Abstract
Middle cerebral artery occlusion (MCAO) using the intraluminal suture technique is a common model used to study cerebral ischemia in rodents. Due to the proximity of the ophthalmic artery to the middle cerebral artery, MCAO blocks both arteries, causing both cerebral and retinal ischemia. While previous studies have shown retinal dysfunction at 48 hours post-MCAO, we investigated whether these retinal function deficits persist until 9 days and whether they correlate with central neurological deficits.
Rats received 90 minutes of transient MCAO followed by electroretinography at 2 and 9 days to assess retinal function. Retinal damage was assessed with cresyl violet staining, immunohistochemistry for glial fibrillary acidic protein (GFAP) and glutamine synthetase, and TUNEL staining.
Rats showed behavioral deficits as assessed with neuroscore that correlated with cerebral infarct size and retinal function at 2 days. Two days after surgery, rats with moderate MCAO (neuroscore < 5) exhibited delays in electroretinogram implicit time, while rats with severe MCAO (neuroscore ≥ 5) exhibited reductions in amplitude. Glutamine synthetase was upregulated in Müller cells 3 days after MCAO in both severe and moderate animals, however, retinal ganglion cell death was only observed in MCAO retinas from severe animals. By 9 days after MCAO, both glutamine synthetase labeling and electroretinograms had returned to normal levels in moderate animals.
Early retinal function deficits correlated with behavioral deficits. However, retinal function decreases were transient and selective retinal cell loss was observed only with severe ischemia, suggesting that the retina is less susceptible to MCAO than the brain. Temporary retinal deficits caused by MCAO are likely due to ischemia-induced increases in extracellular glutamate that impair signal conduction, but resolve by 9 days after MCAO.
Keywords: Middle cerebral artery occlusion, focal ischemia, rat, retina, electroretinogram, retinal ischemia
Introduction
Clinically, ocular ischemia can occur in conjunction with cerebral stroke or arthrosclerosis of the carotid or ophthalmic arteries. Indeed, visual impairments are often a first symptom in these pathologies (Benavente et al., 2001, Mead et al., 2002), and 57% of minor strokes are accompanied by subclinical visual field defects (Falke et al., 1991). Ocular ischemic syndrome, usually caused by severe obstruction of the carotid artery, involves vision loss due to chronically reduced arterial blood flow to the eye (Sturrock and Mueller, 1984). Ocular ischemia can also occur independently of cerebral damage, as is the case in retinal artery occlusion (Rumelt et al., 1999) and anterior ischemic optic neuropathy (Johnson and Arnold, 1994).
Middle cerebral artery occlusion (MCAO) in rodents is a common technique used to study mechanisms and potential treatments of cerebral ischemia. In this model, a filament is inserted into the internal carotid to block the middle cerebral artery (Longa et al., 1989). Due to the proximity of the ophthalmic artery to the middle cerebral artery in rats (Fig. 1), the filament blocks both arteries. The ophthalmic artery branches into the ciliary arteries, which provide blood supply to the outer retina via the choroid, and the central retinal artery, which provides blood supply to the inner retina. Since the ophthalmic artery ultimately provides the blood supply for the entire eye (Smith et al., 2002), it is not surprising that several studies have presented evidence of retinal deficits following MCAO in rodents (Block et al., 1997, Kaja et al., 2003, Cheung et al., 2007, Kalesnykas et al., 2008, Steele et al., 2008, Li et al., 2009). Since common behavioral assessments of MCAO in rodents (i.e., Morris water maze (Andersen et al., 1999); radial arm maze (Lee et al., 2003)) depend on successful interpretation of visual cues and can be affected by visual function deficits (Wong and Brown, 2007), it is imperative to understand how MCAO impacts visual function beyond one week post-ischemia when most behavioral testing is performed.
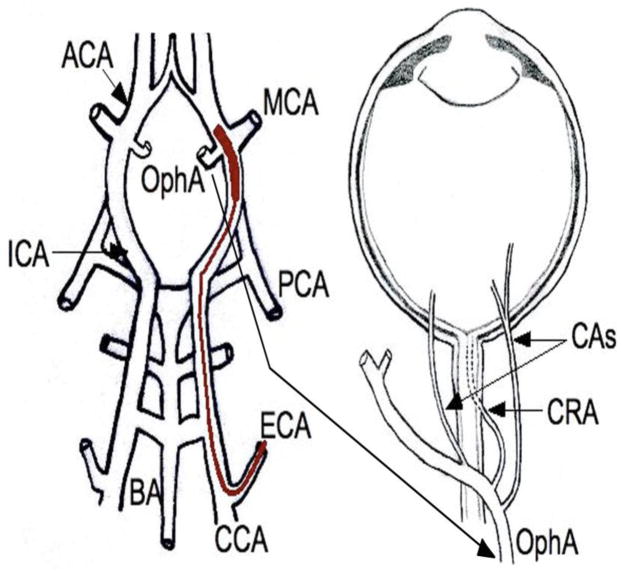
Figure 1.
Diagram of MCAO filament placement. The filament is fed through the external carotid artery (ECA) and into position along the internal carotid artery (ICA) to block the middle cerebral artery (MCA). Note the close proximity of the ophthalmic artery (OphA). CAs, Ciliary Arteries; CRA, Central Retinal Artery; ACA, Anterior Cerebral Artery; PCA, Posterior Cerebral Artery; BA, Basilar Artery; CCA, Common Carotid Artery. Modified from Steele et al., 2008.
Block et al. (1997) reported decreased retinal function as measured by electroretinogram (ERG) a- and b-wave amplitudes and delayed b-wave implicit times during MCAO in rats, with reduced b-wave amplitudes persisting to 48 hours after reperfusion. Additionally, glial fibrillary acidic protein (GFAP) expression (a marker for retinal glial cell reactivity) increased in Müller cells following MCAO in rats (Block et al., 1997, Kalesnykas et al., 2008). However, the eyes of these rats did not show cell loss or changes in retinal thickness with Nissl staining (Block et al., 1997, Kalesnykas et al., 2008), although a few apoptotic cells were found with TUNEL staining in the rat retinal ganglion cell layer (Kaja et al., 2003).
It is interesting that there is no cell death in the inner nuclear layer of the rat retina given that 1) the MCAO model causes a ~50% reduction in bipolar cell function as measured by ERG b-wave at 2 days post-MCAO, and 2) MCAO causes extensive cell death in the brain (Longa et al., 1989). The absence of retinal cell death suggests that functional deficits may recover. This idea is supported by the findings of Block et al. (1997) that photoreceptor dysfunction recovers at 2 days post-MCAO as shown by a-wave amplitude recovery (Block et al., 1997).
Thus, we hypothesized that the retina will exhibit reduced susceptibility to MCAO, with retinal function recovering across time. To test this hypothesis, we investigated the temporal response of the retina to injury and whether retinal function and structure correlate with the severity of brain injury as determined by behavioral deficits. These studies are important in determining whether visual deficits confound behavioral assessments in MCAO rats and in comparing the retinal versus cortical neuron response to MCAO injury.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (n = 31) from Charles River Laboratories were used in this study. Rats were approximately 60 days of age (290–330 grams) at the time of surgery. Littermates that did not receive surgery were used as controls. All animal procedures were approved by the Institutional Animal Care and Use Committee (Emory University protocol #251–2008) and performed in accordance with NIH guidelines and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Rats were housed under a 12:12 reverse light:dark cycle with water and food ad libitum and handled daily for at least 5 days prior to surgery. One rat died during surgery, one was excluded due to an incomplete reperfusion, and one control rat was excluded because of functional abnormalities.
MCAO surgery
MCAO surgery was performed with minor modifications to the previous description (Longa et al., 1989). Briefly, animals were initially anesthetized via inhalation of 5% isoflurane (in a N2/O2 70%/30% mixture) and remained sedated with inhalation of 2% isoflurane. A pulse oximeter (SurgiVet, model V3304; Waukesha, WI, USA) was used to analyze and sustain blood oxygen saturation (SpO2) at 90%. Body temperature was monitored via rectal probe and sustained between 36.5°C and 37.5°C with a heating lamp. A laser-Doppler flowmetry (LDF) probe (Moor Instruments, Wilmington, Delaware, USA), an established and reliable system for monitoring changes in cerebral blood flow due to induction of focal cerebral ischemia (Dirnagl et al., 1989), was used to monitor cerebral blood flow for 5 minutes prior to occlusion through 5 minutes after reperfusion. Because we wanted to investigate correlation between the brain and retina over a range of severities, we did not exclude animals with low % occlusions even though this is a practice commonly used to reduce variability (Sayeed et al., 2006).
A midline incision was made at the ventral surface of the neck, and a 6–0 silk suture was used to separate and ligate the right common carotid arteries. Next, a microvascular clip was used to temporarily occlude the internal carotid and pterygopalatine arteries while a 4–0 silicon-coated monofilament (0.35–0.40 mm long) (Doccol Co., Albuquerque, NM, USA) was inserted through the cut in the external carotid artery and into the internal carotid artery. This filament was pushed an estimated 20 mm distal to the carotid bifurcation to block the opening of the middle cerebral artery and the adjacent ophthalmic artery (Fig. 1) and remained in place for 90 minutes, followed by reperfusion. Upon removal of the filament, the wound was sutured, and each rat was transferred to a heating blanket to recover from anesthesia.
Neurological assessment
Neurological deficit scores (herein referred to as neuroscore) were determined as described (Xia et al., 2006). Briefly, scores ranging from 0 (no neurological deficit) to 8 (stroke-related death) were used to assess neurological deficits at 24 and 72 hours post-occlusion. Neuroscores are presented here as an average of both scores. Animals with a neuroscore of less than 5 were classified as “moderate” (n = 11), while animals with a neuroscore of 5 or more were classified as “severe” (n = 5). Moderate animals were euthanized at 3 (n = 6) and 9 (n = 5) days post-MCAO, while all severe animals were euthanized at 3 days due to IACUC-required endpoints. Animals with a score of 5 “circle or walk spontaneously only to the left” (Xia et al., 2006) and were more likely to be euthanized due to IACUC endpoint requirements.
For rats euthanized at 3 days, brain slices were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO, USA), which differentiates between metabolically active and stroke tissue. Brains were removed and placed in ice-cold saline. Using a rat brain matrix (Harvard Apparatus, Holliston, MA, USA) and beginning 1 mm posterior to the anterior pole, brains were sliced into 7 serial coronal sections (2 mm thick). Slices were stained with 2% TTC in saline for 15 min at 37°C in the dark and then fixed in 10% buffered formalin. Metabolically active tissue reduces TTC to form a red product, while stroke tissue remains white because its metabolic enzymes are compromised. Stained sections were scanned using a high-resolution scanner (Epson Perfection 2400 Photo).
Electroretinogram (ERG)
ERGs were used to measure the retinal response to light stimuli. Rats were dark-adapted overnight and then anesthetized (ketamine 80 mg/kg and xylazine 16 mg/kg). The corneal surface was anesthetized with proparacaine (1%), and the pupils dilated with tropicamide (1%) and phenylephrine hydrochloride (2.5%). Retinal responses were recorded by placing a DTL fiber in contact with the corneal surface of each eye through a layer of methylcellulose. Platinum needle reference and ground electrodes were placed in the cheek below the eye and in the tail, respectively. Using a commercial amplifier and acquisition system (UTAS-E3000, LKC, Gaithersburg, MD, USA), dark-adapted ERG responses were recorded to a series of Ganzfeld strobe flashes (with intensities increasing from 0.00039 to 137 cd s/m2). Interstimulus time increased from 10 to 60 s as light intensity increased, and 3 to 10 responses were averaged per flash stimulus. Amplitudes and implicit times were measured for a-waves (baseline to trough, representative of photoreceptor cell function (Penn and Hagins, 1969, Hood and Birch, 1990), b-waves (trough to peak, representative of bipolar cell function (Robson and Frishman, 1998), and oscillatory potentials (wavelets which likely represent amacrine and ganglion cell function), (Wachtmeister, 1998). Oscillatory potential amplitudes and implicit times are presented as the sum of all 6 oscillatory potentials for each flash stimulus. Reported amplitude averages are for the brightest flash stimulus.
Retinal morphology and immunohistochemistry
Rats were euthanized and their eyes removed. Eyes were fixed in 10% buffered formalin, processed through a series of graded ethanols, and embedded in paraffin. Sections (5 μm) were cut on a rotary microtome. Every 6th slide was stained with 0.1% cresyl violet. Images were captured and inspected for pyknotic nuclei, and counts of total cells (number of cells in the retinal ganglion cell and inner nuclear layers and number of rows of photoreceptor nuclei in the outer nuclear layer) were made in retinal sections containing optic nerve. Three sections were counted and averaged per eye, and 6 fields were counted per section (3 on either side of the optic nerve, spaced approximately 100 microns apart).
To label GFAP and glutamine synthetase (GS) in Müller cells, sections were blocked for 30 minutes in 0.1 M Tris-buffered saline (TBS; pH 7.4) containing 3% normal serum, then incubated in primary antibodies diluted in the blocking serum overnight at 4°C. Primary antibodies included rabbit anti-GFAP (1:500; Millipore; Billarica, MA, USA; AB5804) and mouse anti-GS (1:1000; Millipore; MAB302). For double labeling, primary and secondary antibodies from different species were used simultaneously. Sections were rinsed 3 times with 0.1 M TBS following primary antibody incubation, then incubated for 1 hour at room temperature with the corresponding fluorophore-conjugated secondary antibody solution (goat anti-rabbit; 1:500; Alexa Fluor 546; A11071 and goat anti-mouse; 1:500; Alexa Fluor 488; A11001; Invitrogen, Grand Island, NY, USA). Sections were then rinsed with 0.1 M TBS, mounted with a DAPI mounting medium, and coverslipped. One image per retina, superior to the optic nerve was acquired. GS images were analyzed using the histogram tool of commercial imaging software (Image Pro©, Media Cybernetics; Rockville, MD, USA) to quantify GS labeling intensity. Measurements were normalized to the brightness of the background for each micrograph.
Fluorescent terminal deoxynucleotidyl transferase-mediated 2′deoxyuridine 5′-triphosphate-biotin nick end labeling (TUNEL) staining was used to assess apoptosis in the retina. Briefly, retinal sections were incubated with TUNEL using a DeadEnd Fluorometric TUNEL kit (Promega; Madison, WI, USA) and counter-stained with a mounting medium containing propidium iodide. A fluorescent microscope (Olympus BX41, Olympus America Inc.) was used to acquire all images and staining intensity was quantified using Photoshop.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). ERG, GS expression, and cell count results were analyzed using a two-way repeated measures analysis of variance (ANOVA) followed by Tukey’s test for individual comparisons. All correlations were analyzed using Pearson’s correlation.
