Significance
Bullying is a common childhood experience that affects children at all income levels and racial/ethnic groups. Being a bully victim has long-term adverse consequences on physical and mental health and financial functioning, but bullies themselves display few ill effects. Here, we show that victims suffer from greater increases in low-grade systemic inflammation from childhood to young adulthood than are seen in others. In contrast, bullies showed lower increases in inflammation into adulthood compared with those uninvolved in bullying. Elevated systemic low-grade inflammation is a mechanism by which this common childhood social adversity may get under the skin to affect adult health functioning, even many years later.
Keywords: stress, social functioning, longitudinal, risk factor, epidemiology
Abstract
Bullying is a common childhood experience that involves repeated mistreatment to improve or maintain one’s status. Victims display long-term social, psychological, and health consequences, whereas bullies display minimal ill effects. The aim of this study is to test how this adverse social experience is biologically embedded to affect short- or long-term levels of C-reactive protein (CRP), a marker of low-grade systemic inflammation. The prospective population-based Great Smoky Mountains Study (n = 1,420), with up to nine waves of data per subject, was used, covering childhood/adolescence (ages 9–16) and young adulthood (ages 19 and 21). Structured interviews were used to assess bullying involvement and relevant covariates at all childhood/adolescent observations. Blood spots were collected at each observation and assayed for CRP levels. During childhood and adolescence, the number of waves at which the child was bullied predicted increasing levels of CRP. Although CRP levels rose for all participants from childhood into adulthood, being bullied predicted greater increases in CRP levels, whereas bullying others predicted lower increases in CRP compared with those uninvolved in bullying. This pattern was robust, controlling for body mass index, substance use, physical and mental health status, and exposures to other childhood psychosocial adversities. A child’s role in bullying may serve as either a risk or a protective factor for adult low-grade inflammation, independent of other factors. Inflammation is a physiological response that mediates the effects of both social adversity and dominance on decreases in health.
The social and psychological effects of bullying involvement are independent of other childhood experiences, pleiotropic, and long lasting, with the worst effects for those who are both victims and bullies (e.g., refs. 1–4). To date, the primary focus of bullying research has been on such psychosocial outcomes. Bullied children, however, also have adverse physical health functioning (1, 5–7), including a broad range of somatic issues, such as sleep problems, abdominal pain, appetite suppression, headaches, and frequency of illnesses. In contrast, there is evidence to suggest that those who perpetrate only, pure bullies, may be healthier than their peers, emotionally and physically (6, 8). Little is known about how this social adversity becomes biologically embedded to influence health status.
One potential mechanism is chronic systemic low-grade inflammation (9). Inflammation is activated similarly by a diverse range of health risk behaviors (poor diet, lack of exercise, and sleep disturbance) and environmental challenges [low socioeconomic status (SES), psychosocial stress] (10–14). Elevated inflammation markers are part of the phenomenology of common psychological disorders (particularly depression) across the lifespan (for reviews, refs. 15, 16). One marker of inflammation, C-reactive protein (CRP), has been the focus of extensive epidemiologic investigation because of the association of elevated plasma CRP levels (>3 mg/L) with cardiovascular risk (17, 18) and aspects of metabolic syndrome (19–21). Subclinical levels of inflammation may be a nonspecific marker for a broad range of organismic challenges, but they have not been studied as a mechanism for the social adversity of bullying involvement on health.
The aim of this study was to use a prospective, longitudinal study that has followed a sample of 1,420 children up to nine times to test whether involvement in childhood bullying affects low-grade inflammation as measured by CRP levels short term within childhood/adolescence (ages 9–16) and long term into adulthood (ages 19 and 21). Chronic victims and bully/victims display the worst health and psychosocial outcomes (1, 2, 4). It is hypothesized that both these groups will have more systemic inflammation because of the social strain of victimization. Almost no attention has been paid to the biological consequences to bullying itself in the absence of being a victim. Children may use bullying techniques in efforts to elevate their social status (22). In adults, such elevated social status, measured by income or education level, is associated with lower levels of inflammatory markers (23–25). The role of elevated social status inflammatory markers has not yet been tested, but we expected that pure bullies would display lower levels of CRP than those uninvolved in bullying.
Results
Descriptive Statistics.
By age 21, 8,806 total assessments were completed in the 1,420 study subjects. Blood spots were obtained at 6,087 assessments (69.1%). Comparisons of observations with versus without blood spots indicated no significant differences in any of the bullying measures. Of the 6,087 blood spots collected, 6,001 (98.6%) were assayed successfully for CRP. Of the 1,420 study participants, 1,334 (93.9%) provided blood spot samples assayed for CRP in 1 year or more. The median number of CRP samples provided was 5 [mean 4.77 (SD 2.24); range 1–9].
Table 1 provides the rates of demographic and bullying variables during childhood/adolescent observations (ages 9–16) and young adult observations (ages 19 and 21), as well as mean CRP levels within each period. As shown previously, levels of CRP rise from adolescence to young adulthood (26). There were no baseline differences in CRP levels between bullying groups before bullying involvement or based upon subsequent cumulative involvement.
Table 1.
Descriptive statistics
| Variables | Childhood/adolescent (ages 9–16) | Adulthood (ages 19 and 21) |
| Subjects, n | 1,309 | 759 |
| Observations, % (n) | ||
| Total | 4,870 | 1,131 |
| Female | 52.5 (2,678) | 54.6 (575) |
| Race | ||
| White | 89.7 (3,227) | 89.7 (679) |
| American Indian | 4.4 (1,386) | 4.1 (399) |
| African American | 5.9 (257) | 6.2 (53) |
| Victims, no. of exposures | ||
| 0 | 75.7 (3,568) | 73.7 (810) |
| 1 | 18.9 (950) | 19.2 (213) |
| 2 | 3.9 (249) | 5.1 (70) |
| 3+ | 1.6 (103) | 2.0 (38) |
| Bully, no. of exposures | ||
| 0 | 92.1 (4,317) | 88.9 (964) |
| 1 | 6.8 (440) | 8.9 (127) |
| 2 | 1.0 (100) | 1.9 (32) |
| 3+ | 0.1 (13) | 0.3 (8) |
| Median CRP level, mg/L | 0.20 | 0.75 |
Percentages are weighted, and number of observations is unweighted.
Bullying Involvement and Childhood/Adolescent CRP Levels.
Table 2 summarizes results from models predicting childhood/adolescent (ages 9–16; 4,870 observations of 1,309 subjects) CRP levels from recent bullying involvement. All models accounted for CRP levels at the prior observation; thus, the models predict changes in CRP levels associated with recent bullying involvement. This simple model predicted CRP levels from bully status (dummy coded to compare pure victims, pure bullies, and bully–victims with those uninvolved in bullying). Subsequent models tested whether simple associations were robust to two sets of covariates: (i) variables associated with CRP levels [sex, age, race/ethnicity, time since last interview, body mass index (BMI), recent nicotine use, recent alcohol use, recent drug use, recent medication use, health ailments, low SES] and (ii) variables associated with bullying involvement (sex, low SES, family instability, family dysfunction, maltreatment, depressive disorders, anxiety disorders, disruptive behavior disorders, or substance disorders). All models used weighted linear regression models with robust variance (sandwich-type) estimates to adjust for repeated observations of each subject.
Table 2.
Associations between CRP and bullying involvement within childhood/adolescence (ages 9–16)
| Simple | Adjusted for CRP-related covariates | Adjusted for bullying-related covariates | |||||||
| Bully status | β (SE) | P | Sig. covariates | β (SE) | P | Sig. covariates | β (SE) | P | Sig. covariates |
| Recent status | |||||||||
| Pure bully | 0.07 (0.05) | 0.11 | Prior CRP | 0.05 (0.05) | 0.25 | Prior CRP, race, BMI, med. use | 0.08 (0.05) | 0.12 | Prior CRP, race, family dysfunction |
| Pure victim | 0.03 (0.02) | 0.19 | Prior CRP | 0.03 (0.02) | 0.22 | Prior CRP, race, BMI, med. use | 0.04 (0.02) | 0.10 | Prior CRP, race, family dysfunction |
| Bully–victim | 0.06 (0.07) | 0.35 | Prior CRP | 0.02 (0.07) | 0.78 | Prior CRP, race, BMI, med. use | 0.07 (0.07) | 0.32 | Prior CRP, race, family dysfunction |
| Cumulative | |||||||||
| Pure bully | 0.01 (0.01) | 0.63 | Prior CRP | 0.00 (0.01) | 0.87 | Prior CRP, race, BMI, med. use | 0.01 (0.02) | 0.58 | Prior CRP, race, anxiety, family dysfunction |
| Pure victim | 0.02 (0.01) | 0.01 | Prior CRP | 0.02 (0.01) | 0.01 | Prior CRP, race, BMI, med. use | 0.02 (0.01) | 0.008 | Prior CRP, race, anxiety, family dysfunction |
| Bully–victim | 0.05 (0.05) | 0.32 | Prior CRP | 0.03 (0.06) | 0.66 | Prior CRP, race, BMI, med. use | 0.05 (0.06) | 0.41 | Prior CRP, race, anxiety, family dysfunction |
All models were tested using weighted linear regression. Simple models include current status on the bullying variables and status of CRP at the prior observation. CRP-related covariates also included the following: sex, age, race/ethnicity, time since last interview, BMI, recent nicotine use, recent alcohol use, recent drug use, recent medication use, health ailments, and low SES. Bullying-related covariates include sex, race/ethnicity, low SES, family instability, family dysfunction, maltreatment, depressive disorders, anxiety disorders, disruptive behavior disorders, or substance disorders. Boldface values are significant at the P < 0.05 level.
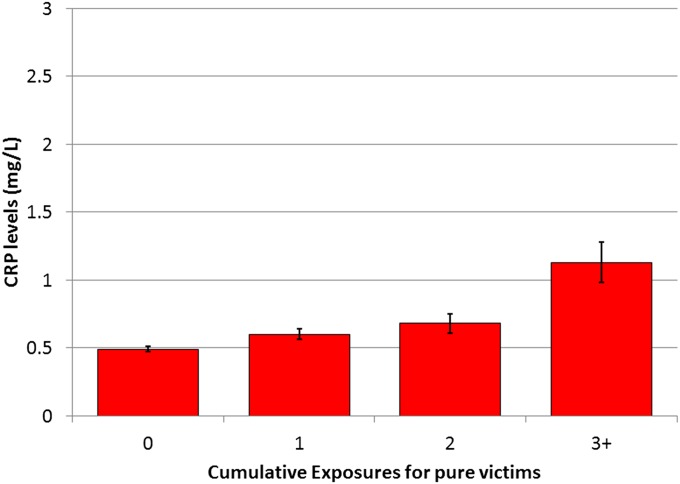
The first series of models focuses on recent bullying involvement only (rows 1–4), meaning that we predicted current CRP levels from recent bullying involvement, controlling for previous CRP. Pure victims, pure bullies, and bully–victims (those who both bullied others and were bullied) were compared with those uninvolved in bullying. Neither pure bullies nor victims differed in CRP changes from those uninvolved in bullying in simple models or in models adjusted for CRP- or bullying-related covariates. Prior levels of CRP were the strongest predictor of current CRP levels in all models. The second series of models (rows 5–8) looks at the effect of cumulative bullying involvement over time, meaning that our bullying variable in these analyses counted the number of assessments during which a particular bullying involvement had been reported to date. For example, children who had not experienced bullying at wave 1, but had experienced it at waves 2 and 3, received a code of “0” at wave 1, “1” at wave 2, and “2” at wave 3. Cumulative exposures for pure victims predicted increased CRP levels in the simple model as well as in the covariate-adjusted models. Neither cumulative bullying nor bully–victim exposures predicted CRP levels. Fig. 1 shows the adjusted mean CRP levels based on cumulative exposures to being bullied. Tables S1 and S2 show results separately by parent and child report.
Fig. 1.
Adjusted mean childhood/adolescent CRP levels (milligrams per liter) based on cumulative exposure to being bullied. These values are adjusted for baseline CRP levels as well as other CRP-related covariates. All analyses used robust SEs to account for repeated observations.
Bullying Involvement and Young Adult CRP Levels.
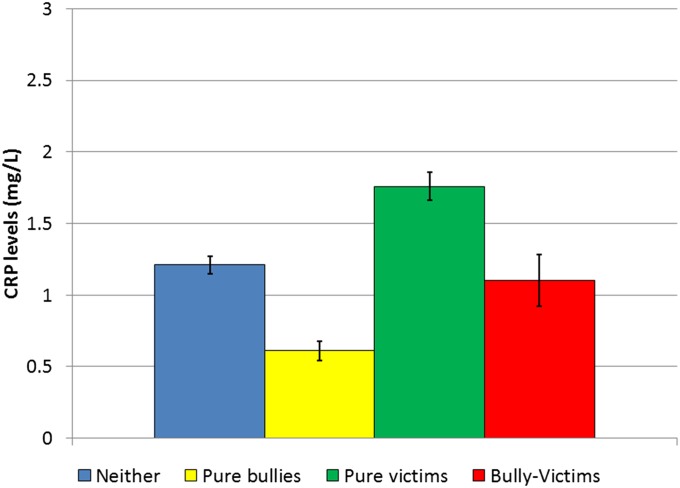
Table 3 summarizes models predicting young adult CRP levels (ages 19–21; 1,131 observations of 759 subjects) from childhood/adolescent bullying involvement (ages 9–16). All analyses predicting early adult CRP levels controlled for baseline CRP levels in childhood; thus, these models predict changes in CRP levels that are associated with childhood/adolescent bullying involvement from childhood to adulthood. The first set of models (rows 1–4) aggregated information about any bullying involvement in childhood/adolescence. For example, if a child had been bullied at any point during ages 9–16, he or she received a code of 1 on this variable. The second part of the table (rows 5–8) looked at the cumulative number of childhood and adolescent observations positive for such involvement. Similar sets of CRP- and bullying-related covariates were used to test for robust associations, except CRP-related covariates were measured in adulthood, whereas bullying-related covariates accounted for childhood hardships and psychiatric problems. Both series of models produced similar results: being a bully in childhood/adolescence predicted lower levels of CRP in young adulthood, and being a victim predicted higher levels of CRP compared with those uninvolved in bullying. Bully–victims, however, did not vary from those uninvolved in bullying. Fig. 2 shows the young adult adjusted mean CRP levels based on childhood/adolescent bullying status. Furthermore, cumulative victimization (victims) in childhood increased CRP levels in adulthood, indicating a dose–response. Tables S3 and S4 show results separately by parent and child report. Analyses were rerun to compare the effect of bullying involvement in childhood (ages 9–13) and adolescence (ages 14–16) separately (Table S5). The finding of lower CRP levels in victims was stronger in childhood and the higher CRP levels for bullies in the adolescent analyses.
Table 3.
Associations between childhood bullying involvement and adult CRP levels (ages 19 and 21)
| Simple | Adjusted for CRP-related covariates | Adjusted for bullying-related covariates | |||||||
| Bully status | β (SE) | P | Sig. covariates | β (SE) | P | Sig. covariates | β (SE) | P | Sig. covariates |
| Childhood status | |||||||||
| Pure bully | −0.13 (0.04) | 0.002 | Prior CRP | −0.09 (0.04) | 0.01 | Prior CRP, sex, race, BMI, med. use | −0.10 (0.04) | 0.01 | Prior CRP, sex, race, SES |
| Pure victim | 0.07 (0.04) | 0.10 | Prior CRP | 0.09 (0.04) | 0.02 | Prior CRP, sex, race, BMI, med. use | 0.07 (0.03) | 0.02 | Prior CRP, sex, race, SES |
| Bully–victim | −0.03 (0.06) | 0.68 | Prior CRP | −0.08 (0.05) | 0.09 | Prior CRP, sex, race, BMI, med. use | −0.03 (0.06) | 0.56 | Prior CRP, sex, race, SES |
| Cumulative | |||||||||
| Pure bully | −0.06 (0.04) | 0.08 | Prior CRP | −0.06 (0.03) | 0.04 | Prior CRP, sex, race, BMI, med. use | −0.05 (0.03) | 0.14 | Prior CRP, sex, race, poverty, dysfunction |
| Pure victim | 0.04 (0.03) | 0.08 | Prior CRP | 0.05 (0.03) | 0.06 | Prior CRP, sex, race, BMI, med. use | 0.05 (0.02) | 0.01 | Prior CRP, sex, race, poverty, dysfunction |
| Bully–victim | −0.04 (0.09) | 0.62 | Prior CRP | −0.04 (0.07) | 0.58 | Prior CRP, sex, race, BMI, med. use | 0.05 (0.07) | 0.44 | Prior CRP, sex, race, poverty, dysfunction |
All models were tested using weighted linear regression. Simple models include current status on the bullying variables and status of CRP at the prior observation. CRP-related covariates include the following: sex, age, time since last interview, BMI, recent nicotine use, recent alcohol use, recent drug use, recent medication use, health ailments, and low SES. Bullying-related covariates controlled for childhood/adolescent covariates of bullying status. These included sex, low SES, family instability, family dysfunction, maltreatment, depressive disorders, anxiety disorders, disruptive behavior disorders, or substance disorders. Boldface values are significant at the P < 0.05 level.
Fig. 2.
Adjusted mean young adult CRP levels (milligrams per liter) based on childhood/adolescent bullying status. These values are adjusted for baseline CRP levels as well as other CRP-related covariates. All analyses used robust SEs to account for repeated observations.
Discussion
This study leverages a prospective, longitudinal design to test whether involvement in bullying—as bully, victim, or both—was associated with low-grade inflammation in the short term within childhood or long term into young adulthood. Short term, there was a dose-dependent relation between the number of times a child had been bullied and CRP levels. This relationship provides a potential mechanism for the observed health problems reported for victims of bullying (1, 5, 6). Childhood bullying involvement as either a pure bully or victim predicted changes in CRP levels that lasted into adulthood. Although CRP levels rose for all participants across this period, being bullied predicted greater increases in CRP levels, whereas bullying others predicted lower increases in CRP compared with those uninvolved in bullying. These long-term effects were robust to adjustment for BMI, substance use, childhood physical and mental health status, and exposures to other early-life psychosocial adversities. Inflammation is a plausible mechanism by which bullying involvement may affect short- and long-term health status.
The finding of greater increases in CRP levels for pure victims is less surprising given previous evidence of short- and long-term impaired health functioning (1, 6, 8) and associations between childhood psychosocial adversity and inflammation levels (27, 28). At the same time, the strength of our findings rests on the following features of this study. First, this study was able to control for preexisting CRP levels in all analyses, allowing us to clarify that observed differences are not attributable to baseline CRP differences and thus preexisting differences between groups. Second, the prospective design allowed us to account for a host of individual and family factors that may explain the observed bullying–CRP associations. Together, these features allow for strong inferences about the causal role of bullying involvement in changing CRP level within an observation study. Finally, bullying is different from other childhood adversities studied. It is a relatively common experience for children and adolescents and the most frequent form of violence experienced outside the home (29, 30), although it still is considered by many to be a harmless rite of passage and by others a modest, time-limited stressor. Our findings suggest this childhood social adversity may disrupt levels of inflammation well into adulthood, similar to what is seen for early traumatic events, such as child maltreatment (9).
Our findings of increases in CRP levels as a function of a cumulative history of being bullied are consistent with changes in hypothalamic–pituitary–adrenal axis function, particularly cortisol levels, reported in victims (31–36). Although not all studies support associations between bullying and cortisol levels (see ref. 37 for null finding), a series of studies suggests that victims, particularly those victimized over long periods, have a blunted cortisol secretion in response to a laboratory social stress test (35, 36), with some evidence that this effect is moderated genetically (34). This provides a potential neuroendocrine mechanism for our observed inflammation findings: a blunted response means lower exposure to the anti-inflammatory effects of cortisol for victimized children. Additional analyses are necessary to test other potential psychosocial and biological mechanisms for this observed effect.
Pure bullies displayed lower levels of CRP when followed into adulthood. Longitudinal studies of early life experiences and biological markers have focused almost exclusively on adversity. This finding may seem surprising, because two groups of children/adolescents often were lumped together in previous research as “bullies,” although they are distinct in many features. If considered separately, one group of bullies—those who also are bullied themselves—the bully–victims have the worst long-term emotional problems and poor health outcomes (1, 2). They most closely resemble those with conduct problems (38, 39). In contrast, there is evidence that those who perpetrate only, pure bullies, gain benefits from bullying others without incurring costs and may be healthier than their peers, emotionally and physically (6, 8). As such, analyses that group bully–victims with pure bullies (as is the case in analyses of children with conduct disorder) may be mixing distinct phenotypes. Our findings also are consistent with studies showing lower inflammation rates for individuals with higher SES (23) and studies with nonhuman primates showing health benefits for those higher in the social hierarchy (40). The clear implication of these findings is that both ends of the continuum of social status are important for inflammation levels and health status.
Strengths and Limitations.
The Great Smoky Mountains Study has several strengths besides its longitudinal, prospective design: a population-based design that minimizes selection biases; bullying variables assessed repeatedly with structured interviews; repeated collection of blood spots, allowing subjects to provide up to nine values of CRP across 12 y; and assessment of a wide range of domains, allowing us to control for covariates of bullying and CRP. However, the study also has limitations: the sample is not representative of the US population, with Native Americans overrepresented and African Americans and Latinos underrepresented. The time between any two assessments was never less than a year, yet both CRP levels and bullying involvement may vary over shorter periods. Finally, adult follow-up was limited to those who were available for in-person interviews and agreed to provide blood spots.
Conclusion
Being bullied is known to have adverse effects on psychological and social development, but it is increasingly being recast as similar to family maltreatment in its potential to disrupt both mental and physical functioning across the lifespan (1, 2). In contrast, bullies experience few downsides and reap biological advantages of increased social status. Social status and disruptions to one’s status may play a central role in physical health functioning through effects on chronic low-grade inflammation, and these effects may persist for decades. Our findings suggest that this mechanism may be a key target for efforts to reduce risk for a bevy of age-related diseases and to promote optimal psychological and physical health functioning.
Materials and Methods
Participants.
The Great Smoky Mountains Study is a longitudinal study of the development of psychiatric disorders and the need for mental health services in rural and urban youth (41, 42). A representative sample of three cohorts of children, ages 9, 11, and 13 at intake, was recruited from 11 counties in western North Carolina. Potential participants were selected from the population of some 12,000 children by using a household equal probability, accelerated cohort design. All children scoring above a predetermined cut point (the top 25% of the total scores) on a behavioral screener, plus a 1-in-10 random sample of the remaining 75% of the total scores, were recruited for detailed interviews. This approach oversamples those at risk for psychiatric problems for the purpose of estimating prevalence rates for uncommon psychiatric disorders. All subjects were assigned a weight inversely proportional to their probability of selection, so all results are representative of the population from which the sample was drawn and not biased from the oversampling procedure. About 8% of the area residents and the sample were African American, less than 1% were Hispanic, and 3% were American Indian. Of all subjects recruited, 80% (n = 1,420) agreed to participate. Subjects were assessed annually to age 16, then again at ages 19 and 21. Across all waves, participation rates averaged 84% (range: 74–94%).
Procedures.
The parent (biological mother for 83% of interviews) and subject were interviewed by trained interviewers separately until the subject was 16, after which only the subjects were interviewed. Before the interviews began, parent and child signed informed consent forms approved by the Duke University Medical Center Institutional Review Board. Each parent and child received an honorarium for their participation.
Using a previously described procedure (43), blood samples were obtained at the beginning of each in-person assessment as follows: two finger-prick samples (yielding 10 spots total per visit) were collected at 20-min intervals, applied to standardized collection paper, immediately refrigerated upon drying, and express shipped (without refrigeration) to the laboratory within 2 wk of collection. Samples then were stored at −28 °C until they were assayed. This protocol is consistent with the rigorous quality control program developed for newborn screening programs (44) and has been used in several epidemiologic studies involving blood spot CRP measures (45, 46).
Assessment.
Bullying involvement.
At each assessment between ages 9 and 16, the child and his or her parent reported on whether the child had been bullied/teased or had bullied others in the 3 mo immediately before the interview as part of the Child and Adolescent Psychiatric Assessment (CAPA) (47). Being bullied or bullying others was counted if reported by either the parent or the child. If the informant reported that the subject had been bullied or had bullied others, then the informant was asked separately how often the bullying occurred in the prior 3 mo in the following three settings: home, school, and the community. The focus in the current paper is on peer bullying in the school context only. Subjects were categorized as only bullying others (pure bullies), only being bullied (pure victims), both bullying others and being bullied (bully–victims), or neither bullying others nor being bullied. Parent and child agreement (kappa = 0.24) was similar to that of other bullying measures (48, 49). Although this value may seem low, a large meta-analysis of parent and self-report of behavioral and emotional functioning shows similar concordance levels (50).
CRP.
Our assay for CRP in whole-blood spots was a biotin–streptavidin-based immunofluorometric system improving on a previously published method (51). One assay was completed for each subject at each observation. A validation study was performed with matched serum and blood spot samples assayed for CRP (n = 38). As has been reported for many other analytes, including CRP (43, 51, 52), a close linear correlation was identified between serum and blood spot CRP values (n = 29; R2 = 0.98; P < 0.0001). Serum equivalents therefore were calculated by using the following algorithm based on the serum–blood spot regression: serum [high sensitivity C-reactive protein (hsCRP)] = 1.38*(blood spot CRP value) – 0.97. Blood spot CRP measures have been used in several epidemiologic studies (45, 46, 53). Observations with values above 10 mg/L indicate frank infection and were removed from statistical analysis (n = 109 from a total of 6,000 observations), whereas values below that index the extent of chronic low-grade systemic inflammation associated with cardiovascular and metabolic risk (54).
CRP-related covariates.
Variables included as covariates were those associated with variation in CRP levels (13, 55, 56) or those used as covariates in other longitudinal studies involving CRP (57, 58). These variables included age, sex, race, BMI, medication use, substance use, low SES, and recent physical ailments. BMI was calculated from interviewer-assessed weight and height measurements completed at each assessment. The substance use assessment of the CAPA and Young Adult Psychiatric Assessment (YAPA) interviews evaluates current nicotine, alcohol, and illicit drug use. Dichotomous variables were included to indicate recent use of these substances. A physical health problems survey adapted from the Centers for Disease Control and Prevention National Health Interview Survey Child Supplement (1988) was administered at all interviews to assess 39 common ailments (e.g., diabetes, anemia, mononucleosis). A binary variable indicating any health ailments within the past 12 mo was used for all analyses. Analyses also were tested by using the following separate health categories: atopic (e.g., food/digestive allergy, asthma, and respiratory allergy), injuries, infections (tonsillitis, ear infection, frequent diarrhea or colitis, and urinary tract infections), and chronic diseases (e.g., diabetes, epilepsy, cancer, and chronic heart disease). Medication use within the prior year also was assessed from the Child and Adolescent Services Assessment (59). That interview focused on psychotropic medications, but it also looked at prescribed medications not related to psychiatric problems. All analyses were tested using a broad-based medication use variable as well as categories for individual medication groups (e.g., antidepressant, stimulant, and other prescribed medications). Low SES coded whether the subject’s family displayed any two of the following three indicators: income below the federal poverty line, low parental educational attainment, and low parental occupational status. Additional physiological covariates studied with CRP in older samples at risk for cardiovascular problems (e.g., blood pressure, lipids, or insulin) were not assessed.
Bullying-related covariates.
To clarify that bullying involvement is an independent risk factor for CRP, it was necessary to account for preexisting family and individual factors. All childhood psychiatric and family hardship variables were assessed by parent and self-report using the CAPA (47). Childhood psychiatric variables included any anxiety disorder, any depressive disorder, any behavioral disorder (conduct disorder, attention-deficit hyperactivity disorder, and oppositional defiant disorder), and any substance abuse or dependence. See ref. 60 for additional details. Four types of family hardships were assessed: low SES, unstable family structure, family dysfunction, and maltreatment. A full description of these variables is available from ref. 2.
Analytic Framework.
CRP values were positively skewed and were log10-transformed after scaling for nonnegative values by adding 1. All models used SAS PROC GENMOD to run weighted linear regression models with robust variance (sandwich-type) estimates derived from generalized estimating equations to adjust the SEs for the stratified design and repeated observations.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health (MH63970, MH63671, MH48085, and MH080230), the National Institute on Drug Abuse (DA/MH11301 and DA023026), the National Alliance for Research on Schizophrenia and Depression (Early Career Award to W.E.C.), the William T. Grant Foundation, and the Economic and Social Research Council in the United Kingdom (ES/K003593/1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.W.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323641111/-/DCSupplemental.
References
- 1.Wolke D, Copeland WE, Angold A, Costello EJ. Impact of bullying in childhood on adult health, wealth, crime, and social outcomes. Psychol Sci. 2013;24(10):1958–1970. doi: 10.1177/0956797613481608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copeland WE, Wolke D, Angold A, Costello EJ. Adult psychiatric outcomes of bullying and being bullied by peers in childhood and adolescence. JAMA Psychiatry. 2013;70(4):419–426. doi: 10.1001/jamapsychiatry.2013.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sourander AMD, et al. Childhood predictors of psychiatric disorders among boys: A prospective community-based follow-up study from age 8 years to early adulthood. J Am Acad Child Adolesc Psychiatry. 2005;44(8):756–767. doi: 10.1097/01.chi.0000164878.79986.2f. [DOI] [PubMed] [Google Scholar]
- 4.Winsper C, Lereya T, Zanarini M, Wolke D. Involvement in bullying and suicide-related behavior at 11 years: A prospective birth cohort study. J Am Acad Child Adolesc Psychiatry. 2012;51(3):271. doi: 10.1016/j.jaac.2012.01.001. e3. [DOI] [PubMed] [Google Scholar]
- 5.Gini G, Pozzoli T. Association between bullying and psychosomatic problems: A meta-analysis. Pediatrics. 2009;123(3):1059–1065. doi: 10.1542/peds.2008-1215. [DOI] [PubMed] [Google Scholar]
- 6.Wolke D, Woods S, Bloomfield L, Karstadt L. Bullying involvement in primary school and common health problems. Arch Dis Child. 2001;85(3):197–201. doi: 10.1136/adc.85.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forero R, McLellan L, Rissel C, Bauman A. Bullying behaviour and psychosocial health among school students in New South Wales, Australia: Cross sectional survey. BMJ. 1999;319(7206):344–348. doi: 10.1136/bmj.319.7206.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumpulainen K, et al. Bullying and psychiatric symptoms among elementary school-age children. Child Abuse Negl. 1998;22(7):705–717. doi: 10.1016/s0145-2134(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 9.Danese A, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry. 2011;16(3):244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Moyna NM, et al. Relation between aerobic fitness level and stress induced alterations in neuroendocrine and immune function. Int J Sports Med. 1999;20(2):136–141. doi: 10.1055/s-2007-971107. [DOI] [PubMed] [Google Scholar]
- 12.Alley DE, et al. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain Behav Immun. 2006;20(5):498–504. doi: 10.1016/j.bbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor M-F, et al. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23(7):887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai J, et al. Adherence to the mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: A twin study. Circulation. 2008;117(2):169–175. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaptoge S, et al. Emerging Risk Factors Collaboration C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah T, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: New data and systematic review of 31 prospective cohorts. