Significance
Individuals subjected to surgical transection of the corpus callosum (“split-brains”) fail to transfer information between the cerebral hemispheres, a condition known as “disconnection syndrome.” On the other hand, subjects born without the callosum (callosal dysgenesis, CD) typically show preserved interhemispheric communication. To clarify this paradox, which has defied neuroscientists for decades, we investigated CD subjects using functional and structural neuroimaging and neuropsychological tests. Results demonstrated the existence of anomalous interhemispheric tracts that cross through the midbrain and ventral forebrain, linking the parietal cortices bilaterally. These findings provide an explanation for the preserved cross-transfer of tactile information between hemispheres in CD. We suggest that this condition is associated with extensive brain rewiring, generating a new circuitry that provides functional compensatory interhemispheric integration.
Keywords: callosal agenesis, callosal plasticity, human connectome
Abstract
Why do humans born without the corpus callosum, the major interhemispheric commissure, lack the disconnection syndrome classically described in callosotomized patients? This paradox was discovered by Nobel laureate Roger Sperry in 1968, and has remained unsolved since then. To tackle the hypothesis that alternative neural pathways could explain this puzzle, we investigated patients with callosal dysgenesis using structural and functional neuroimaging, as well as neuropsychological assessments. We identified two anomalous white-matter tracts by deterministic and probabilistic tractography, and provide supporting resting-state functional neuroimaging and neuropsychological evidence for their functional role in preserved interhemispheric transfer of complex tactile information, such as object recognition. These compensatory pathways connect the homotopic posterior parietal cortical areas (Brodmann areas 39 and surroundings) via the posterior and anterior commissures. We propose that anomalous brain circuitry of callosal dysgenesis is determined by long-distance plasticity, a set of hardware changes occurring in the developing brain after pathological interference. So far unknown, these pathological changes somehow divert growing axons away from the dorsal midline, creating alternative tracts through the ventral forebrain and the dorsal midbrain midline, with partial compensatory effects to the interhemispheric transfer of cortical function.
The human brain connectome is sculpted out of intensive interaction between genes and environment during development (1). Subtle interference in this lengthy interactive process generates individual differences and peculiarities within the normal range of human variation (2). However, more drastic developmental disturbances impose changes that will greatly modify adult brain circuits, with adverse consequences for cognition and behavior (3). This is the case of callosal dysgenesis (CD), a condition well-known for generating gross morphological changes in the brain, and pronounced cognitive and behavioral consequences to the patients (4).
CD differs greatly from callosotomy, however, because the latter is characterized by a clear disconnection syndrome (5), whereas the former displays a considerable degree of interhemispheric communication, despite absence of the corpus callosum (CC) (6, 7). The clinical presentation of CD is highly variable, ranging from subtle, subnormal, cognitive symptoms to severe mental retardation, epilepsy, and somatic deficits (4). Strikingly, however, all cases maintain some degree of interhemispheric transfer (8), possibly mediated by compensatory pathways (9) that would—at least partially—replace the role of the (absent or defective) CC. In fact, some evidence for white-matter circuit reorganization in CD has been produced (10–12), but no rewiring of any kind has been identified in adults subjected to callosal transection (13), although failure to interrupt interhemispheric transfer has been reported after callosotomy in humans and monkeys (14, 15). None of the anomalous tracts in CD, however, has been proven to explain the disconnection paradox observed in these patients, which has remained unsolved since its discovery by Sperry and his collaborators (6, 7).
To approach this puzzle, we followed the hypothesis that the early infliction of the callosal defect would provide the developing brain with a set of anomalous connections that might preserve the crossed transfer of information between cortical areas, and thus assume—at least in part—the functions of the CC. We used multiple neuroimaging techniques and neuropsychological tests on subjects with total (agenesis) and partial CD, and compared them with normal controls. In addition to confirming the presence of the previously known long, aberrant tracts of the white matter, the Probst and the sigmoid bundles (10–12), we describe the trajectory of two so-far unreported homotopic interhemispheric tracts that course through the posterior or anterior commissures, and provide evidence suggestive of their functional role in enabling crossed-transfer of complex tactile function between the hemispheres of these subjects.
Results
Longitudinal and Crossed Heterotopic Abnormal Circuits.
Anatomical MRI showed the typical morphological features of CD, including parallel, enlarged lateral ventricles, downward displacement of the cingulate gyrus, and radial sulci on the medial brain surface. A summary of the main characteristics and experimental approaches for CD subjects are listed in Table S1.
Aberrant fibers forming the Probst bundles were identified by diffusion tensor imaging (DTI/deterministic tractography) (Materials and Methods) in all individuals with CD in our sample (Fig. S1 and Table S1), as described in previous studies (11, 12).
Another abnormal interhemispheric, heterotopic, white-matter pathway (the sigmoid bundle) has also been described in CD (11, 12, 16). DTI tractography analysis showed that this aberrant bundle crosses the midline and connects the occipitoparietal region with the contralateral frontal pole through the rostral remnant of the CC, in subjects with partial CD and a rostral CC remnant (Fig. S2 and Table S1). The improved acquisition and image processing used in the present study showed that the sigmoid bundle is bilateral, rather than unilateral as previously reported (11), albeit strongly asymmetric, the more robust tract connecting the right anterior and the left posterior cerebral cortices (Fig. S2).
Homotopic Abnormal Connections Between the Hemispheres.
The aforementioned abnormal white-matter bundles have been described as morphological features of CD, with aberrant, highly heterotopic trajectories. CD subjects, however, reportedly exhibit normal performance in many interhemispheric functions (4, 6–8), for which a morphological counterpart is still lacking. We searched for direct evidence of alternative interhemispheric pathways that could explain why individuals with CD do not show the complete disconnection syndrome observed in patients who have undergone surgical callosotomy or suffered callosal damage in adulthood. Using DTI and high angular-resolution diffusion imaging (HARDI), as well as tractography [fiber assignment by continuous tracking (FACT) and probabilistic tractography], combined with resting-state functional MRI (rs-fMRI) (Materials and Methods and Supporting Information), we identified two novel alternative commissural pathways (Fig. 1) and revealed their functional surrogates in CD.
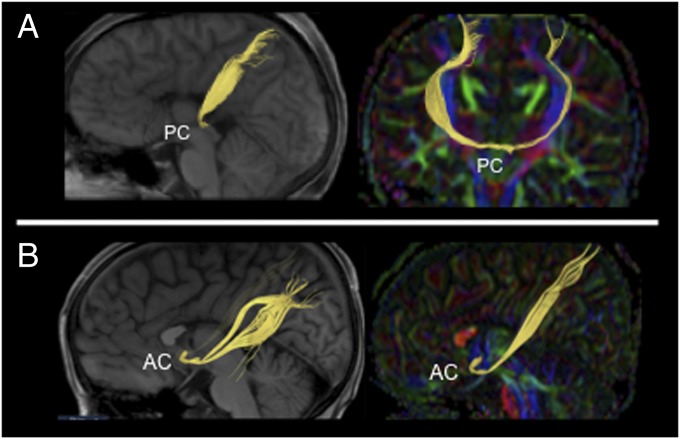
Fig. 1.
DTI deterministic tractography shows anomalous interhemispheric connections via posterior (PC) and anterior (AC) commissures in CD. (A and B) DTI-tractography on T1 sagittal/parasagittal plane (Left), and on coronal or sagittal color-coded FA maps (Right), showing the interhemispheric midbrain bundle (A) and the interhemispheric ventral forebrain bundle (B) in yellow.
Conventional anatomical images did not show any morphological changes in the anterior or posterior commissures in CD subjects compared with controls. Comparisons of color-coded fractional anisotropy (FA) DTI maps, however, showed a more organized (higher FA) posterior commissure in CD subjects, compared with controls. In addition, DTI and FACT tractography revealed an aberrant interhemispheric tract crossing the midline at the level of the posterior commissure (heretofore referred to as the “aberrant midbrain bundle”) in four patients with CD (Fig. 1A, Fig. S3 A–D, and Table S1), and another bundle crossing through the anterior commissure in one CD patient (referred to as the “aberrant ventral forebrain bundle”) (Fig. 1B, Fig. S3E, and Table S1). Both bundles were shown to interconnect the dorsolateral parietal cortices homotopically. In addition, it is remarkable that they follow a trajectory parallel and adjacent to the corticospinal tract, as shown in Fig. S3 A–D.
rs-fMRI data (using independent component analyses) supported these tractographic findings by showing that CD brains exhibited a robust, bilaterally symmetric functional connectivity corresponding to the posterior parietal cortex component of the default mode network (DMN) (Fig. 2A), in close resemblance to controls (Fig. 2B). No differences between patients and controls were found when a parietal region of interest (ROI)-to-ROI functional connectivity analysis was performed (Fig. S4). Moreover, this parietal component closely overlapped with the homotopic regions of the posterior parietal cortex that were shown to be structurally connected by the aberrant midbrain bundle (Fig. 1A and Fig. S3 A–D) or by the aberrant ventral forebrain bundle (Fig. 1B and Fig. S3E).
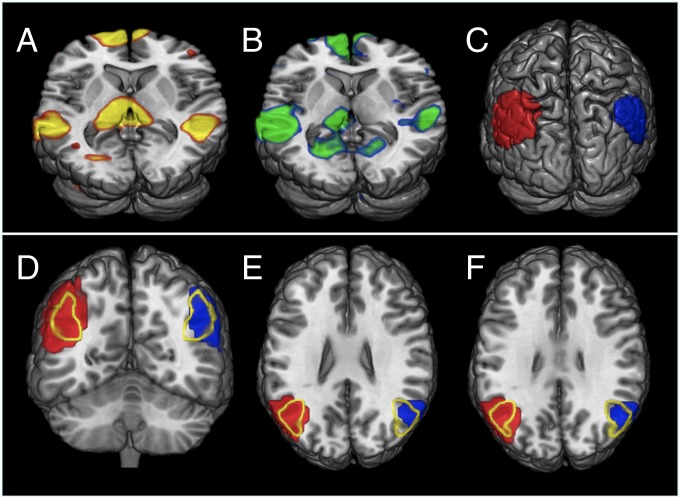
Fig. 2.
rs-fMRI shows functional connectivity between the left and right parietal cortex in CD. DMN rs-fMRI statistical maps in CD (A) and control (B) groups. (C) Surface view of bilateral parietal DMN ROIs obtained from pooled data from both groups. (D–F) Views of the same DMN ROIs, overlapped with BA39 [outlined in yellow; based on Brodmann template, MRIcron V.6 (http://www.cabiatl.com/mricro/mricro/lesion.html#brod)]. Colors in A and B are arbitrary. Red and blue in C–F denote left and right hemisphere resting state connectivity.
Based on these bilateral parietal components of the DMN obtained from both groups, ROIs (Fig. 2C) were then defined and used for further probabilistic tractography analysis in CD and control participants. These parietal regions largely correspond to the angular gyrus [Brodmann’s area 39 (BA39)] and its immediate surroundings, as outlined in Fig. 2 D–F.
DTI and probabilistic tractography showed an abnormal, robust interhemispheric bundle in CD, crossing the midline at the topography of the posterior commissure (close to the mesodiencephalic border) (Fig. 3 A, B, E, and F), overlapping with the bundle revealed by FACT previous analysis in the same group of CD subjects (Fig. 1A and Fig. S3 A–D). Moreover, probabilistic tractography also showed a similar abnormal interhemispheric tract crossing at the level of the anterior commissure and connecting both parietal lobes in one subject with CD (Fig. 3C), replicating the results obtained using FACT (Fig. 1B).
