Abstract
Background
Suicide is a common reason for psychiatric emergency and morbidity, with few effective treatments. Anxiety symptoms have emerged as potential modifiable risk factors in the time before a suicide attempt, but few studies have been conducted using laboratory measures of fear and anxiety. We operationally defined fear and anxiety as the increased in startle reactivity during anticipation of predictable (fear-potentiated startle) and unpredictable (anxiety-potentiated startle) shock. We hypothesized that a lifetime history of suicide attempt (as compared to history of no suicide attempt) would be associated with increased fear-potentiated startle.
Methods
A post-hoc analysis of fear- and anxiety-potentiated startle was conducted in 28 medication-free patients with Major Depressive Disorder (MDD) divided according to suicide attempt history.
Results
The magnitude of fear-potentiated startle was increased in depressed patients with lifetime suicide attempts compared to those without a lifetime history of suicide attempt (F(1,26) = 5.629, p = .025). There was no difference in anxiety-potentiated startle by suicide attempt history.
Limitations
This is a post-hoc analysis of previously analyzed patient data from a study of depressed inpatients. Further replication of the finding with a larger patient sample is indicated.
Conclusions
Increased fear-potentiated startle in suicide attempters suggests the role of amygdala in depressed patients with a suicide attempt history. Findings highlight the importance of anxiety symptoms in the treatment of patients at increased suicide risk.
Keywords: Suicide, major depressive disorder, fear-potentiated startle
Introduction
Suicidal behavior is a leading cause of death and morbidity (Centers for Disease Control and Prevention, 2013) and there are few, if any, effective treatments for patients at risk. Anxiety has emerged as a potential modifiable risk factor for later suicidal behavior (Fawcett et al., 1990, Hawton et al., 2013, Sareen et al., 2005). In a nationally representative sample, anxiety disorders, such as post-traumatic stress disorder (PTSD), were significantly associated with the transition from suicidal thoughts to suicide attempt, an association which was not found for depression(Nock et al., 2010). Anxiety sensitivity, meaning the fear of the physical, social or cognitive consequences of anxiety, is a demonstrated risk factor for suicide attempts in the context of depression (Capron et al., 2013); cognitive behavioral treatment for anxiety sensitivity has been associated with reduced suicidal thoughts and behavior (Capron et al., 2014).
While anxiety is an important symptom and treatment target for suicidal behavior, most of the published research has assessed anxiety through the use of self-report measures or clinical diagnosis of an anxiety disorder. The use of paradigms that assess changes in fear and anxiety for suicide research is relatively rare and primarily measure aversive reactivity to minor threats. For example, one study of affectively modulated startle reflex (to suicide-related, positive and negative visual stimuli) found no differences between depressed controls, ideators, and attempters (Smith et al., 2010). Another found no difference on acoustic startle reflex between depressed suicide attempters and healthy controls (Quednow et al., 2006). To our knowledge, there has been no investigation of an anxiety- or fear-related paradigm with the potential of actual threat in the context of suicidal thoughts and behavior.
Another concern in studying anxiety is the heterogeneity of aversive responses to threat. As an example, fear can be considered a brief response in anticipation to a proximal threat. In contrast, anxiety is considered to be a more sustained response to unpredictable stress. Fear and anxiety have been shown to have different neural correlates, with fear mediated by the amygdala and anxiety mediated by the bed nucleus of the stria terminalis (BNST) (Davis et al., 2010). Fear and anxiety have been investigated empirically by measuring startle reactivity during the threat of predictable and unpredictable shock, respectively (Schmitz and Grillon, 2012). In this paradigm, fear and anxiety are operationally defined as the increase in startle magnitude from a safe condition to periods of predictable (i.e., fear-potentiated startle) and unpredictable (i.e., anxiety-potentiated startle) shock anticipation, respectively. This paradigm has been used as a marker of post-traumatic stress disorder (PTSD) and panic disorder(Grillon et al., 2009, Grillon et al., 2008) and has demonstrated anxious anticipation in patients with MDD, (Grillon et al., 2013), but has never been used in the study of suicide risk.
