Abstract
Background & Aims
Non-alcoholic steatohepatitis (NASH) is characterized by steatosis and inflammation. The transition from steatosis towards NASH represents a key step in pathogenesis, as it will set the stage for further severe liver damage. Under normal conditions, lipoproteins that are endocytosed by Kupffer cells (KCs) are easily transferred from the lysosomes into the cytoplasm. Oxidized LDL (oxLDL) that is taken up by the macrophages in vitro is trapped within the lysosomes, while acetylated LDL (acLDL) is leading to normal lysosomal hydrolysis, resulting in cytoplasmic storage. We have recently demonstrated that hepatic inflammation is correlated with lysosomal trapping of lipids. So far, a link between lysosomal trapping of oxLDL and inflammation was not established. We hypothesized that lysosomal trapping of oxLDL in KCs will lead to hepatic inflammation.
Methods
Ldlr−/− mice were injected with LDL, acLDL and oxLDL and sacrificed after 2, 6 and 24 h.
Results
Electron microscopy of KCs demonstrated that after oxLDL injection, small lipid inclusions were present inside the lysosomes after all time points and were mostly pronounced after 6 and 24 h. In contrast, no lipid inclusions were present inside KCs after LDL or acLDL injection. Hepatic expression of several inflammatory genes and scavenger receptors was higher after oxLDL injections compared with LDL or acLDL.
Conclusions
These data suggest that trapping of oxLDL inside lysosomes of KCs in vivo is causally linked to increased hepatic inflammatory gene expression. Our novel observations provide new bases for prevention and treatment of NASH.
Keywords: inflammation, Kupffer cells, liver, lysosomes, oxLDL
Non-alcoholic fatty liver disease (NAFLD) is a condition ranging from lipid accumulation in the liver (steatosis) to steatosis combined with inflammation. The latter is referred to as non-alcoholic steatohepatitis (NASH) and is viewed as the hepatic event of the metabolic syndrome. As obesity and insulin resistance reach epidemic proportions in industrialized countries, the prevalence of NASH is increasing and is therefore considered a major health hazard. Although steatosis alone is considered as the less aggressive stage of NAFLD, inflammation represents a key step in the pathogenesis of NASH, thereby setting the stage for further liver damage including fibrosis, cirrhosis and liver cancer (1). Currently, the mechanisms by which inflammation develops are poorly understood and therapy options are very poor.
Recent studies point strongly towards the importance of Kupffer cells (KCs), the resident macrophages of the liver, in triggering hepatic inflammation (2). In line with this view, we demonstrated the correlation between hepatic inflammation and the appearance of foamy KCs, analogous to foamy macrophages in atherosclerosis (3). Such cholesterol-loaded foamy macrophages are formed by the uptake of oxidized cholesterol-rich low-density lipoproteins (oxLDL) via scavenger receptors (4). Uptake of oxLDL by macrophages in vitro is shown to be resistant to rapid endolysosomal hydrolysis and is trapped within the lysosomes, while acetylated LDL (acLDL) is leading to normal lysosomal hydrolysis, resulting in cytoplasmic storage of cholesteryl esters (5, 6). We have previously shown that haematopoietic deletion of CD36 and SR-A, two main scavenger receptors for the uptake of oxLDL, sets off a cascade of pro-inflammatory events leading to the initiation of the inflammatory response in the liver (7). Moreover, the reduced inflammatory response was associated with less lysosomal cholesterol accumulation inside KCs (8, 9). However, a causal link between lysosomal cholesterol accumulation in KCs and hepatic inflammation has not yet been established. In the current manuscript, we hypothesize that lysosomal trapping of oxLDL in KCs leads to hepatic inflammation.
To investigate whether oxLDL can directly affect hepatic inflammation in vivo, Ldlr−/− mice were injected with a bolus of oxLDL and sacrificed after 2, 6 and 24 h of injection. Injections with PBS, LDL and acLDL were used as controls. After oxLDL injection, we found that lysosomal trapping of oxLDL was correlated with elevated expression of hepatic inflammatory genes. These data suggest a causal relationship between oxLDL and hepatic inflammation and provide new bases for prevention and treatment of NASH.