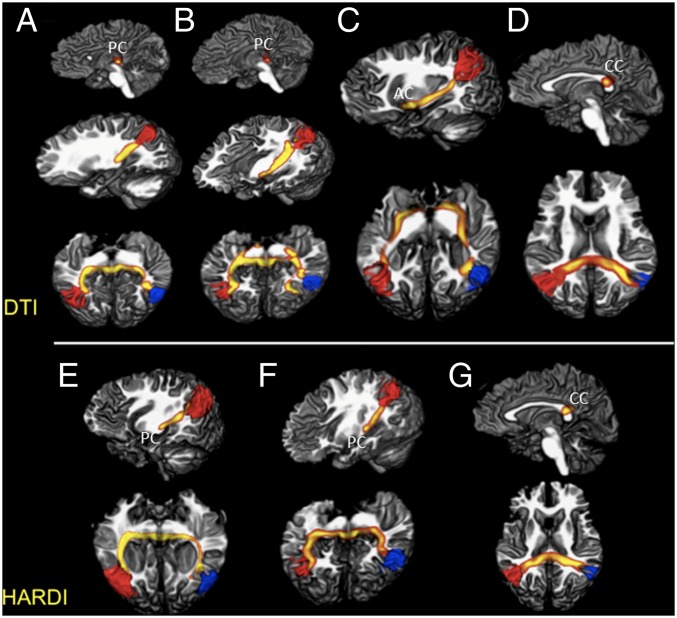
Fig. 3.
DTI and HARDI probabilistic tractography reveal anomalous interhemispheric connections via posterior and anterior commissures. (A–D) DTI analysis. Aberrant midbrain (A and B) and ventral forebrain (C) bundles reconstructed from CD individuals, crossing through the posterior (PC) and the anterior (AC) commissures. Seed ROIs positioned at the posterior parietal cortex as defined by rs-fMRI. (D) Control individual with similarly defined ROIs, showing the expected structural interhemispheric connectivity through the CC. (E–G) HARDI analysis. (E and F) Replication of the aberrant midbrain tracts in patients with CD, both with the posterior commissure bundle. (G) Connectivity analysis performed in a control, showing the expected interhemispheric connections through the CC.
When an equivalent probabilistic tractography analysis was performed in controls, structural interhemispheric connectivity of the parietal lobes was shown through the CC in all individuals, as expected (Fig. 3 D and G), with no evidence for additional pathways.
The specificity of these findings was corroborated by further analyses using lenient thresholds in controls, to increase the probability of appearance of artifactual tracts that might be confounded with true connections. In addition, we ascertained that our diffusion acquisition data (using 32 diffusion directions) provided a successful estimate of two crossing fibers per voxel with reliability in many voxels (Fig. S5). We also scanned two CD patients and one control using HARDI (128 diffusion-sensitizing directions), and found a consistent estimation of three crossing fibers per voxel (Fig. S6). Using the HARDI dataset, probabilistic tractography was performed as described above (based on rs-fMRI ROIs), confirming the findings described with the original diffusion datasets for both patients and controls (Fig. 3 E–G).
One additional key question needed to be faced: To what extent would the structurally abnormal commissural pathways observed in CD be related to the degree of functional integration across hemispheres as indexed by rs-fMRI? This question was tested by correlating the robustness of the abnormal interhemispheric bundles with the degree of rs-fMRI connectivity across hemispheres at the level of the parietal lobes. Strikingly, the structural connectivity distribution values were significantly correlated with the functional connectivity values in CD patients (Fig. 4A), providing further support for the existence of the aberrant interhemispheric bundles and suggesting a direct association between their structural robustness and rs-fMRI indices of interhemispheric functional connectivity in CD patients.
Fig. 4.
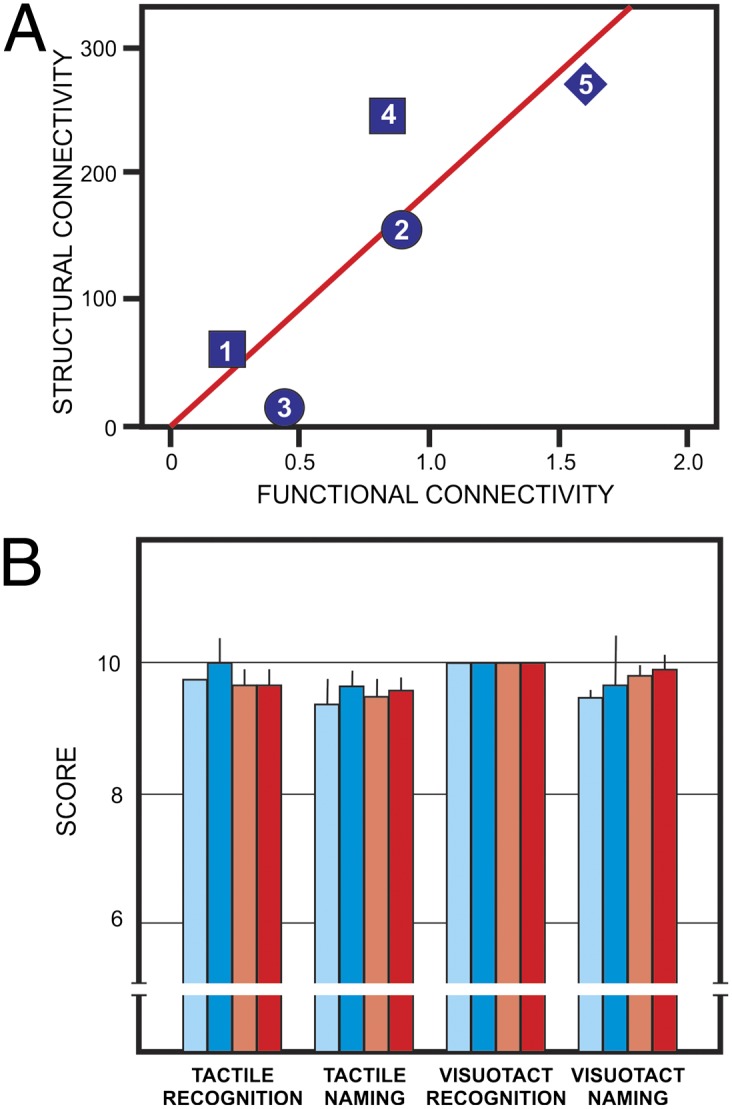
Functional and behavioral correlates of structural connectivity. (A) Statistical correlation between structural connectivity (diffusion connectivity values of the aberrant bundles) and rs-fMRI functional connectivity between hemispheres Fisher z-transformed correlation coefficients; r = 0.858; P = 0.03 (one-tailed). One of the patients (CD06) was not included because rs-fMRI data were not available. Numbered symbols denote the different CD morphological phenotypes: squares for partial dysgenesis, circles for agenesis, diamonds for hypoplasia. The numbers correspond to patient numbers in Table S1. (B) Tactile performance of CD subjects (blue bars) compared with neurotypic controls (red bars). Light bars: left hand; dark bars: right hand. Mean + SD. Performance with left vs. right hand was statistically nonsignificant on all tests, as well as that of CD vs. control subjects.