We reanalyzed data from a previous investigation in patients with Major Depressive Disorder (MDD) (Grillon et al., 2013) to examine the extent to which suicide influenced fear- and anxiety-potentiated startle. Lifetime history of suicide attempt was used as a within-subject factor, as previous attempt is a significant suicide risk factor (Suominen et al., 2004) and anxiety symptoms may be particularly associated with suicidal behavior in patients with depression. We hypothesized that there would be increased fear-potentiated startle in MDD patients with a history of suicide attempts, due to the clinical findings of amygdala pathology in suicidal individuals (Anisman et al., 2008, Hrdina et al., 1993, Maheu et al., 2013) as well as the incidence of negative stressful events in the time immediately before many suicide attempts (Bagge et al., 2013, Cooper et al., 2002). Preliminary findings will have implications for neurological and clinical treatment targets in patients at risk for suicide.
Methods
Participants
Following written informed consent, 28 adult participants between the ages of 18–55 with MDD were enrolled into the protocol, as approved by the Combined Neuroscience Institutional Review Board (CNS-IRB) of the National Institutes of Health (NIH) in accordance with the Declaration of Helsinki. All participants were screened through the Experimental Therapeutics and Pathophysiology Branch (ETPB) of the National Institute of Mental Health (NIMH) Bethesda, Maryland, USA for participation in treatment protocols. Diagnoses were assessed by psychiatrists through clinical interview and confirmed with the Structured Clinical Interview for DSM-IV Diagnoses (SCID) (First, 1997), and all participants had a current, primary diagnosis of MDD without psychotic features, lasting at least 4 weeks in duration. Suicide attempt histories were gathered via clinical interview with participants.
All participants were deemed to be in good physical health following an extensive medical history, physical examination, hematologic laboratory evaluation, electrocardiogram, urinalysis, and toxicology screening. Exclusion criteria included patients with a comorbid substance abuse or dependence disorder (excluding caffeine or nicotine) in the 3 months prior to screening, positive urine toxicology screen, history of antidepressant- or substance- induced hypomania or mania, serious unstable medical disorders or conditions, or concomitant treatment with psychotropic medications or electroconvulsive therapy in the 2 weeks prior to the experiment. Females could not be pregnant or nursing.
Study Design, Stimuli, and Physiologic Responses
A previously published methodological report details the threat of shock paradigm (Schmitz and Grillon, 2012). This paradigm has successfully been used in several articles examining startle potentiation in anxious (Grillon et al., 2006, Grillon et al., 2009) and depressed (Grillon et al., 2013) participants while anticipating shocks. Briefly, participants were exposed to three conditions: no shock (N), predictable shock (P), and unpredictable shock (U), or, the NPU-threat test. Startle reactivity was measured with an electromyograph (EMG) throughout the experiment via eyeblink electrodes that were superficially placed on the skin below the left eye; EMG data was then digitized (1000 Hz) and amplified (bandwidth 30–500 Hz). Initially, participants were habituated to the startle response by receiving a total of nine acoustic startle stimuli every 18–23 seconds via headphones. All acoustic startle stimuli were white noise sounds (40-ms duration, 103-dB). Superficial shock electrodes were then unilaterally attached medially to the supine wrist. A shock work up was initiated to set the intensity of shock to a mildly painful level. Stimulation and recording were controlled by a commercial system (Contact Precision Instruments, London, England).
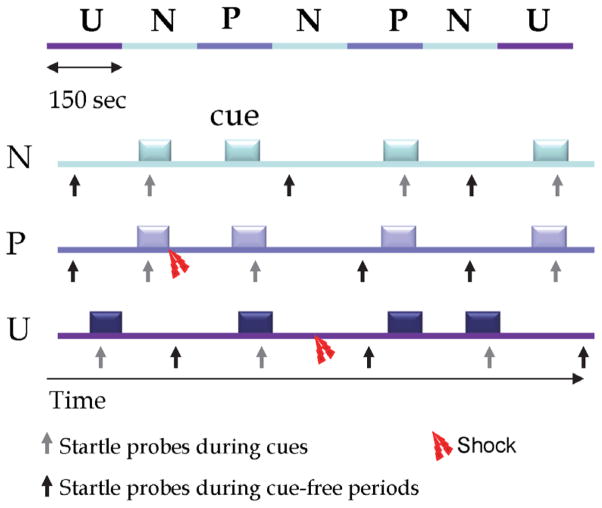
Explicit instructions were then given to participants for the NPU-threat test conditions. A total of three conditions lasting 150-second in duration were administered (Figure 1 for schematic representation). During each condition, an 8-second cue was presented four times on a computer monitor facing the participants. A green circle cue represented the N condition, a red square for P, and a blue triangle for U. Depending on the condition being tested, the following written instructions were continuously displayed on the computer screen: “no shock (N),” “shock only during shape (P),” or “shock at any time (U).” Therefore, the cues signaled the possibility of receiving a shock only in the P condition but the cues had no signal value in the N and U conditions. Each participant was exposed to two blocks of three N, two P, and two U in either the order of P-N-U-N-U-N-P or U-N-P-N-P-N-U. Participants received a total of eight shocks during the session: two in each of the P and U conditions. When delivered in the P conditions, shocks were at the end of the cue; in the U conditions, they were in the absence of the cue.