Results
Retinal function deficits in MCAO animals are transient and correlate with behavioral deficits
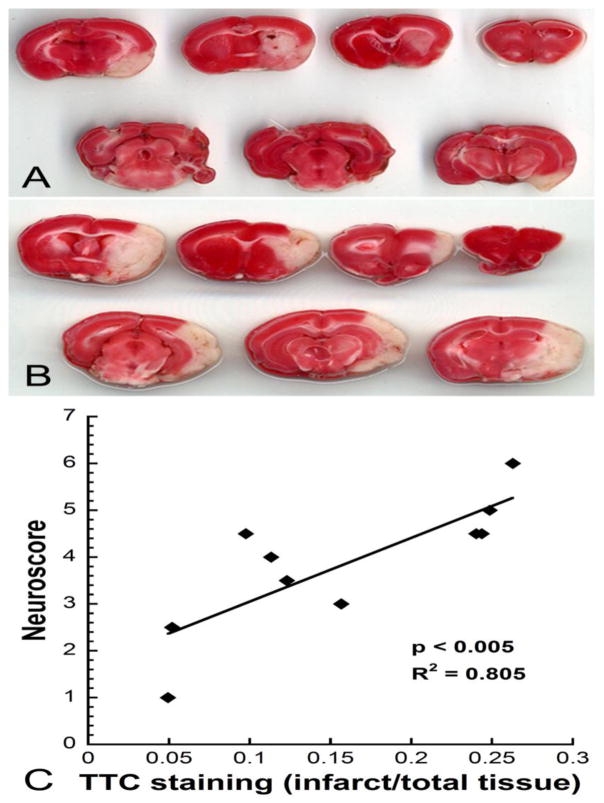
Neuroscores ranging from 1 to 7 (mean = 3.9, SEM = 0.4) were observed in MCAO rats. To demonstrate how the behavioral scores compare to neurological damage, Figure 2 shows representative brain slices stained with TTC from MCAO rats assigned a score of 3 (Fig. 2A) or 6 (Fig. 2B). Behavioral deficits measured with the neuroscore were positively correlated with infarct size (R2 = 0.805, p < 0.005) (Fig. 2C). Occlusions were designated as severe or moderate based on neuroscore, with animals with a neuroscore of less than 5 being classified as “moderate” and animals with a neuroscore of 5 or greater being classified as “severe”. Additionally, % occlusion as measured by LDF was, on average, 15% higher in the severe group versus the moderate group.
Figure 2.
Correlation of infarct size with behavioral responses. Representative sequential coronal slices of MCAO brains treated with TTC in an animal that received a neuroscore of 3 (A) or 6 (B). The infarct (white tissue) is larger in animals with higher neuroscores. C) Neuroscores showed a significant positive correlation with infarct size (R2 = 0.805, p < 0.005).
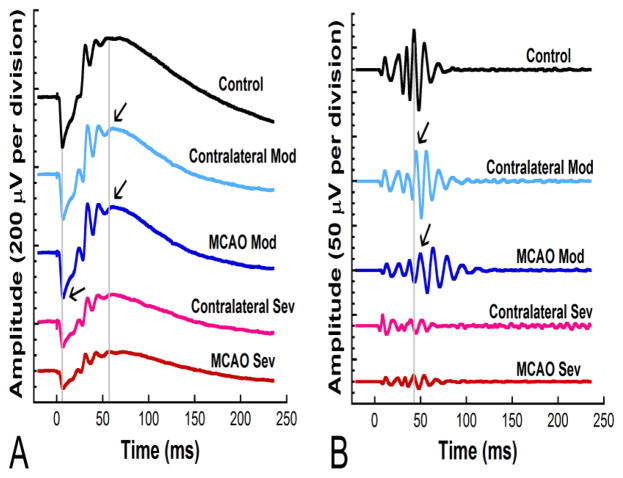
We observed different retinal function responses in moderate versus severe stroke animals. Figure 3 shows representative ERG and oscillatory potential waveforms from MCAO and control rats 2 days after surgery. Moderate animals showed delays in latency in MCAO eyes, while severe animals showed decreases in amplitude in both MCAO and contralateral eyes.
Figure 3.
Representative waveforms from moderate and severe MCAO eyes, contralateral eyes, and naïve controls at 2 days post-MCAO. For both ERG and oscillatory potential waveforms, severe animals showed decreases in amplitude in both MCAO and contralateral eyes, while moderate animals showed delays in latency in MCAO eyes. A) ERG waveforms in response to 137 cd s/m2 flash stimuli. The two gray lines mark the control a- wave and b- wave, respectively. Arrows indicate delayed responses. B) Oscillatory potentials (OPs) from filtered ERGs in response to 137 cd s/m2 flash stimuli. The gray line marks OP4 for the control trace. Arrows indicate delayed responses.
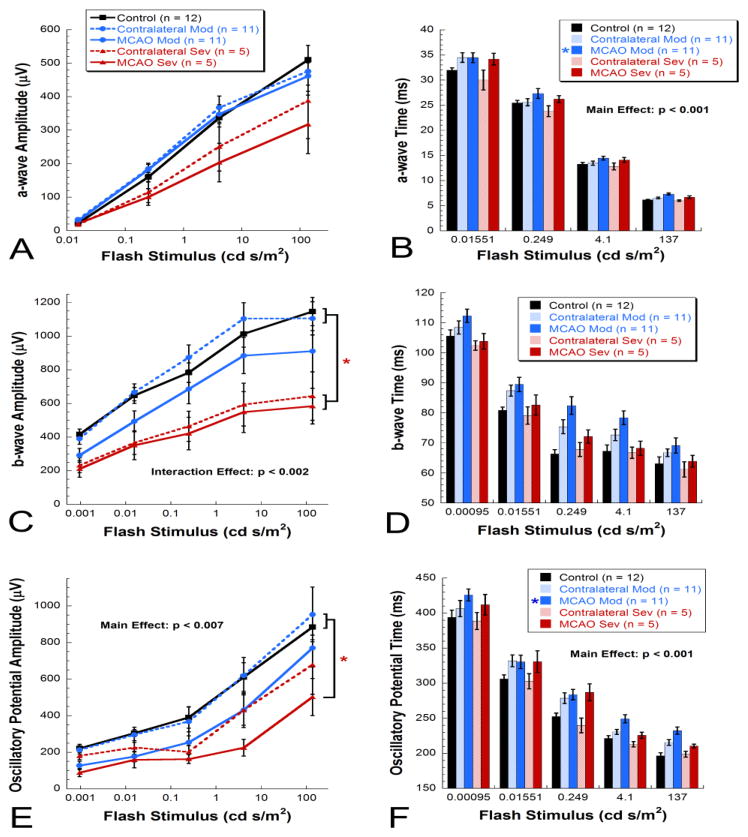
ERG quantification 2 days after MCAO surgery revealed that rats with severe MCAO showed a trend for reductions (38%) in dark-adapted a-wave amplitudes in MCAO eyes (317 ± 87 μV) and contralateral eyes (388 ± 113 μV), while both moderate MCAO (462 ± 46 μV) and contralateral eyes (476 ± 41 μV) showed similar levels to controls (510 ± 43 μV) [Repeated measures ANOVA, F(4, 114) = 2.131, p < 0.01] (Fig. 4A). Amplitude changes at 2 days after surgery were accompanied by significant delays in a-wave implicit time in MCAO eyes from the moderate group and a trend for delay in MCAO eyes from the severe group compared with all other groups [Repeated measures ANOVA main effect, F(4, 111) = 5.650, p < 0.001](Fig. 4B).
Figure 4.
