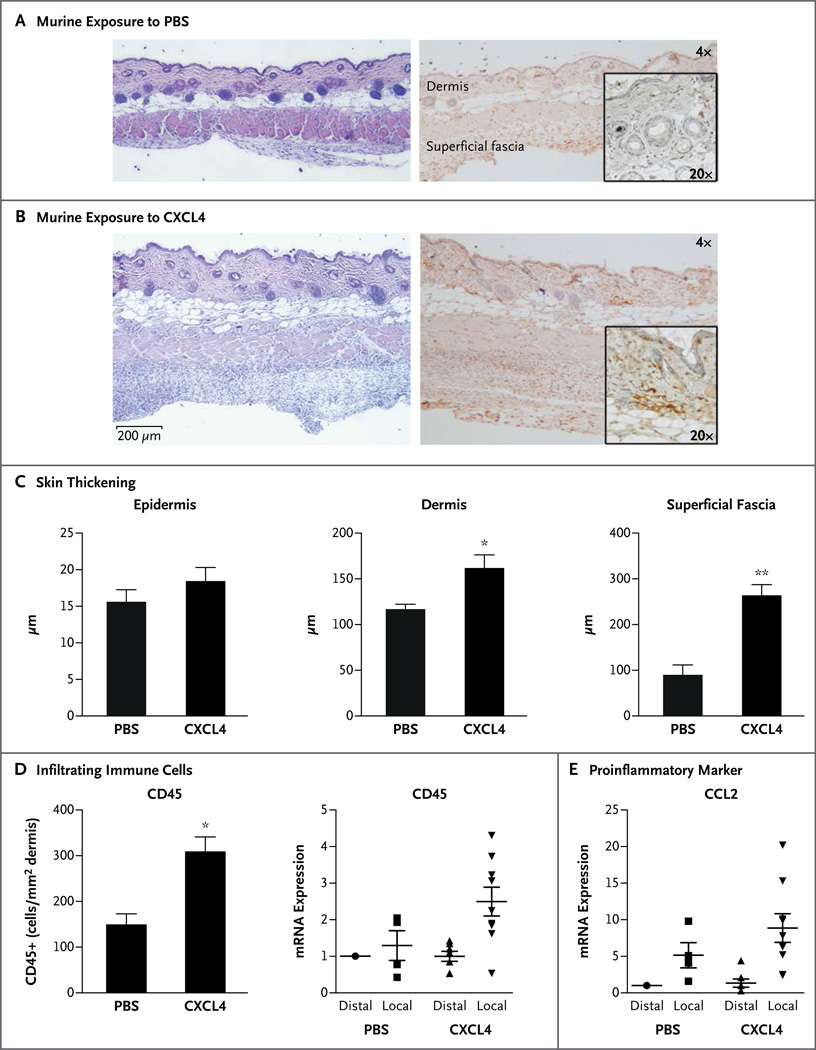
To examine the possible role of CXCL4 in vivo, we used a recently described murine subcutaneouspump model.25 The subcutaneous administration of CXCL4 for 7 days resulted in a marked infiltration of inflammatory cells in the dermis and subdermis, as compared with that seen in mice infused with phosphate-buffered saline as a control (Fig. 4A and 4B). In addition, CXCL4 administration resulted in a significant increase in the thickness of dermal and subdermal layers after 7 days (Fig. 4C). CXCL4-induced dermal thickening was also observed in a longer, 28-day model of daily subcutaneous injection (Fig. S3D in the Supplementary Appendix). The influx of inflammatory cells was confirmed by an increased number of dermal CD45+ cells in the CXCL4-treated skin, as well as increased expression of CD45 mRNA (Fig. 4D). Furthermore, we observed that CXCL4 induced the expression of the highly proinflammatory CCL2 in the two models (Fig. 4E, and Fig. S3F in the Supplementary Appendix). During an investigation of the gene-expression profile in mice that were treated with CXCL4 for 7 days, we found increased mRNA expression of tumor necrosis factor α, IFIT1, and MX2, the products of type I interferon-inducible genes; of the profibrotic marker PAI-1; and of the antiangiogenic factor thrombospondin 1 (Fig. S3A, S3B, and S3C in the Supplementary Appendix).
Figure 4. (facing page). Inflammatory Skin Changes Mimicking Those in Systemic Sclerosis Induced by CXCL4 In Vivo in Mice.