Crossed Transfer of Tactile Function in CD.
The above findings strongly suggest that alternative interhemispheric pathways develop in CD subjects, connecting the dorsolateral parietal cortices bilaterally. Because the superior and inferior parietal lobules (BA7, BA39, BA40) are known to play a key role in complex tactile information processing (17, 18), we tested for interhemispheric transfer in CD individuals using neuropsychological tests that probe cross-hemispheric integration of tactile object recognition and naming with each hand individually (Fig. S7; see Materials and Methods and Supporting Information for details).
There were no significant differences between control and CD subjects on these tests (Fig. 4B). In addition, CD subjects and controls performed equally on ideomotor praxis, writing, and handedness assessments (Supporting Information and Table S2). This behavioral performance of CD subjects confirms that they developed compensatory ways of sustaining the crossed transfer of tactile and visuotactile abilities at the level of neurotypic subjects. The finding fits with the functional imaging evidence, involving BA39 and the neighboring cortex in interhemispheric transfer through the abnormal bundles in CD, and suggesting that they may have acquired the compensatory role for maintaining these interhemispheric functions.
Discussion
Together, the longtime known Probst bundle (10), the more recently described sigmoid bundle (11), and the two novel aberrant midbrain and ventral forebrain tracts here reported make up the fundamental brain circuitry of CD subjects (12, 19). We focused on the abnormal commissural interhemispheric bundles and showed that they link structurally and functionally the two homotopically synchronized posterior parietal areas (primarily BA39), cortical sectors involved in tactile object recognition (17, 18, 20). Finally, we tested for the crossed transfer of this cognitive ability, confirming that CD subjects perform as proficiently as controls concerning interhemispheric transfer of tactile cognition. This pattern suggests that although deficits may be caused by heterotopic aberrant connections, the alternative, homotopic commissural tracts may compose, at least in part, the set of compensatory pathways hypothesized to provide some of the preserved interhemispheric communication of dyscallosals since Sperry’s description of their condition (4, 6–9, 21).
The formation of anomalous tracts after developmental insults has been inferred by many authors out of subtle changes documented in the white matter of human subjects with different neuropsychological disorders (22), such as schizophrenia, autism, and epilepsy. To our knowledge, however, this is the first time they are morphologically described, proved functional, and associated with specific cognitive abilities. This anomalous circuitry is conceptualized as a result of long-distance plasticity, a property of the developing nervous system, which is different from the better known microstructural changes that take place at the cellular or synaptic level (23). For long-distance plasticity to occur, an early in utero interference would have to be imposed on pivotal cellular and molecular mechanisms of axon growth and guidance, so that entirely new pathways and connections are established during development. In accordance with this possibility, some human disorders of axon guidance have been described (24), and their genetic and molecular determinants have begun to be revealed.
In the case of the CC, many of these mechanisms are known (see refs. 25–27 and references therein). In short, callosal neurons are specified early during embryogenesis under control of specific transcription factors that supposedly determine their future connectivity. Callosal axons emerge during radial migration of pyramidal neurons into the cortex, grow toward the white matter, and fasciculate over preexisting fiber tracts. Upon reaching the white matter, callosal pioneers from the cingulate cortex deflect medially toward the midline, whereas followers from dorsolateral cortical sectors fasciculate onto them after antipodal bifurcation, and supposedly preserve their medial branch thereafter, while pruning the exuberant lateral branch that grew toward the internal capsule.
Growth cones at the tip of elongating axons are sensitive to different signals that attract or guide them to the midplane before crossing, and repel them as long as they reach the other hemisphere (25). These signals are often similar to those produced in other strategic points in the developing brain, such as the dorsal midplane for callosal and hippocampal axons, the ventral midplane for anterior commissure fibers, and the internal capsule for different descending and ascending pathways (26). In addition, callosal fibers tend to fasciculate onto themselves and other fiber tracts, driven by a Wnt receptor expressed ubiquitously in the brain, and guided by interactive contacts with adhesion molecules on their surface (27).
It is conceivable, therefore, that in CD a so-far unknown disturbance inflicted to one or more of these guidance cues at the dorsal forebrain midline could divert callosal axons longitudinally, forcing them to fasciculate onto the cingulum fibers and form the Probst bundles bilaterally (Fig. S1); in addition, some of the early callosal axons that find a remnant of the CC rostrally would succeed crossing into the opposite hemisphere, but head caudally in the wrong direction, forming the sigmoid bundle (Fig. S2). These phenomena are compatible with the recent finding that aberrant bundles can be seen in the brain of CD human fetuses as early as 20 gestation weeks (28). Furthermore, in these individuals, dorsolateral callosal neurons might conceivably have some of their bifurcating medial branches pruned because of unsuccessful guidance, whereas the lateral branches would then navigate toward and into the internal and external capsules, attracted by the same signals expressed at the dorsal midline. The neurons would then fasciculate onto descending corticospinal fibers, until diverting again toward the midline ventrally at the posterior commissure (Fig. 1A and Fig. S3 A–D). Some of these lateral fibers would proceed further laterally along the subcortical white matter toward the external capsule, and be attracted to the ventral forebrain midline to follow the anterior commissure pathway (Fig. 1B and Fig. S3E). Both fascicles growing laterally would form the interhemispheric bundles herein described, connecting homotopic sectors of the angular gyrus, in the inferior parietal lobule.