Mean ERG results at 2 days post-MCAO. A) A trend for reduction in dark-adapted a-wave amplitudes was observed in severe MCAO and contralateral eyes [Repeated measures ANOVA, F(4, 114) = 2.131, p < 0.01]. B) Significant delays were observed in a-wave implicit time in moderate MCAO eyes with a trend for delay in severe MCAO eyes [Repeated measures ANOVA, F(4, 111) = 5.650, p < 0.001]. C) A significant reduction in dark-adapted b-wave amplitudes was observed for severe MCAO and contralateral eyes while a trend for reduction in amplitude was observed in moderate MCAO eyes [Repeated measures ANOVA, F(4, 156) = 5.117, p < 0.002]. D) For b-wave implicit times, a trend for delay was observed in contralateral and MCAO eyes from the moderate group. E) A significant reduction in dark-adapted oscillatory potential amplitudes was observed in severe MCAO eyes with a trend for a decrease in severe contralateral and moderate MCAO eyes [Repeated measures ANOVA, F(4, 156) = 4.166, p < 0.007]. F) For oscillatory potential implicit times, we observed a significant delay in moderate MCAO eyes versus control and severe contralateral eyes [Repeated measures ANOVA, F(4,153) = 7.422, p < 0.001].
Both MCAO (584 ± 106 μV) and contralateral eyes (644 ± 144 μV) from severe rats had significant reductions in dark-adapted b-wave amplitudes (49% and 43%, respectively) compared with control (1146 ± 84 μV) and moderate contralateral eyes (1106 ± 99 μV) [Repeated measures ANOVA interaction effect, F(4, 156) = 5.117, p < 0.002] (Fig. 4C). For moderate animals, a trend for a decrease (21%) in amplitude was observed in MCAO eyes (911 ± 123 μV) but not contralateral eyes (Fig. 4C). For b-wave implicit times, we observed a trend for delay in contralateral and MCAO eyes from the moderate group (Fig. 4D).
For dark-adapted summed oscillatory potentials, severe MCAO eyes (502 ± 101 μV) showed significant reductions (43%) in amplitude compared with control eyes (885 ± 70 μV) and moderate contralateral eyes (953 ± 104 μV) while severe contralateral and moderate MCAO eyes showed a trend for a reduction in amplitude [Repeated measures ANOVA main effect, F(4, 156) = 4.166, p < 0.007](Fig. 4E). For summed oscillatory potential implicit times, we observed a significant delay in moderate MCAO eyes versus control and severe contralateral eyes [Repeated measures ANOVA main effect, F(4,153) = 7.422, p < 0.001](Fig. 4F). Delays in light-adapted b-wave implicit times were observed in moderate MCAO eyes as well (data not shown).
By 9 days after surgery, we observed a complete recovery of function in the moderate group (data not shown). There were no significant differences in any ERG parameters at this time point.
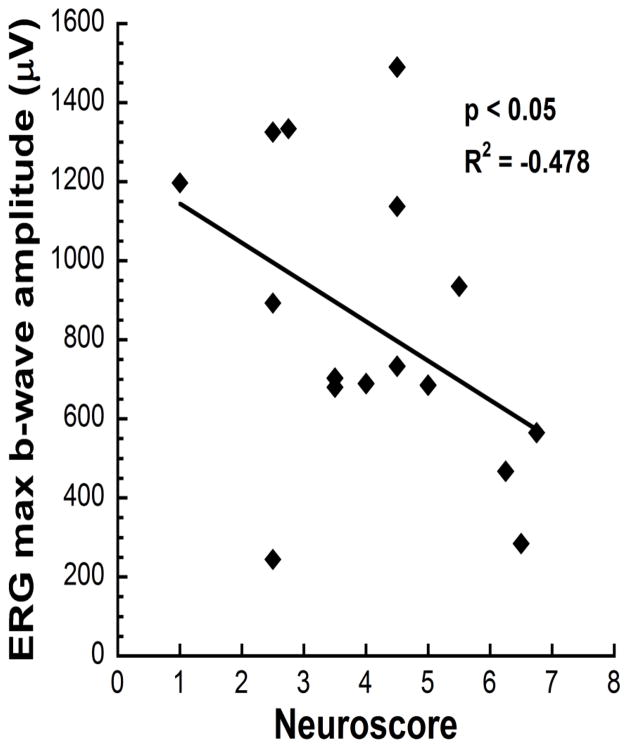
A significant correlation was observed between the neuroscore and retinal function measured with the ERG b-wave amplitude to the brightest flash intensity (137 cd s/m2; R2 = −0.478, p < 0.05)(Fig. 5), with more reduced b-wave amplitudes occurring in animals with higher (worse) neuroscores.
Figure 5.
ERG b-wave responses at the brightest flash stimulus (137 cd s/m2) showed a significant correlation with neurological deficit scores (R2 = −0.478, p < 0.05).
Selective apoptosis in retinal ganglion cells from severe MCAO animals
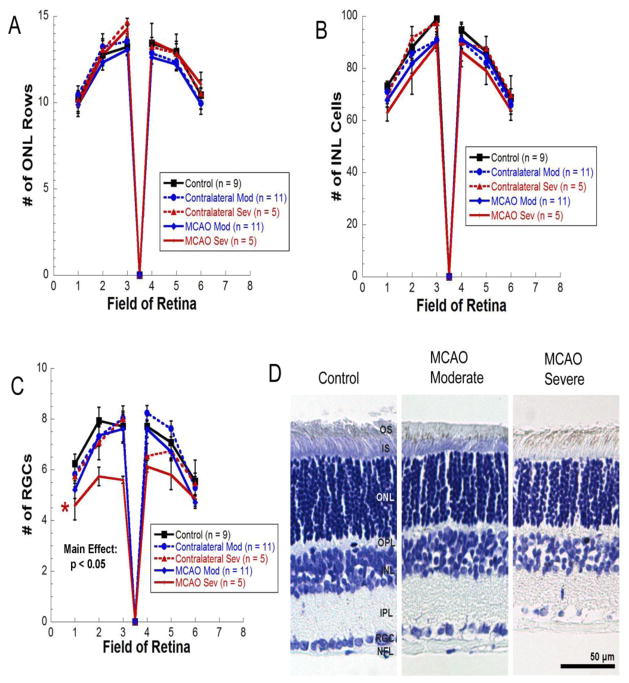
As shown in Figure 6, cresyl violet staining revealed no differences in morphology, no pyknotic cells, and no differences in cell numbers between groups in the outer nuclear layer (Fig. 6A) or the inner nuclear layer (Fig. 6B). However, there was a significant reduction in retinal ganglion cells (Fig. 6C) in severe MCAO eyes versus moderate contralateral and control eyes [Repeated measures ANOVA main effect, F(4, 180) = 2.940, p < 0.05]. Like control eyes, MCAO and contralateral eyes from both moderate and severe MCAO groups all contained a few scattered TUNEL positive cells with no significant differences between groups (data not shown).
Figure 6.
A–C) No significant differences were observed in number of rows of photoreceptor nuclei in the outer nuclear layer (A) or in cell counts in the inner nuclear layer (B). C) A significant reduction in retinal ganglion cells was observed in severe MCAO retinas versus moderate contralateral and controls [Repeated measures ANOVA, F(4, 180) = 2.940, p < 0.05]; ON, optic nerve. D) Representative micrographs of cresyl violet staining in a control retina, a moderate MCAO retina, and a severe MCAO.