Material and methods
Mice
The mice were housed under standard conditions and given free access to food and water. Experiments were performed according to Dutch regulations and approved by the Committee for Animal Welfare of Maastricht University. Twelve-week-old female Ldlr−/− mice were injected in the tail vein with a bolus of human LDL, oxLDL or acLDL (200 lg for each) (Intracel, Frederick, MD, USA) and sacrificed after 2, 6 and 24 h of injection (n = 8 per group). The control group was injected with PBS. The mice were sacrificed by cervical dislocation. Tissues were then isolated and snap-frozen in liquid nitrogen and stored at −80°C.
RNA isolation and quantitative PCR
RNA isolation and cDNA synthesis were performed as described previously (3, 7). All applications were performed according to the manufacturers’ protocols. For each gene, a standard curve was generated with a serial dilution of a liver cDNA pool. To standardize for the amount of cDNA, Cyclophillin A (Ppia) was used as the reference gene. Primer sets for the selected genes were developed with Primer Express version 2.0 (Applied Biosystems, Foster City, CA, USA) using default settings. Primer sequences were as followed: Ppia forward 5′-TTCCTCCTTTCACAGAATTATTCCA; Ppia reverse 3′-CCGCCAGTGCCATTATGG; Tnf-α forward 5′-CATCTTCTCAAAATTCGAGTGACAA; Tnf-α reverse 3′-TGGGAGTAGACAAGGTACAACCC; Saa-1 forward 5′-GGCTGCTGAGAAAATCAGTGATG; Saa-1 reverse 3′-TCAGCAATGGTGTCCTCATGTC; Tlr-4 forward 5′-TATCCAGGTGTGAAATTGAAACAATT; Tlr-4 reverse 3′-GGGTTTCCTGTCAGTATCAAGTTTG; Icam forward 5′-CTACCATCACCGTGTATTCGTTTC; Icam reverse 3′-CGGTGCTCCACCATCCA; Hmox forward 5′-CCGCCTTCCTGCTCAACAT; Hmox reverse 3′-ATCTGTGAGGGACTCTGGTCTTTG; Cd36 forward 5′-GCCAAGCTATTGCGACATGA; Cd36 reverse 3′-AAAA GAATCTCAATGTCCGAGACTTT; Sr-a forward 5′-CA TACAGAAACACTGCATGTCAGAGT; Sr-a reverse 3′-TTCTGCTGATACTTTGTACACACGTT. Data from qPCR were analysed according to the relative standard curve method.
Liver histology
A detailed overview about liver histology was described previously (7). In brief, frozen liver sections (7 μm) were fixed in acetone and stained with rat-anti-mouse Mac1 (M1/70) (generous gifts from Prof. Kraal, Free University, Amsterdam, the Netherlands). Immune cells were counted in six microscopic views (magnification 200×) and were noted as cells/mm2. Pictures were taken with a Nikon DMX1200 digital camera and ACT-1 version 2.63 software (Nikon Instruments Europe, Amstelveen, the Netherlands).
Electron microscopy
Livers were freshly isolated from the mice and perfused and fixed overnight with 2.5% glutaraldehyde (Ted Pella, Redding, CA, USA). Tissue fragments were washed and post-fixed in 1% osmium tetroxide. Tissues were subsequently dehydrated through 100% ethanol, cleared with propylene oxide and embedded in epoxy resin. Next, sections of 70–90 nm were cut on an ultra-microtome, mounted on Formvar-coated (1595E, Merck) 75-mesh copper grids and counter-stained with uranyl acetate and lead citrate before analysis on a Philips CM100 transmission electron microscope.
Statistics
Data were analysed using Graphpad Prism 4.0.3 software. Groups were compared using the unpaired t-test. The data were expressed as the mean and standard error of the mean and were considered significantly different at *P < 0.05; **P < 0.01; or ***P < 0.001.