Panels A and B show the results of histologic analyses of skin from wild-type C57BL/6 mice that were treated with phosphate-buffered saline (PBS) as a control (Panel A) or with CXCL4 (Panel B) for 7 days with the use of a subcutaneous-pump model, shown at 4× and 20× magnification (hematoxylin and eosin, left; CD45 immunohistochemical staining, right). The murine sample that was treated with CXCL4 shows marked infiltration of inflammatory cells in the dermis and subdermis, as compared with the control sample. Panel C shows quantification of the thickening of skin layers after CXCL4 treatment for 7 days with the use of the pump model, as compared with the PBS controls. Shown are mean values for three analyses in each group, with T bars indicating standard errors. Panel D shows the quantification of infiltrating immune cells after 7-day exposure to CXCL4 or PBS, in which CD45+ cells in the dermis were counted after immunohistochemical staining; the means of three analyses per group are shown (at left). In addition, the influx of inflammatory cells is confirmed by increased CD45 messenger RNA (mRNA) expression in CXCL4-exposed skin isolated from the distal or proximal (local) area to the pump outlet, as measured on quantitative polymerasechain-reaction (PCR) assay (at right). In Panels C and D, a single asterisk denotes P<0.05 for the between-group comparison; double asterisks denote P<0.01. Panel E shows the mRNA expression of proinflammatory marker CCL2 induced after 7-day exposure to CXCL4, as compared with PBS, also measured on quantitative PCR assay. In Panels D and E, the mRNA analyses included 4 samples for PBS and 7 samples for CXCL4. The horizontal lines indicate means, and I bars standard errors.
Discussion
In this study, we found that CXCL4 levels in patients with systemic sclerosis not only correlate strongly with both skin and pulmonary disease but also appear to predict progression in systemic sclerosis phenotypes. Collectively, the identification of CXCL4 as a marker for fibrosis and pulmonary arterial hypertension may be helpful in early diagnosis and risk assessment, an important factor in patients who require aggressive treatment. CXCL4 was one of the most highly and differentially expressed genes in a genomewide association study involving patients with systemic sclerosis who had pulmonary arterial hypertension and idiopathic pulmonary arterial hypertension, as compared with healthy controls.26
CXCL4 is a 70-amino acid, lysine-rich, 7.8-kDa protein that was first identified as a product of megakaryocytes and comprises 2 to 3% of the protein content of activated platelets.27 CXCL4 is generally well accepted as one of the most potent antiangiogenic chemokines, influencing angiogenesis through an integrin-dependent mechanism.28 The high levels of CXCL4 that were found in patients with systemic sclerosis are of great interest, since plasma obtained from these patients also showed antiangiogenic activity, and the disease is characterized by rarefaction of vessels despite the presence of multiple proangiogenic factors.
In addition to its antiangiogenic activity, CXCL4 inhibits the expression of the antifibrotic cytokine interferon-γ (a type 1 helper T cell) and up-regulates profibrotic cytokines interleukin-4 and interleukin-13 (type 2 helper T cells).29 It also stimulates the proliferation of T regulatory cells with impaired suppressive function.30 Such data might indicate that the high levels of CXCL4 in patients with systemic sclerosis could be linked to features of immune dysfunction that have been observed in the disease.31 Accumulating evidence suggests a role for CXCL4 in other chronic fibroproliferative and inflammatory conditions. CXCL4 was shown to be an important mediator in atherosclerosis, both in vivo and in vitro,32 and increased CXCL4 levels have been associated with progressive liver fibrosis.33 Intriguingly, despite the fact that CXCL4 induced skin inflammation in vivo in mice, fibrosis was not observed. In this light, it is tempting to speculate that although CXCL4 may sensitize various cells to inflammatory stimuli, culminating in fibrosis, the presence of CXCL4 alone is not sufficient. This hypothesis is supported by our observation that increased levels of CXCL4 were found in patients with Raynaud’s phenomenon, most of whom do not have progression to systemic sclerosis.
Our data from cell culture and the murine model lead us to speculate that plasmacytoid dendritic cells, through the production of CXCL4, are pivotal in the onset and perpetuation of systemic sclerosis. In this respect, the downregulation of FLI1 is of particular interest, since selective endothelial-cell deletion of Fli-1 in mice leads to down-regulated expression of classic endothelial-cell markers, which are also seen to be down-regulated in the vasculature of patients with systemic sclerosis. The phenotype of Fli-1 knockout mice indicates a profound role for Fli-1 in vessel formation, maturation, and stabilization.34 In addition, CXCL4 induces the expression of thrombospondin 1 and attenuates the effects of vascular endothelial growth factor. These observations might explain the absence of neovascularization in systemic sclerosis, despite the presence of high levels of vascular endothelial growth factor. Thus, we speculate that CXCL4 may play a major role in vasculopathy associated with systemic sclerosis through altered FLI1 expression. The effect of FLI1 expression on fibroblasts may also be important, since FLI1 regulates elevated collagen synthesis, the hallmark of fibrosis.35
Several previous small studies have hinted at increased levels of CXCL4 and other inflammatory markers in the circulation of patients with systemic sclerosis.23,24,36–39 In those studies, the observed CXCL4 levels were attributed to platelet activation, a conclusion inconsistent with increased mRNA expression by peripheral-blood mononuclear cells, increased secretion of CXCL4 by plasmacytoid dendritic cells, and a lack of association between increased β-thromboglobulin levels and increased CXCL4 levels in patients with systemic sclerosis. Our data show that CXCL4 levels correlated highly with disease phenotype and disease progression in five large, independent, and clinically well-characterized patient cohorts, whereas levels of CCL2, CXCL10, CCL5, von Willebrand factor, and CCL18 did not show such correlation.