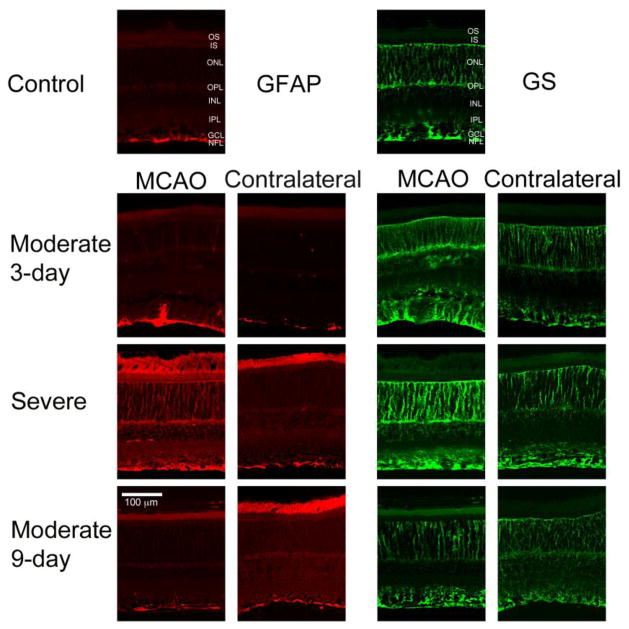
Upregulation of GFAP and GS in MCAO retinas
GFAP and GS were upregulated in the retina following MCAO (Fig. 7). GFAP labeling was observed in Müller cells in severe and some moderate MCAO retinas 3 days after surgery. In 9-day MCAO and contralateral eyes and intact controls, GFAP staining was observed only in the nerve fiber layer, where GFAP-expressing astrocytes typically present (Fig. 7).
Figure 7.
Representative micrographs from sections immunostained for GS and GFAP. GFAP and GS were upregulated in the retina following MCAO. GFAP labeling was observed in Müller cells in severe and some moderate MCAO retinas at 3 days. In all other groups, GFAP staining was observed only in the nerve fiber layer (NFL), where GFAP-expressing astrocytes typically present. Increased GS labeling was observed in severe MCAO and severe contralateral eyes and in moderate MCAO eyes at 3 days. Moderate contralateral at 3 days and moderate MCAO and contralateral at 9 days showed levels similar to controls. GCL, Ganglion Cell Layer; IPL, Inner Plexiform Layer; INL, Inner Nuclear Layer; OPL, Outer Plexiform Layer; ONL, Outer Nuclear Layer; IS, Inner Segments; OS, Outer Segments.
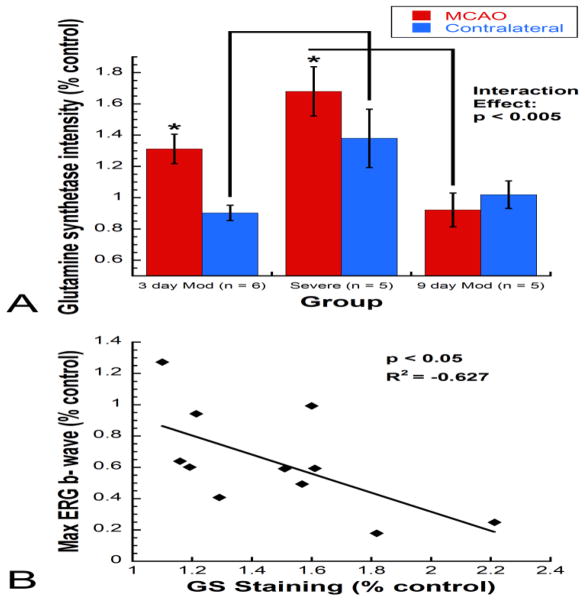
A significant increase in GS intensity was observed between MCAO eyes and contralateral eyes in the moderate 3-day (n = 6) and severe groups (n = 5) but not the moderate 9-day group (n = 5) [Repeated measures ANOVA interaction effect, F(2, 13) = 8.006, p < 0.005] (Fig. 8A). GS intensity was significantly increased in severe MCAO eyes compared with 9-day moderate MCAO eyes and in severe contralateral eyes compared with 3-day moderate contralateral eyes. Additionally, there was a significant inverse correlation between GS staining intensity and ERG max b- wave (R2 = −0.627, p < 0.05) (Fig. 8B).
Figure 8.
Mean GS immunolabeling intensity as percent of naïve control for severe and moderate MCAO eyes versus contralateral eyes. A) A significant increase in GS intensity was observed between MCAO eyes and contralateral eyes within the severe group and moderate 3-day group but not the moderate 9-day group. Comparisons between severity and time points revealed that GS intensity was significantly increased in severe MCAO eyes compared with 9-day moderate MCAO eyes and in severe contralateral eyes compared with 3-day moderate contralateral eyes [Repeated measures ANOVA interaction effect, F(2, 13) = 8.006, p < 0.005]. B) A significant inverse correlation was observed between GS staining intensity and ERG b- wave at the bright flash stimulus (137 cd s/m2; R2 = −0.627, p < 0.05).
Discussion
To determine whether neurons in the retina and brain respond similarly to MCAO, and whether MCAO resulted in sustained visual deficits, we investigated retinal function and structure at different times after MCAO with varying severity of stroke and the correlation with brain injury and behavioral deficits.
Retinal dysfunction without cell death with moderate MCAO
We found retinal deficits are dependent upon the severity of the occlusion at 2 days – such that moderate injuries resulted in ERG delays, while severe occlusions caused ERG amplitude reductions. Our observations extended the findings of others (Block et al., 1997, Kaja et al., 2003, Cheung et al., 2007, Kalesnykas et al., 2008, Steele et al., 2008, Li et al., 2009) who found that MCAO surgery resulted in retinal dysfunction, such as decreased ERG amplitude (Block et al., 1997). The Block et al. (1997) study showed recovery of photoreceptor cell function as measured by the a-wave by 2 days after MCAO, while we observed the additional recovery of the b-wave (bipolar cell function) and oscillatory potentials (amacrine and ganglion cell function) by 9 days (i.e., a full ERG recovery) in moderate animals.
While large amounts of pyknotic cells, cell loss, and TUNEL-positive cells have been observed in both the retinal ganglion cell and inner nuclear layers in the mouse retina at 1 day following MCAO (Cheung et al., 2007, Steele et al., 2008, Li et al., 2009), rat retinas consistently showed no cell death, no major morphological changes with Nissl staining (Block et al., 1997, Kalesnykas et al., 2008), and very few, if any, TUNEL-positive cells in the inner nuclear and photoreceptor layers (Kaja et al., 2003, Kalesnykas et al., 2008), even in studies showing retinal function deficits (Block et al., 1997). Similarly, in our study the functional and morphological differences following MCAO were not accompanied by retinal cell death in the inner and outer nuclear layers, which are responsible for generating the b- wave and a- wave, respectively. We did, however, observe cell loss in the retinal ganglion cell layer in severe MCAO retinas. In addition to differences in retinal cell loss, mice differ from rats in cerebral response after MCAO, requiring greatly reduced occlusion times (30 minutes versus 90–120 minutes in rats) to induce a comparable cerebral infarct (Carmichael, 2005). These results suggest important species differences in response to ischemia in both the retina and the brain, with mice showing more susceptibility to ischemia in both tissues. Such differences could inform interpretations of studies designed to evaluate the outcome of neuroprotective agents given in the acute stage of an ischemic brain injury.
Transient retinal function deficits may be caused by sub-lethal increases in extracellular glutamate
By investigating retinal function in MCAO rats across time, we were able to show that retinal function deficits following MCAO are transient and recover by 9 days in animals with moderate stroke injury. Since cell death was not detected in the photoreceptor or inner nuclear layers, it appears that these neurons may undergo a transient dysfunctional state. We were unable to examine retinal morphology of the severe group at 9 days, but given that no cell death was detected at 3 days in the photoreceptor or inner nuclear layers, which contain neurons that generate the ERG, it is likely that the severe group would have experienced an eventual recovery of function as well.
Although retinal cell death did not occur in the inner and outer nuclear layer following MCAO, we observed increases in GFAP at 3 days post-MCAO, which is a general sign of retinal stress and pathology (Bringmann et al., 2009). Increases in GFAP have been observed previously both in rat retinas at 2 and 5 days post-MCAO and in mouse retinas at 1 day post-MCAO (Block et al., 1997, Cheung et al., 2007, Kalesnykas et al., 2008). Additionally, we observed increases in GS, a more specific response that occurs following retinal injuries that involve changes in levels of extracellular glutamate (Bringmann et al., 2009). Glutamate is the neurotransmitter used by photoreceptors, bipolar cells, and retinal ganglion cells (Massey and Redburn, 1987), and increased levels of extracellular glutamate could affect the function and/or signal conduction of these cells (Kalloniatis, 1995). When retinal neurons fire, they release glutamate into the synapse. This extracellular glutamate is taken up by nearby Müller cells via the Müller-specific glutamate transporter, GLAST (GLutamate ASpartate Transporter). Then GS, a substrate-regulated enzyme present in Müller cells, converts glutamate to glutamine (Pow and Barnett, 1999).
Ischemia has been shown to induce increases in extracellular glutamate in both the brain (Hillered et al., 1989) and the retina (Perlman et al., 1996) that if uncontrolled can result in excitotoxicity (Beal, 1992, Kalloniatis, 1995). MCAO may cause increased levels of extracellular glutamate in the retina, and the Müller cells respond by upregulating GS, as shown in other retinal injury models that involve increased extracellular glutamate (Bringmann et al., 2009). We hypothesize that even with these increased levels of GS, enough glutamate remains in the extracellular space to impair signal conduction, resulting in decreased retinal function, but not cell death.
The effects of sub-lethal levels of glutamate on ERG function have been documented previously (Barnett and Pow, 2000). When GLAST is inhibited, extracellular glutamate is increased due to the lack of Müller cell uptake. The result is decreased ERG responses yet no cell death. We postulate that a similar increase in glutamate occurs at 2 days post-MCAO as evidenced by the increased GS. By 9 days post-MCAO, GS levels returned to normal, suggesting that GS may clear all of the excess glutamate and allow for recovery of retinal function.
Similarly, increases in GS have been observed from 3 hours to 3 days after cerebral ischemia (Petito et al., 1992, Tanaka et al., 1992, Hoshi et al., 2006, Verma et al., 2010), and these increases appear to be transient, returning to baseline levels by 5 days (Tanaka et al., 1992). Additionally, treatments that enhance the activity of glutamate transporter (Verma et al., 2010) and GS (Zhang et al., 2011) have been shown to reduce brain injury in ischemia-reperfusion models, providing further support for a protective role of GS in reducing levels of extracellular glutamate after ischemic injury.
We also observed increased GS and decreased retinal function in contralateral eyes as well as MCAO eyes. This result could be an example of diaschisis, that is, the undamaged neural tissue is losing function due to damage to distant, but connected, neural tissue (Stein et al., 1983, Feeney and Baron, 1986). Additionally, the decreased function and increased retinal glutamate in contralateral eyes could be due to indirect effects of the cerebral infarct, such as increased systemic levels of glutamate, which have been shown to be 3-fold higher after permanent MCAO, a model that causes a less severe stroke than our transient MCAO model (Puig et al., 2000). Furthermore, in the permanent occlusion model, which involves permanent occlusion at a point distal to the internal carotid such that the middle cerebral artery alone is blocked and the ophthalmic artery is unaffected, changes in the retina (i.e., a slight upregulation of HIF-1a) were still detected (Kalesnykas et al., 2008). The fact that retinal changes were observed independent of ophthalmic artery occlusion, coupled with the fact that we observed retinal function deficits in both the occluded and contralateral eye in MCAO rats, suggests that the retinal injury caused by MCAO is at least partially systemic in nature.
Our finding of increased GS and decreased retinal function in the contralateral eye has interesting clinical implications, because while it is unlikely that a clot would block both the middle cerebral and ophthalmic arteries in humans, it is possible that increased systemic levels of glutamate are the cause of the transient vision loss that often accompanies stroke in people (Mead et al., 2002).
The retina is less susceptible to MCAO although early retinal function deficits correlate with deficits in brain function
Utilizing a range of stroke severity allowed us to investigate the correlation between retinal function and brain function. We observed a significant correlation between retinal function responses at 2 days and neuroscores (Fig. 2), which clearly established similar ischemic effects after MCAO at this early time point. However, while retinal function recovers by 9 days, we know that neurological deficits after MCAO are more lasting (Modo et al., 2000). Further, we showed that cell death occurred in brains from both moderate and severe animals but only in the retinal ganglion cell layer in retinas from severe animals. Therefore, it appears that the retina (particularly the inner and outer nuclear layers) shows less susceptibility than the brain to the ischemic injury caused by MCAO. It is also possible that the brain and the retina receive different levels of occlusion. One limitation of this study is that we only took laser-Doppler readings from the MCA but not the ophthalmic artery. However, Steele et al. (2008) showed that almost no retinal perfusion occurs during MCAO in the mouse. Future work should compare perfusion to the brain and retina during MCAO in rats.
The retina has been shown to have more or less susceptibility to injury than the brain depending on the type of insult. The endothelial cells of the retina have been shown to be more susceptible than brain-derived endothelial cells to oxidative stress and increased vascular permeability, as measured by glutathione peroxidase activity, superoxide production and superoxide dismutase levels, and junctional protein levels in bovine endothelial cell cultures (Grammas and Riden, 2003). The retina is also more sensitive than either the brain or the liver to dietary changes in lipid levels, with the retina but not the brain showing an incorporation of trans DHA (Docosahexaenoic acid) and a reduction in cis DHA as well as ERG defects following a diet high in trans fatty acids (Acar et al., 2006). In the event of ischemia, however, the retina is less susceptible than the brain, showing a much greater tolerance time to ischemic injury. In the brain, permanent damage is caused by 3–7 minutes of global ischemia in both animal models and humans (Weinberger L, 1940, Kabat H, 1941, Brock, 1956, Meyer, 1956), while the retina exhibits tolerance times that vary from less than 30 minutes to as long as 98 minutes (Reinecke et al., 1962, Fujino and Hamasaki, 1967, Hayreh and Weingeist, 1980, Faberowski et al., 1989, Lafuente et al., 2001, Zhu et al., 2002). This resilience could be because the retina and the vitreous have stores of glucose and glycogen while the brain relies on the cerebral vasculature, or because reflow through occluded vasculature happens more readily in the retina than the brain (Hayreh and Weingeist, 1980).
We observed a difference in susceptibility among the cell types within the retina as well. Retinal cell types have been shown to have differential susceptibilities previously, with retinal ganglion cells showing more cell death with aging than either photoreceptors or cells of the inner nuclear layer (Lei et al., 2011). It is interesting that retinal ganglion cells, like the cells of the brain, show death in the MCAO model, given that the blood supply and blood retinal barrier for retinal ganglion cells is more similar to that of the brain, while the photoreceptors rely on the choroid for blood supply and the retinal pigmented epithelium for blood retina barrier (Cunha-Vaz, 1976).
Conclusions
The transient MCAO model provides a unique opportunity to investigate cerebral and retinal ischemia simultaneously due to the functional deficits that occur in both brain and retina. Moreover, it creates a useful model for the study of mechanisms and treatments of ocular ischemic syndrome in rats. Retinal function deficits occur at 2 days post-MCAO and correlate with behavioral deficits. Decreased b-wave amplitudes occurred in both MCAO and contralateral eyes in severe animals, suggesting the injury is at least partially systemic. With the addition of the 9-day post-injury evaluations, we were able to show that the retinal function deficit following MCAO is transient and recovers by 9 days with moderate occlusion. This finding suggests that behavioral testing at this time is not confounded by visual deficits.