Although an interhemispheric bundle through the anterior commissure has long been hypothesized by previous investigators (4, 8, 9), an alternative pathway through the posterior commissure has remained relatively unsuspected. The anterior commissure is a prosencephalic tract connecting the rostral sectors of the temporal cortex, but the posterior commissure is an exclusively subcortical, mesodiencephalic bundle (29) that makes direct connections with the nucleus of Darkschewitsch and the red nucleus, as well as with the habenular nuclei.
It is possible that other developing cortical fibers in CD (e.g., from the visual, extrastriate cortex and auditory, superior temporal gyrus) may also take the way through the anterior commissure; it is conceivable, as well, that visual and auditory fibers may cross abnormally through the posterior commissure or even the intertectal commissure. These possibilities would still be compatible with the reported evidence of visual and auditory compensation in CD (6–9).
We hypothesize, along with Owen et al. (19), that the reported abnormal tracts, and perhaps additional ones yet to be described in the white matter of CD patients, result from abnormal molecular mechanisms taking place in the natural course of events in ontogenesis, leading to an anomalous network of connections in these subjects.
Knowing the extensive, distinct connection network in CD, it becomes important to establish whether or not they are functional. To approach this issue, we have used rs-fMRI. Results showed: (i) bilaterally symmetric resting state functional connectivity between homotopic regions of the posterior parietal cortex (Fig. 2), compatible with the fields linked by the interhemispheric midbrain and ventral forebrain bundles (Fig. 3); and (ii) significant correlation between the strength of structural and functional connectivities (Fig. 4A).
These findings indicate that the anomalous fiber tracts of subjects with CD become functional during development, establishing active contact between homotopic dorsolateral parietal cortices of both hemispheres through the interhemispheric bundles. It is noticeable that the functional connectivity found in homotopic parietal sectors of the cerebral cortex involves the angular gyrus bilaterally (BA39 and surroundings) (Figs. 2 and 3), which plays a key role in tactile object recognition (18).
One important limitation of the techniques used in this work is that they do not reveal precisely which functions are conveyed by the described abnormal tracts. In addition, there is still some ongoing controversy concerning the role of CC in promoting and maintaining crossed functional connectivity between the hemispheres, and whether other commissures may contribute as well to this coupling, as reported for nonhuman primates (14, 15). To circumvent these limitations, we studied the neuropsychological performance of CD subjects, focusing on those functions associated to the dorsolateral parietal cortices that are typically connected by the intercortical bundles, because the functional properties of these regions have been extensively explored (17, 18).
The dorsolateral parietal cortex (BA39, BA40, BA7, and others) has been associated with tactile object recognition (17, 18). When object recognition is expressed by verbal language, it usually requires contralateral (left hemisphere) processing when the right hand is tested, and crossed interhemispheric transfer when the left hand is tested. It has been noticed for a long time that, surprisingly, this kind of tactile ability is preserved in subjects lacking the posterior body of the CC since birth (30), which is the case of our patients. Consistently, surgical transection of this callosal region in adults causes failure of cross-linking the parietal, somatosensory cortices, thus leading to impaired transfer of tactile information across hemispheres (31). By testing tactile object recognition—verbally and nonverbally—in our subjects, we tackled the hypothesis that the alternative intercortical bundles through the posterior and anterior commissures would provide the interhemispheric communication necessary for normal crossed processing of tactile information. In our study, we have not used neuropsychological tests sensitive to response times or crossed-uncrossed differences. However, we speculate that the higher crossed-uncrossed differences of CD patients previously reported (32) could be explained, in light of our findings, by the much longer trajectories of the interhemispheric aberrant tracts here reported, compared with the shorter distances of callosal connections in normotypic subjects.
Results showed that indeed dyscallosal subjects were proficient in recognizing and naming objects by active touch with either hand (Fig. 4B), with and without visual assistance, with and without verbal expression. Therefore, because they lack the posterior body of the CC, we concluded that compensatory plasticity took place in their brains, most probably by recruiting the functional interhemispheric bundles formed along development, shown to connect homotopically the dorsolateral parietal regions involved in these functions. It cannot be excluded, however, that other tracts may exist and compensate for the transfer of other sensory modalities, or, alternatively, that subcortical decussations may play a role in these compensatory phenomena, as suggested by Sperry himself (6, 7).
In sum, using multiple morphological and functional approaches, we described some of the anomalous tracts that constitute brain circuitry of subjects with CD, establishing that they are functionally active, and that some of them may provide the basis for the normal performance of these subjects on the interhemispheric transfer of tactile object recognition and naming. These results help to solve the Sperry paradox, a long-standing puzzle in the literature (4, 6–9, 21), explaining the preservation of interhemispheric communication in CD by the function of alternative intercortical axonal pathways crossing through the anterior and posterior commissures. We propose that the anomalous circuitry in CD is a result of long-distance plasticity, originating by early interference on developmental mechanisms that divert growing axons through anomalous pathways, some of which exert compensatory effects, as is the case of the interhemispheric midbrain and ventral forebrain bundles.
Materials and Methods
A detailed account of the experiments and techniques used in this work is provided in Supporting Information.