Figure 1.
Schematic representation of sequences of stimulus presentation during each condition in one block of the NPU-threat test.
The top of the figure represents a complete block, including two P (predictable), two U (unpredictable) and three N (no shock) conditions (order U-N-P-N-P-N-U as shown; or, alternatively administered as P-N-U-N-U-N-P). The remaining figure shows each condition, including cues (8-s duration), startle probes presented during cues (grey arrow pointing up) or during cue-free periods (dark arrow pointing up), and shocks. Image originally adapted from reference (Grillon et al., 2009) and taken from Grillon et al. 2013.
At the beginning of each block, four acoustic habituation startle stimuli were delivered. During each of the seven individual conditions (N, P, or U), a total of six acoustic startle stimuli were delivered; three during cue-free periods (known as the inter-trial intervals, or ITI) and one during three of the four total cues, 5–7 seconds following cue onset. The mean inter-startle interval was 21 seconds (ranging from 18–25 seconds). All acoustic startle stimuli were given at least 8 seconds after an aversive shock stimulus in order to avoid potential short-term sensitization of startle. Following each block, participants subjectively rated their anxiety level in the presence and absence of the cue in each condition (N, P, U) on an analog scale ranging from 0 (no anxiety) to 10 (extreme anxiety).
On the morning of experimentation, participants completed a packet of questionnaires, including the Beck Depression Inventory (Beck and Beamesderfer, 1974). In addition, all participants completed the clinician-administered Montgomery-Asberg Depression Rating Scale (Montgomery and Asberg, 1979), with a required score of ≥20 to quantify current depression status.
Data Analysis
Overall differences in demographics and clinical characteristics between suicide attempters and non-attempters were evaluated using univariate analyses. Startle magnitude was analyzed using within-subject T-Scores via a Group (non-attempters vs. attempters) X Condition (N, P, U) X Stimulus Type (Cue, ITI) ANOVA. In interactions involving more than two levels, Greenhouse-Geisser corrections were used. A similar 3-way ANOVA was also conducted for retrospective anxiety ratings collected during the study. As the aim of this analysis was to evaluate fear and anxiety-potentiated startle in individuals who made suicide attempts, any significant group interactions were investigated using group contrasts. Fear-potentiated startle was calculated as the difference in startle magnitude during the Cue minus ITI in the Predictable (P) condition. Anxiety-potentiated startle was calculated as the difference in ITI startle between the Unpredictable condition (U) and the No Shock (N) condition. As number of suicide attempts may represent another signifier of suicide risk, this variable was compared to measures of fear and anxiety via correlational analysis. Spearman correlations were used due to nonparametric distribution of suicide attempts in the sample. Significance was measured at the .05 alpha level and all analyses were conducted using SPSS 21.
Results
Sample characteristics are presented in Table 1. There were no significant demographic or clinical differences between suicide attempters and non-attempters, including a measure of current suicidal ideation from the BDI.
Table 1.