Results
The injected oxLDL is trapped inside lysosomes of Kupffer cells
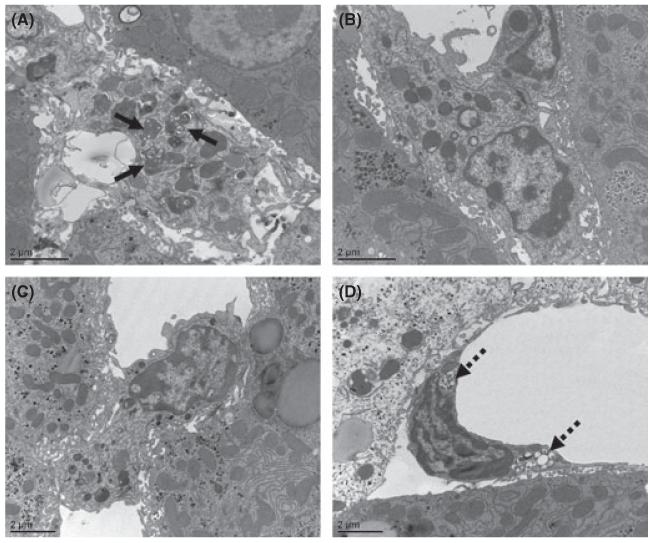
The direct role of oxLDL in hepatic inflammation was investigated by intravenous injection of oxLDL in Ldlr−/− mice. Normal LDL, acLDL and PBS were used as control. Electron microscopy was performed to explore the intracellular distribution of oxLDL, LDL and acLDL inside KCs (Fig. 1A–C). After oxLDL injection, small lipid inclusions were present inside the lysosomes of KCs in all time points and were mostly pronounced after 6 and 24 h of oxLDL injection (Fig. 1A). LDL could not be detected inside the KCs after all time points (Fig. 1B), while acLDL was mainly located inside the endothelial cells of the liver after 2 and 6 h of injection (Fig. 1D), instead of in KCs (Fig. 1C). Altogether, these data demonstrate that oxLDL is trapped inside lysosomes of KCs in vivo.
Fig. 1.
Electron microscopy after acLDL, oxLDL and LDL injections. (A–C) Representative electron microscopy pictures of Kupffer cells 24 h after oxLDL, LDL and acLDL injections respectively. (D) Representative electron microscopy picture of an endothelial cell 2 h after acLDL injection. Oxidized LDL inside lysosomes of KCs is indicated by solid arrows, acetylated LDL inside endothelial cells by broken arrows.
Hepatic inflammation is increased after oxLDL injection
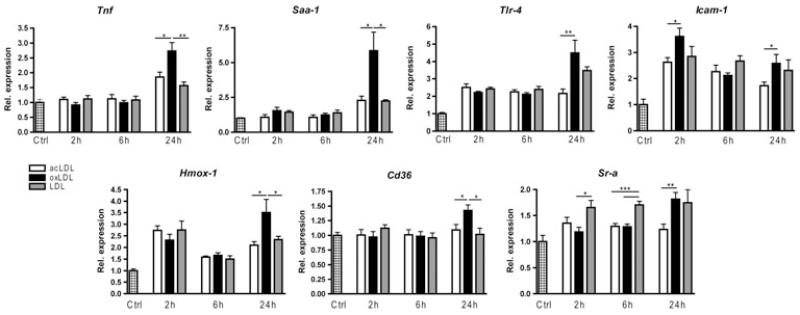
After 24 h of injection, hepatic gene expression of the inflammatory markers tumour necrosis factor alpha (Tnf-α), serum amyloid A1 (Saa-1), toll-like receptor 4 (Tlr-4) and intracellular adhesion molecule-1 (Icam-1) was significantly higher in mice that received a bolus of oxLDL compared with acLDL (Fig. 2). The expression of Tnf-α and Saa-1 of oxLDL-injected mice was also higher than LDL-injected mice after 24 h. Moreover, Icam-1 was already significantly increased after 2 h of oxLDL injection compared with acLDL. The antioxidant haem oxygenase 1 (Hmox-1) was also higher after 24 h of oxLDL injection compared with LDL and acLDL. Infiltration of monocytes/macrophages and neutrophils (Cd11b staining) was not different between the groups, which is in line with the unchanged mRNA expression of Integrin alpha-M (Itgam) (Supplemental Figure 1). Together, these data demonstrate that the inflammatory gene expression in liver is increased after oxLDL injection compared with LDL and acLDL.
Fig. 2.
Hepatic gene expression after acLDL, oxLDL and LDL injections. Gene expression analysis by qPCR of tumour necrosis factor alpha (Tnf-α), serum amyloid A1 (Saa-1), toll-like receptor 4 (Tlr-4), intracellular adhesion molecule-1 (Icam-1), haem oxygenase 1 (Hmox-1) and scavenger receptors Cd36 and Sr-a. Gene expression data are shown relative to the control injected group. *P < 0.05, **P < 0.01 and ***P < 0.001.
Next, mRNA expression of the two main scavenger receptors CD36 and Sr-A was performed to explore their response upon oxLDL injection. After 24 h of oxLDL injection, expression of Cd36 was significantly increased compared with LDL and acLDL, while expression of Sr-a was increased compared with acLDL. However, after 2 and 6 h of LDL injection, expression of Sr-a was increased compared with oxLDL injection (Fig. 2). Altogether, these data demonstrate that both scavenger receptors CD36 and Sr-A are involved in the inflammatory response upon oxLDL administration in vivo (Fig. 3).
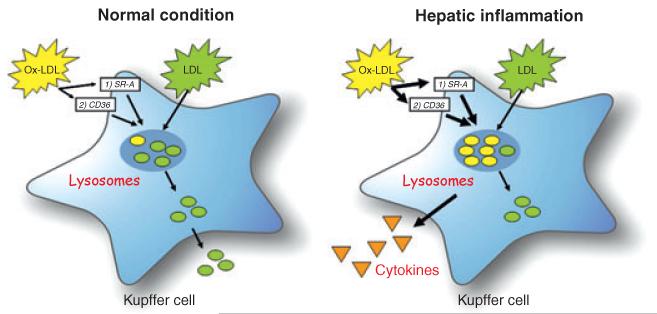
Fig. 3.
Uptake of modified lipoproteins by Kupffer cells (KCs). Under normal conditions, circulating cholesterol is taken up by KCs and initially directed to lysosomes. After hydrolyzation by lysosomal enzymes, free cholesterol is transferred into the cytoplasm. There, free cholesterol can be converted into cholesterol esters, or excreted from the cell by efflux mechanisms. Oxidized LDL (oxLDL) can be taken up by scavenger receptors CD36 and Sr-A on the KCs. OxLDL is then directed to the lysosomes of the KCs, where it will accumulate via unknown mechanisms. Therefore, oxLDL cannot be converted into free cholesterol and will be trapped inside the lysosomes, triggering an inflammatory response.
Discussion
This study demonstrates for the first time the in vivo effects of oxLDL on hepatic inflammatory gene expression. We have previously shown the association between lysosomal trapping of oxLDL and inflammation. However, a causal link was never established. In the current manuscript, we investigated the specific effect of oxLDL on the development of hepatic inflammation. While injection of normal LDL or acLDL was not associated with lysosomal trapping or inflammation, we demonstrated for the first time that in a mouse model of NASH, oxLDL is trapped within the lysosomes of KCs and is associated with an inflammatory response. These data provide new bases for prevention and treatment of NASH.
Oxidative modification of LDL transforms LDL to a pro-inflammatory, immunogenic and cytotoxic oxLDL that is generally held as a key component in the development of atherosclerotic foam cells (10). Oxidation of LDL has been found to take place in the arterial wall (11) and in plasma (12). By functioning as scavenger organ for circulating oxLDL, the liver reduces the harmful effects of oxLDL in other tissues (13). However, there is some evidence that high levels of circulating oxLDL can impair the function of the liver itself (14), and when injected into the general circulation of mice, oxLDL can induce several pathophysiological events in the hepatic sinusoids (15). Although these findings support the idea that oxLDL is very harmful for the liver, the authors did not investigate the link with lysosomal cholesterol accumulation.
The clearance of native LDL from serum proceeds at a low rate (13). When LDL is oxidized or acetylated and injected into rats, this leads to a markedly increased removal from the blood circulation paralleled with an increased uptake in the liver (13). The KC uptake of oxLDL 10 min after injection is much higher than for acLDL, leading to KCs as the main liver site for oxLDL uptake (13). Furthermore, oxLDL that is taken up by macrophages in vitro is trapped within the lysosomes, while acLDL is leading to normal lysosomal hydrolysis, resulting in cytoplasmic storage of cholesteryl esters (5, 6). Therefore, although acLDL is not naturally present in vivo, we have used it in the current experiment as a control for lysosomal cholesterol accumulation achieved by oxLDL. We demonstrated that the injected oxLDL is trapped inside lysosomes of KCs, while acLDL is not present in the cytoplasm of KCs, but is mainly found in the endothelial cells of the liver. These data are also in agreement with previous observations in literature, demonstrating that acLDL is mainly taken up by the endothelial cells in the liver, and not by KCs (13, 16). It has been suggested that unlike acLDL, uptake of oxLDL by macrophages leads to impaired lysosomal degradation (17), and thereby expansion of and a decrease in the density of the lysosomal compartments in macrophages (18). In the current manuscript, we show for the first time that accumulation of oxLDL inside lysosomes of KCs can also trigger an inflammatory response. Although the hepatic inflammatory gene expression was already affected after several hours of oxLDL injection, infiltration of inflammatory cells in the liver (CD11b staining) was not observed. This lack of inflammatory cell infiltration is probably related to the rapid clearance of oxLDL from the circulation (13), and therefore we could only observe changes in the inflammatory gene expression upon injections as this is rapidly influenced. These data are also in line with our expectations, as the accumulation of oxLDL inside the lysosomes will first lead to activation of the KCs, which in turn will lead to a rapid release of a wide range of inflammatory mediators and signalling molecules for the attraction of inflammatory cells (19).
The two main macrophage scavenger receptors responsible for binding and internalization of oxLDL, namely CD36 and Sr-A showed elevated hepatic mRNA expression upon oxLDL injection. These data are also in line with previous reports on the effect of oxLDL on scavenger receptors, where functional expression of CD36 and Sr-A was increased in macrophages upon oxLDL loading (20, 21). We have previously demonstrated that both the scavenger receptors are involved in lysosomal trapping of oxLDL (9). When oxLDL is trapped inside lysosomes because of impaired cholesteryl ester hydrolysis or a change in the acidic pH of lysosomes (22), it has the potential to damage lysosomal membranes (23). Endocytosed oxLDL particles partially inactivate lysosomal enzymes and cause relocation of these enzymes to the cytosol (24), as well as activation of the NLRP3 inflammasome (25-27). Moreover, when there are high levels of circulating oxLDL, as observed in foamy macrophages found in inflamed atherosclerotic plaques, cholesterol is not transferred into the cytoplasm, but rather accumulates inside lysosomes (22, 28, 29). Several lines of evidence also indicate a strong association between lysosomal cholesterol accumulation and inflammation (23, 25, 27, 30, 31). Our current study is the first one to provide a causal relationship between lysosomal oxLDL trapping and elevation of hepatic inflammatory gene expression in the context of NASH.
Taken altogether, our novel observations point towards the link between lysosomal oxLDL accumulation inside KCs and hepatic inflammatory gene expression. Therefore, inhibition of oxLDL itself or the redirection of lysosomal cholesterol accumulation can provide a new basis for prevention and treatment of NASH.
Supplementary Material
Acknowledgement
Financial support: The Netherlands Organisation for Scientific Research (NWO): Veni grant (916.76.070) and Vidi grant (016.126.327); Dutch GI/Liver Foundation (MLDS): WO 08-16; 09-46 and 11-35.
Abbreviations
- acLDL
acetylated low-density lipoprotein
- CE
cholesteryl ester
- HMOX
haem oxygenase
- ICAM
intracellular adhesion molecule
- KC
Kupffer cell
- LDL(R)
low-density lipoprotein (receptor)
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- oxLDL
oxidized low-density lipoprotein
- SAA
serum amyloid A
- SR-A
scavenger receptor A
- TLR
toll-like receptor
- TNF-α
tumour necrosis factor alpha.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Infiltrating macrophages. Hepatic gene expression of Integrin alpha-M (Itgam) and quantification of histology for infiltrating macrophages (Mac1 staining) after 24 h of injection.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol. 2006;12:7413–20. doi: 10.3748/wjg.v12.i46.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wouters K, van Gorp PJ, Bieghs V, et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474–86. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 5.Yancey PG, Jerome WG. Lysosomal sequestration of free and esterified cholesterol from oxidized low density lipoprotein in macrophages of different species. J Lipid Res. 1998;39:1349–61. [PubMed] [Google Scholar]
- 6.Yancey PG, Miles S, Schwegel J, Jerome WG. Uptake and trafficking of mildly oxidized LDL and acetylated LDL in THP-1 cells does not explain the differences in lysosomal metabolism of these two lipoproteins. Microsc Microanal. 2002;8:81–93. doi: 10.1017/s1431927601020013. [DOI] [PubMed] [Google Scholar]
- 7.Bieghs V, Wouters K, van Gorp PJ, et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholicsteatohepatitis in hyperlipidemic mice. Gastroenterology. 2010;138:2477–86. 2486 e1–3. doi: 10.1053/j.gastro.2010.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bieghs V, van Gorp PJ, Walenbergh S, et al. Specific immunization strategies against oxidized low-density lipoprotein: a novel way to reduce nonalcoholic steatohepatitis in mice. Hepatology. 2012;56:894–903. doi: 10.1002/hep.25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bieghs V, Verheyen F, van Gorp PJ, et al. Internalization of modified lipids by CD36 and Sr-A leads to hepatic inflammation and lysosomal cholesterol storage in Kupffer cells. PLoS ONE. 2012;7:e34378. doi: 10.1371/journal.pone.0034378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–6. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 11.Yla-Herttuala S, Palinski W, Rosenfeld ME, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–95. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avogaro P, Bon GB, Cazzolato G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis. 1988;8:79–87. [PubMed] [Google Scholar]
- 13.Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem. 1991;266:2282–9. [PubMed] [Google Scholar]
- 14.Itabe H. Oxidized low-density lipoproteins: what is understood and what remains to be clarified. Biol Pharm Bull. 2003;26:1–9. doi: 10.1248/bpb.26.1. [DOI] [PubMed] [Google Scholar]
- 15.Oteiza A, Li R, McCuskey RS, Smedsrod B, Sorensen KK. Effects of oxidized low-density lipoproteins on the hepatic microvasculature. Am J Physiol Gastrointest Liver Physiol. 2011;301:G684–93. doi: 10.1152/ajpgi.00347.2010. [DOI] [PubMed] [Google Scholar]
- 16.Nagelkerke JF, Barto KP, van Berkel TJ. in vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983;258:12221–7. [PubMed] [Google Scholar]
- 17.Yancey PG, Jerome WG. Lysosomal cholesterol derived from mildly oxidized low density lipoprotein is resistant to efflux. J Lipid Res. 2001;42:317–27. [PubMed] [Google Scholar]
- 18.Lougheed M, Moore ED, Scriven DR, Steinbrecher UP. Uptake of oxidized LDL by macrophages differs from that of acetyl LDL and leads to expansion of an acidic endolysosomal compartment. Arterioscler Thromb Vasc Biol. 1999;19:1881–90. doi: 10.1161/01.atv.19.8.1881. [DOI] [PubMed] [Google Scholar]
- 19.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl. 1):S54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida H, Quehenberger O, Kondratenko N, Green S, Steinberg D. Minimally oxidized low-density lipoprotein increases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arterioscler Thromb Vasc Biol. 1998;18:794–802. doi: 10.1161/01.atv.18.5.794. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Hajjar DP, Febbraio M, Nicholson AC. Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor, CD36. J Biol Chem. 1997;272:21654–9. doi: 10.1074/jbc.272.34.21654. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz G, Grandl M. Endolysosomal phospholipidosis and cytosolic lipid droplet storage and release in macrophages. Biochim Biophys Acta. 2009;1791:524–39. doi: 10.1016/j.bbalip.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Yuan XM, Li W, Olsson AG, Brunk UT. The toxicity to macrophages of oxidized low-density lipoprotein is mediated through lysosomal damage. Atherosclerosis. 1997;133:153–61. doi: 10.1016/s0021-9150(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Yuan XM, Olsson AG, Brunk UT. Uptake of oxidized LDL by macrophages results in partial lysosomal enzyme inactivation and relocation. Arterioscler Thromb Vasc Biol. 1998;18:177–84. doi: 10.1161/01.atv.18.2.177. [DOI] [PubMed] [Google Scholar]
- 25.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–56. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajamaki K, Lappalainen J, Oorni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin EE, Ullery JC, Cox BE, Jerome WG. Aggregated LDL and lipid dispersions induce lysosomal cholesteryl ester accumulation in macrophage foam cells. J Lipid Res. 2005;46:2052–60. doi: 10.1194/jlr.M500059-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Jerome WG. Advanced atherosclerotic foam cell formation has features of an acquired lysosomal storage disorder. Rejuvenation Res. 2006;9:245–55. doi: 10.1089/rej.2006.9.245. [DOI] [PubMed] [Google Scholar]
- 30.Weissmann G. The role of lysosomes in inflammation and disease. Annu Rev Med. 1967;18:97–112. doi: 10.1146/annurev.me.18.020167.000525. [DOI] [PubMed] [Google Scholar]
- 31.Yan C, Lian X, Li Y, et al. Macrophage-specific expression of human lysosomal acid lipase corrects inflammation and pathogenic phenotypes in lal−/− mice. Am J Pathol. 2006;169:916–26. doi: 10.2353/ajpath.2006.051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.