Six patients with CD aged 6–33 y (three males), with no associated malformations of the central nervous system, were included (Table S1): two had callosal agenesis (complete lack of the CC), two showed callosal hypoplasia, and two had partial CD (lack of the body and splenium, with a small rostral remnant). Despite the anatomopathological difference between the cases, most (four of six) clearly lacked the posterior callosum and, for the purpose of somatosensory crossed integration, were considered a homogeneous group. A total of 12 individuals with no evidence of neurological disease served as neurotypic controls. All subjects provided informed written consent to participate of the study. Procedures were approved by the ethics committee of the D’Or Institute for Research and Education (Rio de Janeiro, Brazil), and were performed according to international regulations (33).
Neuropsychological Testing.
Background neuropsychological tests were used in all CD subjects and controls for praxis and writing, daily functional capacity, severe impairment of memory, language, motor performance, conceptualization, and general knowledge, as well as handedness and general intelligence (Tables S1 and S2). The Supporting Information provides details on the behavioral protocols.
Tactile and visuotactile recognition and naming tests were applied to specifically evaluate interhemispheric tactile abilities of patients and controls (8). For each hand, 10 different small objects, previously selected from a larger pool of common objects used in a pilot study with normal children, were used to perform this test (Fig. S7A). In the Tactile Naming Test the subjects were asked to name each object by active touch inside a wooden box and without the aid of vision. In case of failure, a card with the pictures of all test objects was shown and he or she was asked to point to the one they felt (Fig. S7B) with the hand opposite to that used in active touch (visuotactile recognition). In addition, when an object was correctly named in the first trial, it was assumed that the participant was naturally able to also recognize it by touch and the trial was scored as correct for both conditions (tactile recognition and naming). The same scoring procedure was adopted for visuotactile recognition and visuotactile naming. Performance of patients and controls were compared. Statistical analyses are detailed in Supporting Information.
Image Acquisition and Processing.
Acquisitions were conducted on an Achieva 3T Philips MR scanner in CD patients and in six age- and sex-matched subjects of the control group. Image protocol was composed by DTI, HARDI, rs-fMRI, and anatomical sequences, including a high resolution T1-weighted volumetric sequence [TR/TE = 7.2/3.4 s; voxel size = 1 mm3; field of view (FOV) = 240 mm; 170 sagittal slices]. An eight-channel SENSE head coil was used. For each subject, two diffusion-weighted images were acquired and averaged, using a single-shot, spin-echo, echo-planar sequence (TR/TE = 9,500/60 ms; voxel size = 2 mm3; FOV = 232 mm; 60 axial slices; NSA = 2; scan time = 12 min), with diffusion sensitization gradients applied in 32 noncollinear directions (b factor = 1,000 s/mm2). HARDI images were acquired in two patients and one control using a single-shot, spin-echo, echo-planar sequence, (TR/TE = 12,126/70 ms; voxel size = 2 mm3; FOV = 224 mm; 60 axial slices; scan time = 26 min), with diffusion sensitization gradients applied in 128 directions (b factor = 1,500 s/mm2), through Persistent Angular Structure implementation (34), computing the probability density function of particle displacement on each voxel, lying on a 3D sphere in Fourier space. Two nondiffusion volumes were also acquired (b factor = 0) and averaged. Functional sequences consisted of single-shot, fast field echo, echo-planar imaging (EPI) (TR/TE = 2,000/22 ms; flip angle 90°; FOV = 240 mm; voxel size = 3 mm2; 36 contiguous axial slices; 180 volumes; scan time = 6 min), including five “dummy” scans for signal stabilization purpose. Before image analysis, subjects’ datasets were anonimized, randomized across the subjects and groups, and images were visually inspected for artifacts.
Data processing was performed using FMRIB Software Library (FSL) (35), DtiStudio (36), PRIDE software (Philips Research Integrated Development Environment software, PRIDE research platform), and CONN Toolbox framework (Functional Connectivity Toolbox v.13.0, http://web.mit.edu/swg/software.htm).
Diffusion tensor tractography (fiber-tracking).
Fiber-tracking was performed in DtiStudio or PRIDE software using the FACT method (37). The diffusion tensor for each voxel was calculated based on the eigenvectors (v1, v2, v3) and eigenvalues (λ1, λ2, λ3) using multivariate fitting and diagonalization (36, 38). Tracking was initiated at an FA value of 0.2 and was terminated when FA fell below 0.2 or the angle between two adjacent eigenvectors was greater than 40°. In CD subjects, tractography was performed to investigate the fiber topography of the cingulum, Probst, sigmoid, corticospinal, and interhemispheric abnormal bundles (11, 39). The connectivity of the latter bundles through anterior and posterior commissures was tracked using a multiple ROI approach, such as placing a bilateral ROI in parasagittal slices in the topography of the anterior or posterior commissures in CD subjects and controls. ROIs were placed in T1 and T2 images and loaded on the FA maps.
rs-fMRI.
Probabilistic independent component analysis (ICA) was used to decompose the rs-fMRI time-course data into different temporal and spatial components, according to the standard FEAT (fMRI Expert Analysis Tool) (40) and MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) (40, 41) pipeline in FSL software tools. For each subject, all nonbrain voxels were removed and a mean EPI brain volume was extracted (42). These EPI brain mean volumes were then used to create binary brain masks, which were registered to each individual high-resolution anatomical brain volumes. Each subject’s anatomical data were registered to the standard stereotactic Montreal Neurological Institute (MNI) anatomical template, using 12° nonlinear transformations for optimal accuracy. Finally, the generated transformation matrix was applied to functional data to convert the rs-fMRI volumes into MNI space (43). For ICA-based analysis, a multisubject (CD and controls) temporal concatenation of the resting-state blood oxygen level-dependent (BOLD) signals was used according to the MELODIC pipeline (44), to find resting-state networks from multisubject rs-fMRI datasets (variance-normalized time courses). Before the ICA estimation and to avoid overfitting, the number of components from the concatenated data were calculated using Bayesian estimators (44) and then reduced using principal components analysis.
Subsequently, the spatial component from the estimated ICA group analysis, which describes the most common effect to all subjects (CD and controls) was selected as an a priori ROI. This selected spatial component represents the common regions found in the DMN. A thresholded binary mask was used to discriminate the DMN regions into specific binary mask files and the bilateral parietal components of the DMN (right and left hemisphere) were selected as ROIs. These ROIs were then transformed into each subject’s native space by applying an inverse transformation native-to-MNI matrix (or MNI-to-subject matrices) (45) and used for further analyses.
Based on the DMN parietal ROIs from ICA fMRI analysis, the average temporal BOLD signal for each ROI was extracted and the functional connectivity measures between these two ROIs were obtained. ROI-to-ROI connectivity matrices were computed for each subject using bivariate correlation (Fisher z-transformed correlation coefficients) as a measure of “total” functional connectivity between the two ROIs. In the group analysis, all connectivity matrices from all subjects were entered into a general linear model for statistics. Contrasts of interest were tested by comparing the patterns of functional connectivity between groups. The results were thresholded at P < 0.05, corrected for multiple comparisons across ROIs, using analysis-wise method to control for false positives (false discovery rate). In addition, these parietal ROIs were then finally used as seeds for the probabilistic tractography approach as described below.
Probabilistic tractography.
The DTI probabilistic tractography was performed using DTIFit 2.0, FDT [FMRIB’s Diffusion Toolbox (46)]. After the FA maps were calculated from the eigenvalues, color-coded maps were generated from the FA values and three vector elements of v1 to visualize the white-matter tract orientation. FA images were brain-extracted (46) and registered to a common space (MNI 152), using constrained nonlinear registration [Image Registration Toolkit (47)]. The fiber estimation per voxel was then verified [Markov chain Monte Carlo sampling technique (48)] and, as shown in Fig. S5, the 32-diffusion direction acquisition could successfully support two fibers per voxel with good reliability.
Original individual FA maps were registered to the FA template in the MNI space (FMRIB58 FA template), using a nonlinear registration tool [FNIRT, FSL (43)]. The probabilistic tractography was performed (Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques, modeling for crossing fibers, PROBTRACKX, FSL) in the native space of each subject (48). To investigate the interhemispheric connectivity, a two-ROI-approach probabilistic tractography was performed and results were compared among CD subjects and controls. For the two-ROIs approach, the bilateral parietal ROIs (obtained from the DMN functional network as defined above) were used as seeds in each subject.
Next, to directly correlate structural and functional connectivity in CD, the connectivity probabilistic streamlines distribution values between two ROIs, obtained from probabilistic tractography, were correlated to the rs-fMRI Fisher z-transformed correlation coefficients (Pearson correlation).
HARDI processing.
To best ensure that differences between groups could not be caused by missing major populations of fiber crossings, three fibers were modeled in each voxel and the automatic relevance determination (48) parameter was set to 0.5. To visualize voxels where multiple fibers are supported, a threshold f-value of 0.05 was used. As shown in Fig. S6, the HARDI acquisition (128 diffusion-encoding directions) could successfully estimate three-way crossing fibers per voxel with good reliability. Confirmatory analysis of interhemispheric connectivity was performed using HARDI probabilistic tractography with a two-ROI approach in two rescanned CD individuals and one control, for comparison. As described above, the seed ROIs were based on DMN functional parietal network of each participant.
Supplementary Material
Acknowledgments
We thank Debora O. Lima for coordination of patient visits, and Marcia Triunfol (at Publicase) for help with the manuscript. The project was supported by grants and fellowships provided by the Brazilian Council for Development of Science and Technology, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Foundation of the Brazilian Ministry of Education, and the Rio de Janeiro Foundation for the Support of Research. F.T.-M., R.L., and J.M. are members of the National Institute of Translational Neuroscience, Brazilian Ministry of Science, Technology and Innovation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400806111/-/DCSupplemental.
References
- 1.Hagmann P, Grant PE, Fair DA. MR connectomics: A conceptual framework for studying the developing brain. Front Syst Neurosci. 2012;6:43. doi: 10.3389/fnsys.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Short SJ, et al. Associations between white matter microstructure and infants’ working memory. Neuroimage. 2013;64:156–166. doi: 10.1016/j.neuroimage.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staudt M. Reorganization after pre- and perinatal brain lesions. J Anat. 2010;217(4):469–474. doi: 10.1111/j.1469-7580.2010.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul LK, et al. Agenesis of the corpus callosum: Genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8(4):287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- 5.Gazzaniga MS, Bogen JE, Sperry RW. Some functional effects of sectioning the cerebral commissures in man. Proc Natl Acad Sci USA. 1962;48:1765–1769. doi: 10.1073/pnas.48.10.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperry RW. Plasticity of neural maturation. Dev Biol. 1968;(suppl 2):306–327. [Google Scholar]
- 7.Sperry RW. In: in Early Experience in Visual Information Processing in Perceptual and Reading Disorders. Young FA, Lindsley DB, editors. New York: National Academy of Sciences; 1970. pp. 167–178. [Google Scholar]
- 8.Lassonde M, Sauerwein H, Chicoine AJ, Geoffroy G. Absence of disconnexion syndrome in callosal agenesis and early callosotomy: Brain reorganization or lack of structural specificity during ontogeny? Neuropsychologia. 1991;29(6):481–495. doi: 10.1016/0028-3932(91)90006-t. [DOI] [PubMed] [Google Scholar]
- 9.Barr MS, Corballis MC. The role of the anterior commissure in callosal agenesis. Neuropsychology. 2002;16(4):459–471. [PubMed] [Google Scholar]
- 10.Probst M. Ueber den Blau des balkenlosen Grosshirns, sowie uber Mikrogirie un Heterotopie der grauen substanz. Arch Psychiatr Nervenkr. 1901;34:709–786. [Google Scholar]
- 11.Tovar-Moll F, et al. Neuroplasticity in human callosal dysgenesis: A diffusion tensor imaging study. Cereb Cortex. 2007;17(3):531–541. doi: 10.1093/cercor/bhj178. [DOI] [PubMed] [Google Scholar]
- 12.Wahl M, et al. Variability of homotopic and heterotopic callosal connectivity in partial agenesis of the corpus callosum: A 3T diffusion tensor imaging and Q-ball tractography study. AJNR Am J Neuroradiol. 2009;30(2):282–289. doi: 10.3174/ajnr.A1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazzaniga MS. Forty-five years of split-brain research and still going strong. Nat Rev Neurosci. 2005;6(8):653–659. doi: 10.1038/nrn1723. [DOI] [PubMed] [Google Scholar]
- 14.Uddin LQ, et al. Residual functional connectivity in the split-brain revealed with resting-state functional MRI. Neuroreport. 2008;19(7):703–709. doi: 10.1097/WNR.0b013e3282fb8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Reilly JX, et al. Causal effect of disconnection lesions on interhemispheric functional connectivity in rhesus monkeys. Proc Natl Acad Sci USA. 2013;110(34):13982–13987. doi: 10.1073/pnas.1305062110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SK, et al. Diffusion tensor MR imaging visualizes the altered hemispheric fiber connection in callosal dysgenesis. AJNR Am J Neuroradiol. 2004;25(1):25–28. [PMC free article] [PubMed] [Google Scholar]
- 17.Reed CL, Shoham S, Halgren E. Neural substrates of tactile object recognition: An fMRI study. Hum Brain Mapp. 2004;21(4):236–246. doi: 10.1002/hbm.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed CL, Klatzky RL, Halgren E. What vs. where in touch: An fMRI study. Neuroimage. 2005;25(3):718–726. doi: 10.1016/j.neuroimage.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 19.Owen JP, et al. The structural connectome of the human brain in agenesis of the corpus callosum. Neuroimage. 2013;70:340–355. doi: 10.1016/j.neuroimage.2012.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyszka JM, Kennedy DP, Adolphs R, Paul LK. Intact bilateral resting-state networks in the absence of the corpus callosum. J Neurosci. 2011;31(42):15154–15162. doi: 10.1523/JNEUROSCI.1453-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duquette M, Rainville P, Alary F, Lassonde M, Lepore F. Ipsilateral cortical representation of tactile and painful information in acallosal and callosotomized subjects. Neuropsychologia. 2008;46(8):2274–2279. doi: 10.1016/j.neuropsychologia.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Shizukuishi T, Abe O, Aoki S. Diffusion tensor imaging analysis for psychiatric disorders. Magn Reson Med Sci. 2013;12(3):153–159. doi: 10.2463/mrms.2012-0082. [DOI] [PubMed] [Google Scholar]
- 23.Klug A, et al. How do short-term changes at synapses fine-tune information processing? J Neurosci. 2012;32(41):14058–14063. doi: 10.1523/JNEUROSCI.3348-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nugent AA, Kolpak AL, Engle EC. Human disorders of axon guidance. Curr Opin Neurobiol. 2012;22(5):837–843. doi: 10.1016/j.conb.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chédotal A, Richards LJ. Wiring the brain: The biology of neuronal guidance. Cold Spring Harb Perspect Biol. 2010;2(6):a001917. doi: 10.1101/cshperspect.a001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant E, Hoerder-Suabedissen A, Molnár Z. Development of the corticothalamic projections. Front Neurosci. 2012;6:53. doi: 10.3389/fnins.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalil K, Li L, Hutchins BI. Signaling mechanisms in cortical axon growth, guidance, and branching. Front Neuroanat. 2011;5:62. doi: 10.3389/fnana.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasprian G, et al. Assessing prenatal white matter connectivity in commissural agenesis. Brain. 2013;136(1):168–179. doi: 10.1093/brain/aws332. [DOI] [PubMed] [Google Scholar]
- 29.Keene MFL. The connexions of the posterior commissure. J Anat. 1938;72(Pt 4):488–501. [PMC free article] [PubMed] [Google Scholar]
- 30.Dennis M. Impaired sensory and motor differentiation with corpus callosum agenesis: A lack of callosal inhibition during ontogeny? Neuropsychologia. 1976;14(4):455–469. doi: 10.1016/0028-3932(76)90074-9. [DOI] [PubMed] [Google Scholar]
- 31.Fabri M, et al. Contribution of posterior corpus callosum to the interhemispheric transfer of tactile information. Brain Res Cogn Brain Res. 2005;24(1):73–80. doi: 10.1016/j.cogbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Lassonde M, Sauerwein HC, Lepore F. In: in The Parallel Brain. Zaidel E, Iacoboni M, editors. Cambridge: MIT Press; 2003. pp. 357–369. [Google Scholar]
- 33. (2000) World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 284(23):3043–3045. [PubMed]
- 34.Jansons KM, Alexander DC. Persistent angular structure: New insights from diffusion MRI data. Dummy version. Inf Process Med Imaging. 2003;18:672–683. doi: 10.1007/978-3-540-45087-0_56. [DOI] [PubMed] [Google Scholar]
- 35.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81(2):106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Pajevic S, Pierpaoli C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: Application to white matter fiber tract mapping in the human brain. Magn Reson Med. 1999;42(3):526–540. [PubMed] [Google Scholar]
- 39.Wakana S, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 41.Hyvärinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. 1999;10(3):626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- 42.Tipping ME, Bishop CM. Probabilistic principal component analysis. J R Stat Soc, B. 1999;61(Part 3):611–612. [Google Scholar]
- 43.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 44.Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage. 2005;25(1):294–311. doi: 10.1016/j.neuroimage.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 45.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behrens TEJ, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 47.Rueckert D, et al. Nonrigid registration using free-form deformations: Application to breast MR images. IEEE Trans Med Imaging. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 48.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.