Demographics and Clinical Characteristics of Study Sample
| Total Sample | Non-attempters (n = 22) | Attempters (n = 6) | X2 | p | |
|---|---|---|---|---|---|
| Male gender | 11(39) | 9(41) | 2(33) | .11 | .74 |
| African American ethnicity | 3(11) | 2(9) | 1(17) | .94 | .62 |
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | t | p | |
|
| |||||
| Age | 35.50(10.47) | 37.05(10.80) | 29.83(7.25) | 1.53 | .14 |
| Illness duration | 16.60(11.33) | 17.00(12.62) | 15.33(5.82) | .31 | .76 |
| BDI without suicide item | 28.71(.10.44) | 27.70(10.69) | 33.75(8.46) | −1.06 | .30 |
| BDI suicide item | .54(.51) | 0.50(.51) | 0.75(.50) | −.89 | .38 |
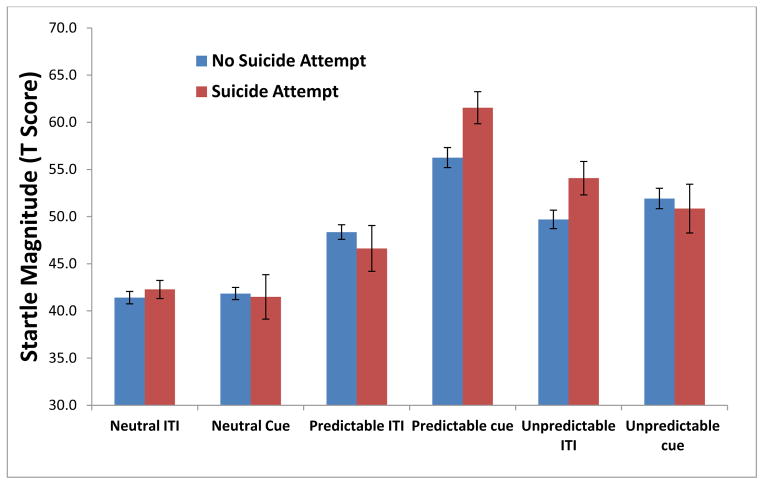
On three-way ANOVA analysis, there was a significant Group X Condition X Stimulus Type interaction, F(2,52) = 4.86, p = .016. Startle magnitude across these conditions is presented in Figure 2. Follow-up analyses separately examined fear-potentiated startle and anxiety-potentiated startle. Fear-potentiated startle was significantly larger in suicide attempters compared to non-attempters, F(1,26) = 5.629, p = .025. Anxiety-potentiated startle did not differ significantly between the two groups (p>.20).
Figure 2.
Startle Response during NPU (T-Scores) by Lifetime History of Suicide Attempt
A similar Group X Condition X Stimulus Type ANOVA was calculated for subjective anxiety ratings. There was a significant Group X Condition interaction F(2,52) = 4.22, p = .029, but the three-way interaction was not significant (p > .20). Subjective anxiety increased from the No Shock condition to the Unpredictable condition and this difference was larger in patients with a lifetime history of suicide attempt, t(26) = −2.66, p = .013. Additionally, subjective anxiety increased from the No Shock to the Predictable condition but this difference was not significantly larger in patients with a lifetime history of suicide attempt (p > .15).
In correlational analyses of fear- and anxiety-potentiated startle magnitude, there was a significant correlation between number of lifetime suicide attempts and fear-potentiated startle (r = .43, p = .021), but no significant correlation with anxiety-potentiated startle (r = .23, p = .241).
Discussion
In this preliminary post-hoc analysis, we found that a lifetime history of suicide attempt was associated with increased fear-potentiated startle to predictable shock. There was no significant difference between the two groups for anxiety-potentiated startle to unpredictable shock. Analysis of retrospective subjective anxiety ratings revealed that patients with lifetime suicide attempts reported more anxiety during the unpredictable condition than patients without a suicide attempt and patients with suicide attempts also reported more fear, although this trend did not reach significance, likely due to the small sample size. The results highlight the presence of laboratory-induced fear in patients at risk for suicide, which may be more generalizable to real-life stressful events than less aversive stimuli such as images or loud sounds (Grillon et al., 2013).
This analysis is the first known study of a fear- or anxiety-related paradigm in the context of actual threat in evaluating MDD patients with a suicide attempt history. As fear-potentiated startle has been shown to be mediated by the amygdala, these findings implicate the amygdala in patients at risk for suicidal behavior. In support of a neurobiological underpinning to suicide, several previous studies have found physiological differences in the amygdala from those who have attempted or died by suicide compared to people who have not. For example, the expression of serotonin receptors in the amygdala has varied between those who have died by suicide and healthy controls (Anisman et al., 2008, Hrdina et al., 1993). The expression of proteins responsible for roles in neuroplasticity (doublecortin and brain-derived neurotropic factor) was shown to differ in the amygdala of depressed individuals who did and did not die by suicide (Maheu et al., 2013). Additionally, gene and protein expression of FKBP5 and glucocorticoid receptors, which have been implicated in depression and anxiety, were significantly reduced in the amygdala of suicide victims compared to controls (Perez-Ortiz et al., 2013). The results of our study, in combination with these previous studies, provide support for the potential role of altered amygdala activity being associated with suicide. With a better understanding of the association between amygdalar function and suicide, future treatments may be able to target the amygdala and its processes for suicide prevention.
Findings concerning anxiety and fear in individuals who have attempted suicide may be considered counter-intuitive in light of current psychological theory. In his Interpersonal Theory of Suicide, Joiner has posited that the capability to attempt suicide is acquired, suggesting individuals with multiple suicide attempts, painful experiences and trauma develop a type of “fearlessness” or habituation to the fear and pain involved in a suicide attempt (Joiner, 2005). This theory has been supported by reduced reported pain perception and fear in individuals with a suicide attempt history (Franklin et al., 2011, Smith et al., 2010). In contrast, these laboratory results using a mildly painful stimulus found that individuals with a suicide attempt reported more subjective anxiety in the unpredictable shock condition than depressed individuals without a suicide attempt. These findings may indicate, as others have suggested (Smith et al., 2010), that the Interpersonal Theory may be more relevant to self-report than physiological measures. Further work may be indicated to compare fear ratings and startle responses in individuals with a suicide attempt history across a range of circumstances and conditions to fully evaluate this theory.
Limitations of this study include a post hoc analysis of a small patient sample. This study was not designed to evaluate differences in fear- or anxiety-potentiated startle by history of suicide attempt and was limited to patients with MDD. Prospective recruitment of patients with lifetime suicide attempts across several mood or anxiety disorder diagnoses may be indicated to replicate this finding. Second, the assessment of lifetime suicide attempts was conducted via clinician interview as part of inpatient psychiatric assessment and SCID. Further studies would benefit from including systematic assessment of suicide attempts, such as the Columbia Suicide Severity Rating Scale (Posner et al., 2011). Third, results for startle and for the subjective ratings did not fully converge, a finding that is consistent with the literature (Grillon et al., 2006, Grillon et al., 2009). These two measures may capture different components of fear and anxiety (e.g., cortical vs. subcortical). In addition, startle is an online measure whereas the subjective ratings were taken retrospectively. Lastly, this analysis was of lifetime history of suicide attempt and findings cannot be inferred to predict future suicidal behavior. However, this study represents a key first step in replicating self-report findings with laboratory measures of fear and anxiety in the study of suicide.
Ultimately, the discovery of relevant biomarkers may facilitate the diagnosis and treatment of psychiatric disorders (Niciu et al., 2013). As part of the Research Domain Criteria (RDoC), acute threat (“fear”) and potential threat (“anxiety”) are constructs within the domain of negative valence systems (Morris and Cuthbert, 2012). Such research domains act as a guide for understanding the physiology of mental illness. By utilizing the threat-of-shock paradigm, we uncovered a difference in fear-potentiated (but not anxiety-potentiated) startle in depressed subjects with a lifetime history of suicide attempt from depressed subjects with no suicide history. This physiologic difference between groups improves the understanding of subgroups within depression, paving the way for future improvements in our comprehension of suicide risk.
Acknowledgments
Role of Funding Source: Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH), by a NARSAD Independent Investigator Award to CAZ, and by the Brain & Behavior Mood Disorders Research Award to CAZ.
Footnotes
Disclosures: The authors have no conflict of interest to disclose, financial or otherwise.
Contributors:
EB conceptualized the study design, ran statistical analyses, interpreted the results, drafted and edited the manuscript.
DI conceptualized the study design, interpreted the results and edited the manuscript.
JV conceptualized the study design, interpreted the results and edited the manuscript.