Taken together, our observations suggest that CXCL4 and plasmacytoid dendritic cells are central to the pathogenesis of systemic sclerosis. The levels of CXCL4 correlated well with the level of fibrosis and the occurrence and progression of pulmonary arterial hypertension, the two clinical hallmarks of this disorder. CXCL4 levels were also increased in several other fibrosing or inflammatory conditions, suggesting that CXCL4 may play a role in many pathologic conditions.
Supplementary Material
Acknowledgments
Supported by awards from the Dutch Arthritis Association (to Dr. Radstake), the Netherlands Organization for Scientific Research (to Drs. Radstake and Affandi), the Niels Stensen Foundation (to Dr. Radstake), the European Research Council (to Dr. Radstake), the European Community’s FP6 (to Drs. Hessel-strand and Saxne), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant no. IP50AR060780, to Dr. Lafyatis), and the German Research Foundation and the European Union (to Dr. Homey).
We thank Dr. J.W.J. Jacobs for his review of an earlier version of the manuscript and assistance with the statistical analysis.
Appendix
The authors’ full names and degrees are as follows: Lenny van Bon, M.D., Alsya J. Affandi, M.Sc., Jasper Broen, M.D., Ph.D., Romy B. Christmann, M.D., Ph.D., Renoud J. Marijnissen, Ph.D., Lukasz Stawski, M.Sc., Giuseppina A. Farina, M.D., Ph.D., Giuseppina Stifano, M.D., Allison L. Mathes, Ph.D., Marta Cossu, M.D., Michael York, M.D., Cindy Collins, M.A., Mark Wenink, M.D., Richard Huijbens, M.Sc., Roger Hesselstrand, M.D., Ph.D., Tore Saxne, M.D., Ph.D., Mike DiMarzio, M.Sc., Dirk Wuttge, M.D., Ph.D., Sandeep K. Agarwal, M.D., Ph.D., John D. Reveille, M.D., Ph.D., Shervin Assassi, M.D., Maureen Mayes, M.D., M.P.H., Yanhui Deng, Ph.D., Joost P.H. Drenth, M.D., Ph.D., Jacqueline de Graaf, M.D., Ph.D., Martin den Heijer, M.D., Ph.D., Cees G.M. Kallenberg, M.D., Ph.D., Marc Bijl, M.D., Ph.D., Arnoud Loof, M.Sc., Wim B. van den Berg, Ph.D., Leo A.B. Joosten, Ph.D., Vanessa Smith, M.D., Ph.D., Filip de Keyser, M.D., Ph.D., Rafaella Scorza, M.D., Ph.D., Claudio Lunardi, M.D., Ph.D., Piet L.C.M. van Riel, M.D., Ph.D., Madelon Vonk, M.D., Ph.D., Waander van Heerde, Ph.D., Stephan Meller, M.D., Bernhard Homey, M.D., Lorenzo Beretta, M.D., Ph.D., Mark Roest, Ph.D., Maria Trojanowska, Ph.D., Robert Lafyatis, M.D., and Timothy R.D.J. Radstake, M.D., Ph.D.
The authors’ affiliations are as follows: the Arthritis Center (L. van Bon, A.J.A., J.B., R.B.C., L.S., G.A.F., G.S., A.L.M., M.Y., C.C., M.D., M.T., R.L., T.R.D.J.R.) and the Flow Cytometry Core Facility (Y.D.), Boston University School of Medicine, Boston; the Department of Rheumatology and Clinical Rheumatology and Laboratory of Translational Immunology (L. van Bon, A.J.A., J.B., R.J.M., M.C., M.W., R. Huijbens, W.B.B., T.R.D.J.R.) and the Research Laboratory of the Department of Clinical Chemistry and Hematology (M.R.), University Medical Center Utrecht, Utrecht, the Departments of Gastroenterology and Hepatology (J.P.H.D.), Internal Medicine (J.G.), and Endocrinology (M.H.) and the Central Laboratory of Hematology (A.L., W.H.) and Internal Medicine (L.A.B.J.), Radboud University Nijmegen Medical Center, and the Department of Rheumatology, University Medical Center Nijmegen (P.L.C.M.R., M.V.), Nijmegen, and the Department of Rheumatology and Clinical Immunology, University Medical Center Groningen, Groningen (C.G.M.K., M.B.) — all in the Netherlands; the Referral Center for Systemic Autoimmune Diseases, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico di Milano, Milan (M.C., R.S., L. Beretta), and the University of Verona, Verona (C.L.) — both in Italy; the Department of Rheumatology, Lund University Hospital, Lund, Sweden (R. Hesselstrand, T.S., D.W.); the Division of Rheumatology, Department of Internal Medicine, University of Texas Health Science Center, Houston (S.K.A., J.D.R., S.A., M.M.); Ghent University Hospital, Ghent, Belgium (V.S., F.K.); and the Department of Dermatology, Heinrich-Heine-University, Duesseldorf, Germany (S.M., B.H.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 2.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 3.Leroy EC. Connective tissue synthesis by scleroderma skin fibroblasts in cell culture. J Exp Med. 1972;135:1351–1362. doi: 10.1084/jem.135.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lafyatis R, York M. Innate immunity and inflammation in systemic sclerosis. Curr Opin Rheumatol. 2009;21:617–622. doi: 10.1097/BOR.0b013e32832fd69e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu YS, Kong J, Cheema GS, Keen CL, Wick G, Gershwin ME. The immunobiology of systemic sclerosis. Semin Arthritis Rheum. 2008;38:132–160. doi: 10.1016/j.semarthrit.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Gourh P, Agarwal SK, Divecha D, et al. Polymorphisms in TBX21 and STAT4 increase the risk of systemic sclerosis: evidence of possible gene-gene interaction and alterations in Th1/Th2 cytokines. Arthritis Rheum. 2009;60:3794–3806. doi: 10.1002/art.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rueda B, Broen J, Simeon C, et al. The STAT4 gene influences the genetic predisposition to systemic sclerosis phenotype. Hum Mol Genet. 2009;18:2071–2077. doi: 10.1093/hmg/ddp119. [DOI] [PubMed] [Google Scholar]
- 8.Radstake TR, Gorlova O, Rueda B, et al. Genome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locus. Nat Genet. 2010;42:426–429. doi: 10.1038/ng.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieudé P, Guedj M, Wipff J, et al. Association between the IRF5 rs2004640 functional polymorphism and systemic sclerosis: a new perspective for pulmonary fibrosis. Arthritis Rheum. 2009;60:225–233. doi: 10.1002/art.24183. [DOI] [PubMed] [Google Scholar]
- 10.York MR, Nagai T, Mangini AJ, Lemaire R, van Seventer JM, Lafyatis R. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56:1010–1020. doi: 10.1002/art.22382. [Erratum, Arthritis Rheum 2007;56:1675.] [DOI] [PubMed] [Google Scholar]
- 11.Rönnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008;17:394–399. doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottenberg JE, Cagnard N, Lucchesi C, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. Proc Natl Acad Sci U S A. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [Erratum, Proc Natl Acad Sci U S A 2006;103:5242.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Pouw Kraan TC, Wijbrandts CA, van Baarsen LG, et al. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Ann Rheum Dis. 2007;66:1008–1014. doi: 10.1136/ard.2006.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eloranta ML, Franck-Larsson K, Lövgren T, et al. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann Rheum Dis. 2010;69:1396–1402. doi: 10.1136/ard.2009.121400. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, Peck A, Santer D, et al. Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum. 2008;58:2163–2173. doi: 10.1002/art.23486. [DOI] [PubMed] [Google Scholar]
- 16.Preliminary criteria for the classification of systemic sclerosis (scleroderma): subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 17.Holewijn S, den Heijer M, Swinkels DW, Stalenhoef AF, de Graaf J. The metabolic syndrome and its traits as risk factors for subclinical atherosclerosis. J Clin Endocrinol Metab. 2009;94:2893–2899. doi: 10.1210/jc.2009-0084. [DOI] [PubMed] [Google Scholar]
- 18.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 20.van der Heijde D, Bellamy N, Calin A, Dougados M, Khan MA, van der Linden S. Preliminary core sets for endpoints in ankylosing spondylitis. J Rheumatol. 1997;24:2225–2229. [PubMed] [Google Scholar]
- 21.Distler O, Rinkes B, Hohenleutner U, et al. Expression of RANTES in biopsies of skin and upper gastrointestinal tract from patients with systemic sclerosis. Rheumatol Int. 1999;19:39–46. doi: 10.1007/s002960050098. [DOI] [PubMed] [Google Scholar]
- 22.Kahaleh MB, Osborn I, LeRoy EC. Increased factor VIII/von Willebrand factor antigen and von Willebrand factor activity in scleroderma and in Raynaud’s phenomenon. Ann Intern Med. 1981;94:482–484. doi: 10.7326/0003-4819-94-4-482. [DOI] [PubMed] [Google Scholar]
- 23.Kodera M, Hasegawa M, Komura K, Yanaba K, Takehara K, Sato S. Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis: a sensitive indicator of active pulmonary fibrosis. Arthritis Rheum. 2005;52:2889–2896. doi: 10.1002/art.21257. [DOI] [PubMed] [Google Scholar]
- 24.Antonelli A, Ferri C, Fallahi P, et al. CXCL10 (alpha) and CCL2 (beta) chemokines in systemic sclerosis — a longitudinal study. Rheumatology (Oxford) 2008;47:45–49. doi: 10.1093/rheumatology/kem313. [DOI] [PubMed] [Google Scholar]
- 25.Farina G, York M, Collins C, Lafyatis R. dsRNA activation of endothelin-1 and markers of vascular activation in endothelial cells and fibroblasts. Ann Rheum Dis. 2011;70:544–550. doi: 10.1136/ard.2010.132464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajkumar R, Konishi K, Richards TJ, et al. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;298:H1235–H1248. doi: 10.1152/ajpheart.00254.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 28.Aidoudi S, Bujakowska K, Kieffer N, Bikfalvi A. The CXC-chemokine CXCL4 interacts with integrins implicated in angiogenesis. PLoS One. 2008;3(7):e2657. doi: 10.1371/journal.pone.0002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romagnani P, Maggi L, Mazzinghi B, et al. CXCR3-mediated opposite effects of CXCL10 and CXCL4 on TH1 or TH2 cytokine production. J Allergy Clin Immunol. 2005;116:1372–1379. doi: 10.1016/j.jaci.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 30.Liu CY, Battaglia M, Lee SH, Sun QH, Aster RH, Visentin GP. Platelet factor 4 differentially modulates CD4+CD25+ (regulatory) versus CD4+CD25- (nonregulatory) T cells. J Immunol. 2005;174:2680–2686. doi: 10.4049/jimmunol.174.5.2680. [DOI] [PubMed] [Google Scholar]
- 31.Radstake TR, van Bon L, Broen J, et al. Increased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFbeta expression. PLoS One. 2009;4(6):e5981. doi: 10.1371/journal.pone.0005981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitsilos S, Hunt J, Mohler ER, et al. Platelet factor 4 localization in carotid atherosclerotic plaques: correlation with clinical parameters. Thromb Haemost. 2003;90:1112–1120. doi: 10.1160/TH03-02-0069. [DOI] [PubMed] [Google Scholar]
- 33.Zaldivar MM, Pauels K, von Hundelshausen P, et al. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatology. 2009;51:1345–1353. doi: 10.1002/hep.23435. [DOI] [PubMed] [Google Scholar]
- 34.Asano Y, Stawski L, Hant F, et al. Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am J Pathol. 2010;176:1983–1998. doi: 10.2353/ajpath.2010.090593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubo M, Czuwara-Ladykowska J, Moussa O, et al. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am J Pathol. 2003;163:571–581. doi: 10.1016/S0002-9440(10)63685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farina G, Lafyatis D, Lemaire R, Lafyatis R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2010;62:580–588. doi: 10.1002/art.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kowal-Bielecka O, Kowal K, Lewszuk A, Bodzenta-Lukaszyk A, Walecki J, Siera-kowski S. Beta thromboglobulin and platelet factor 4 in bronchoalveolar lavage fluid of patients with systemic sclerosis. Ann Rheum Dis. 2005;64:484–486. doi: 10.1136/ard.2004.022970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheja A, Akesson A, Geborek P, et al. Von Willebrand factor propeptide as a marker of disease activity in systemic sclerosis (scleroderma) Arthritis Res. 2001;3:178–182. doi: 10.1186/ar295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasegawa M, Fujimoto M, Matsushita T, Hamaguchi Y, Takehara K, Sato S. Serum chemokine and cytokine levels as indicators of disease activity in patients with systemic sclerosis. Clin Rheumatol. 2011;30:231–237. doi: 10.1007/s10067-010-1610-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.