We have also presented data suggesting that ischemia-induced increases in extracellular glutamate may be the mechanism behind the temporary retinal function reduction after MCAO in rats and this represents a potential mechanism behind temporary retinal function deficits that occur following stroke in humans. Recovery of function may be explained by the transient increase in GS, which may act to overturn sub-lethal levels of extracellular glutamate.
Highlights.
Early retinal function deficits correlate with behavioral deficits.
Severe MCAO reduces retinal function and causes retinal ganglion cell death.
Moderate MCAO causes delays in retinal function that fully recover within 9 days.
Sub-lethal increases in extracellular glutamate may cause transient retinal deficits.
Contralateral eyes also show glutamine synthetase increases and retinal dysfunction.
Acknowledgments
H. Allen and Company, Atlanta VA Rehab R&D Center of Excellence, The Abraham J. and Phyllis Katz Foundation, Foundation Fighting Blindness, Research to Prevent Blindness, NIH National Eye Institute R01EY014026, R01EY016470, R24EY017045, P30EY006360, and T32EY007092-24.
We thank Paul Choi for his help with sectioning and ERGs and Leslie McCann for her help with editing this manuscript.
Abbreviations
- MCAO
middle cerebral artery occlusion
- GFAP
glial fibrillary acidic protein
- LDF
laser-Doppler flowmetry
- ERG
electroretinogram
- GS
glutamine synthetase
- GLAST
GLutamate ASpartate Transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rachael S. Allen, Email: restewa@emory.edu.
Iqbal Sayeed, Email: isayeed@emory.edu.
Heather A. Cale, Email: hcale@u.washington.edu.
Katherine C. Morrison, Email: cillakat@gmail.com.
Jeffrey H. Boatright, Email: jboatri@emory.edu.
Machelle T. Pardue, Email: mpardue@emory.edu.
References
- Acar N, Bonhomme B, Joffre C, Bron AM, Creuzot-Garcher C, Bretillon L, Doly M, Chardigny JM. The retina is more susceptible than the brain and the liver to the incorporation of trans isomers of DHA in rats consuming trans isomers of alpha-linolenic acid. Reproduction, nutrition, development. 2006;46:515–525. doi: 10.1051/rnd:2006033. [DOI] [PubMed] [Google Scholar]
- Andersen MB, Zimmer J, Sams-Dodd F. Specific behavioral effects related to age and cerebral ischemia in rats. Pharmacology, biochemistry, and behavior. 1999;62:673–682. doi: 10.1016/s0091-3057(98)00204-4. [DOI] [PubMed] [Google Scholar]
- Barnett NL, Pow DV. Antisense knockdown of GLAST, a glial glutamate transporter, compromises retinal function. Investigative ophthalmology & visual science. 2000;41:585–591. [PubMed] [Google Scholar]
- Beal MF. Mechanisms of excitotoxicity in neurologic diseases. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1992;6:3338–3344. [PubMed] [Google Scholar]
- Benavente O, Eliasziw M, Streifler JY, Fox AJ, Barnett HJ, Meldrum H. Prognosis after transient monocular blindness associated with carotid-artery stenosis. The New England journal of medicine. 2001;345:1084–1090. doi: 10.1056/NEJMoa002994. [DOI] [PubMed] [Google Scholar]
- Block F, Grommes C, Kosinski C, Schmidt W, Schwarz M. Retinal ischemia induced by the intraluminal suture method in rats. Neuroscience letters. 1997;232:45–48. doi: 10.1016/s0304-3940(97)00575-2. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Progress in retinal and eye research. 2009;28:423–451. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Brock R. Hypothermia and open cardiotomy. Proceedings of the Royal Society of Medicine. 1956;49:347–352. [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx: the journal of the American Society for Experimental Neuro Therapeutics. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AK, Lo AC, So KF, Chung SS, Chung SK. Gene deletion and pharmacological inhibition of aldose reductase protect against retinal ischemic injury. Experimental eye research. 2007;85:608–616. doi: 10.1016/j.exer.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG. The blood-retinal barriers. Documenta ophthalmologica Advances in ophthalmology. 1976;41:287–327. doi: 10.1007/BF00146764. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Kaplan B, Jacewicz M, Pulsinelli W. Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1989;9:589–596. doi: 10.1038/jcbfm.1989.84. [DOI] [PubMed] [Google Scholar]
- Faberowski N, Stefansson E, Davidson RC. Local hypothermia protects the retina from ischemia. A quantitative study in the rat. Investigative ophthalmology & visual science. 1989;30:2309–2313. [PubMed] [Google Scholar]
- Falke P, Abela BM, Jr, Krakau CE, Lilja B, Lindgarde F, Maly P, Stavenow L. High frequency of asymptomatic visual field defects in subjects with transient ischaemic attacks or minor strokes. Journal of internal medicine. 1991;229:521–525. doi: 10.1111/j.1365-2796.1991.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Baron JC. Diaschisis. Stroke; a journal of cerebral circulation. 1986;17:817–830. doi: 10.1161/01.str.17.5.817. [DOI] [PubMed] [Google Scholar]
- Fujino T, Hamasaki DI. Effect of intraocular pressure on the electroretinogram. Archives of ophthalmology. 1967;78:757–765. doi: 10.1001/archopht.1967.00980030759013. [DOI] [PubMed] [Google Scholar]
- Grammas P, Riden M. Retinal endothelial cells are more susceptible to oxidative stress and increased permeability than brain-derived endothelial cells. Microvascular research. 2003;65:18–23. doi: 10.1016/s0026-2862(02)00016-x. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina. IV: Retinal tolerance time to acute ischaemia. The British journal of ophthalmology. 1980;64:818–825. doi: 10.1136/bjo.64.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillered L, Hallstrom A, Segersvard S, Persson L, Ungerstedt U. Dynamics of extracellular metabolites in the striatum after middle cerebral artery occlusion in the rat monitored by intracerebral microdialysis. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1989;9:607–616. doi: 10.1038/jcbfm.1989.87. [DOI] [PubMed] [Google Scholar]
- Hood DC, Birch DG. A quantitative measure of the electrical activity of human rod photoreceptors using electroretinography. Visual neuroscience. 1990;5:379–387. doi: 10.1017/s0952523800000468. [DOI] [PubMed] [Google Scholar]
- Hoshi A, Nakahara T, Kayama H, Yamamoto T. Ischemic tolerance in chemical preconditioning: possible role of astrocytic glutamine synthetase buffering glutamate-mediated neurotoxicity. Journal of neuroscience research. 2006;84:130–141. doi: 10.1002/jnr.20869. [DOI] [PubMed] [Google Scholar]
- Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy. Population-based study in the state of Missouri and Los Angeles County, California. Journal of neuro-ophthalmology: the official journal of the North American Neuro-Ophthalmology Society. 1994;14:38–44. [PubMed] [Google Scholar]
- Kabat HDC, Baher AB. Recovery of function following arrest of the brain circulation. Am J Physiol. 1941;112:737–747. [Google Scholar]
- Kaja S, Yang SH, Wei J, Fujitani K, Liu R, Brun-Zinkernagel AM, Simpkins JW, Inokuchi K, Koulen P. Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections. Investigative ophthalmology & visual science. 2003;44:3155–3162. doi: 10.1167/iovs.02-1204. [DOI] [PubMed] [Google Scholar]
- Kalesnykas G, Tuulos T, Uusitalo H, Jolkkonen J. Neurodegeneration and cellular stress in the retina and optic nerve in rat cerebral ischemia and hypoperfusion models. Neuroscience. 2008;155:937–947. doi: 10.1016/j.neuroscience.2008.06.038. [DOI] [PubMed] [Google Scholar]
- Kalloniatis M. Amino acids in neurotransmission and disease. Journal of the American Optometric Association. 1995;66:750–757. [PubMed] [Google Scholar]
- Lafuente MP, Villegas-Perez MP, Sobrado-Calvo P, Garcia-Aviles A, Miralles de Imperial J, Vidal-Sanz M. Neuroprotective effects of alpha(2)-selective adrenergic agonists against ischemia-induced retinal ganglion cell death. Investigative ophthalmology & visual science. 2001;42:2074–2084. [PubMed] [Google Scholar]
- Lee B, Choi Y, Kim H, Kim SY, Hahm DH, Lee HJ, Shim I. Protective effects of methanol extract of Acori graminei rhizoma and Uncariae Ramulus et Uncus on ischemia-induced neuronal death and cognitive impairments in the rat. Life sciences. 2003;74:435–450. doi: 10.1016/j.lfs.2003.06.034. [DOI] [PubMed] [Google Scholar]
- Lei Y, Garrahan N, Hermann B, Fautsch MP, Johnson DH, Hernandez MR, Boulton M, Morgan JE. Transretinal degeneration in ageing human retina: a multiphoton microscopy analysis. The British journal of ophthalmology. 2011;95:727–730. doi: 10.1136/bjo.2010.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Fu ZJ, Ma H, Jang WC, So KF, Wong D, Lo AC. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Investigative ophthalmology & visual science. 2009;50:836–843. doi: 10.1167/iovs.08-2310. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke; a journal of cerebral circulation. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Massey SC, Redburn DA. Transmitter circuits in the vertebrate retina. Progress in neurobiology. 1987;28:55–96. doi: 10.1016/0301-0082(87)90005-0. [DOI] [PubMed] [Google Scholar]
- Mead GE, Lewis SC, Wardlaw JM, Dennis MS. Comparison of risk factors in patients with transient and prolonged eye and brain ischemic syndromes. Stroke; a journal of cerebral circulation. 2002;33:2383–2390. doi: 10.1161/01.str.0000029827.93497.97. [DOI] [PubMed] [Google Scholar]
- Meyer A. Neuropathological aspects of anoxia. Proceedings of the Royal Society of Medicine. 1956;49:619–622. [PubMed] [Google Scholar]
- Modo M, Stroemer RP, Tang E, Veizovic T, Sowniski P, Hodges H. Neurological sequelae and long-term behavioural assessment of rats with transient middle cerebral artery occlusion. Journal of neuroscience methods. 2000;104:99–109. doi: 10.1016/s0165-0270(00)00329-0. [DOI] [PubMed] [Google Scholar]
- Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969;223:201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Perlman JI, McCole SM, Pulluru P, Chang CJ, Lam TT, Tso MO. Disturbances in the distribution of neurotransmitters in the rat retina after ischemia. Current eye research. 1996;15:589–596. doi: 10.3109/02713689609008898. [DOI] [PubMed] [Google Scholar]
- Petito CK, Chung MC, Verkhovsky LM, Cooper AJ. Brain glutamine synthetase increases following cerebral ischemia in the rat. Brain research. 1992;569:275–280. doi: 10.1016/0006-8993(92)90639-q. [DOI] [PubMed] [Google Scholar]
- Pow DV, Barnett NL. Changing patterns of spatial buffering of glutamate in developing rat retinae are mediated by the Müller cell glutamate transporter GLAST. Cell and tissue research. 1999;297:57–66. doi: 10.1007/s004410051333. [DOI] [PubMed] [Google Scholar]
- Puig N, Davalos A, Adan J, Piulats J, Martinez JM, Castillo J. Serum amino acid levels after permanent middle cerebral artery occlusion in the rat. Cerebrovascular diseases (Basel, Switzerland) 2000;10:449–454. doi: 10.1159/000016106. [DOI] [PubMed] [Google Scholar]
- Reinecke RD, Kuwabara T, Cogan DG, Weis DR. Retinal vascular patterns. V. Experimental ischemia of the cat eye. Archives of ophthalmology. 1962;67:470–475. doi: 10.1001/archopht.1962.00960020470015. [DOI] [PubMed] [Google Scholar]
- Robson JG, Frishman LJ. Dissecting the dark-adapted electroretinogram. Documenta ophthalmologica Advances in ophthalmology. 1998;95:187–215. doi: 10.1023/a:1001891904176. [DOI] [PubMed] [Google Scholar]
- Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. American journal of ophthalmology. 1999;128:733–738. doi: 10.1016/s0002-9394(99)00359-1. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Annals of emergency medicine. 2006;47:381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Smith RS, John SWM, Nishina PM. Posterior segment and orbit. In: Smith RS, John SWM, Nishina PM, Sundberg JP, editors. Systemic Evaluation of the Mouse Eye: Anatomy, Pathology, and Biomethods. Boca Raton, FL: CRC Press; 2002. pp. 25–44. [Google Scholar]
- Steele EC, Jr, Guo Q, Namura S. Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke; a journal of cerebral circulation. 2008;39:2099–2104. doi: 10.1161/STROKEAHA.107.504357. [DOI] [PubMed] [Google Scholar]
- Stein DG, Finger S, Hart T. Brain damage and recovery: problems and perspectives. Behavioral and neural biology. 1983;37:185–222. doi: 10.1016/s0163-1047(83)91216-5. [DOI] [PubMed] [Google Scholar]
- Sturrock GD, Mueller HR. Chronic ocular ischaemia. The British journal of ophthalmology. 1984;68:716–723. doi: 10.1136/bjo.68.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Araki M, Masuzawa T. Reaction of astrocytes in the gerbil hippocampus following transient ischemia: immunohistochemical observations with antibodies against glial fibrillary acidic protein, glutamine synthetase, and S-100 protein. Experimental neurology. 1992;116:264–274. doi: 10.1016/0014-4886(92)90006-c. [DOI] [PubMed] [Google Scholar]
- Verma R, Mishra V, Sasmal D, Raghubir R. Pharmacological evaluation of glutamate transporter 1 (GLT-1) mediated neuroprotection following cerebral ischemia/reperfusion injury. European journal of pharmacology. 2010;638:65–71. doi: 10.1016/j.ejphar.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Progress in retinal and eye research. 1998;17:485–521. doi: 10.1016/s1350-9462(98)00006-8. [DOI] [PubMed] [Google Scholar]
- Weinberger LGM, Gibbon JH. Temporary arrest of the circulation to the central nervous system: I. Physiologic effects. II. Pathologic effects. Arch Neurol Psychiat. 1940;43:615–634. 961–686. [Google Scholar]
- Wong AA, Brown RE. Age-related changes in visual acuity, learning and memory in C57BL/6J and DBA/2J mice. Neurobiology of aging. 2007;28:1577–1593. doi: 10.1016/j.neurobiolaging.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Xia CF, Smith RS, Jr, Shen B, Yang ZR, Borlongan CV, Chao L, Chao J. Postischemic brain injury is exacerbated in mice lacking the kinin B2 receptor. Hypertension. 2006;47:752–761. doi: 10.1161/01.HYP.0000214867.35632.0e. [DOI] [PubMed] [Google Scholar]
- Zhang W, Miao Y, Zhou S, Jiang J, Luo Q, Qiu Y. Neuroprotective effects of ischemic postconditioning on global brain ischemia in rats through upregulation of hippocampal glutamine synthetase. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2011;18:685–689. doi: 10.1016/j.jocn.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ohlemiller KK, McMahan BK, Gidday JM. Mouse models of retinal ischemic tolerance. Investigative ophthalmology & visual science. 2002;43:1903–1911. [PubMed] [Google Scholar]