ES assisted in the interpretation of the results and editing the manuscript.
JF-C consented and screened participants and assisted in editing the manuscript.
CZ conceptualized the study design and edited the manuscript.
CG developed the study paradigm, conceptualized the study design, interpreted the results and edited the manuscript.
All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, Merali Z, Poulter MO. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- Bagge CL, Glenn CR, Lee HJ. Quantifying the impact of recent negative life events on suicide attempts. J Abnorm Psychol. 2013;122:359–368. doi: 10.1037/a0030371. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Capron DW, Norr AM, Macatee RJ, Schmidt NB. Distress tolerance and anxiety sensitivity cognitive concerns: testing the incremental contributions of affect dysregulation constructs on suicidal ideation and suicide attempt. Behav Ther. 2013;44:349–358. doi: 10.1016/j.beth.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Capron DW, Norr AM, Zvolensky MJ, Schmidt NB. Prospective Evaluation of the Effect of an Anxiety Sensitivity Intervention on Suicidality among Smokers. Cogn Behav Ther. 2014;43:72–82. doi: 10.1080/16506073.2013.777466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers For Disease Control And Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS) 2013. [Google Scholar]
- Cooper J, Appleby L, Amos T. Life events preceding suicide by young people. Soc Psychiatry Psychiatr Epidemiol. 2002;37:271–275. doi: 10.1007/s001270200019. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Scheftner WA, Fogg L, Clark DC, Young MA, Hedeker D, Gibbons R. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147:1189–1194. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- First M, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Franklin JC, Hessel ET, Prinstein MJ. Clarifying the role of pain tolerance in suicidal capability. Psychiatry Res. 2011;189:362–367. doi: 10.1016/j.psychres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Franco-Chaves JA, Mateus CF, Ionescu DF, Zarate CA. Major depression is not associated with blunting of aversive responses; evidence for enhanced anxious anticipation. PLoS One. 2013;8:e70969. doi: 10.1371/journal.pone.0070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, Mcdowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawton K, Casanas IC, Haw C, Saunders K. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord. 2013;147:17–28. doi: 10.1016/j.jad.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res. 1993;614:37–44. doi: 10.1016/0006-8993(93)91015-k. [DOI] [PubMed] [Google Scholar]
- Joiner TE. Why people die by suicide. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Maheu ME, Davoli MA, Turecki G, Mechawar N. Amygdalar expression of proteins associated with neuroplasticity in major depression and suicide. J Psychiatr Res. 2013;47:384–390. doi: 10.1016/j.jpsychires.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Mathews DC, Nugent AC, Ionescu DF, Furey ML, Richards EM, Machado-Vieira R, Zarate CA. Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants. Depress Anxiety. 2013 doi: 10.1002/da.22224. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Hwang I, Sampson NA, Kessler RC. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:868–76. doi: 10.1038/mp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Ortiz JM, Garcia-Gutierrez MS, Navarrete F, Giner S, Manzanares J. Gene and protein alterations of FKBP5 and glucocorticoid receptor in the amygdala of suicide victims. Psychoneuroendocrinology. 2013;38:1251–1258. doi: 10.1016/j.psyneuen.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Westheide J, Kuhn KU, Werner P, Maier W, Hawellek B, Wagner M. Normal prepulse inhibition and habituation of acoustic startle response in suicidal depressive patients without psychotic symptoms. J Affect Disord. 2006;92:299–303. doi: 10.1016/j.jad.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Afifi TO, De Graaf R, Asmundson GJ, Ten Have M, Stein MB. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62:1249–1257. doi: 10.1001/archpsyc.62.11.1249. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nat Protoc. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PN, Cukrowicz KC, Poindexter EK, Hobson V, Cohen LM. The acquired capability for suicide: a comparison of suicide attempters, suicide ideators, and non-suicidal controls. Depress Anxiety. 2010;27:871–977. doi: 10.1002/da.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suominen K, Isometsa E, Suokas J, Haukka J, Achte K, Lonnqvist J. Completed suicide after a suicide attempt: a 37-year follow-up study. Am J Psychiatry. 2004;161:562–563. doi: 10.1176/appi.ajp.161.3.562. [DOI] [PubMed] [Google Scholar]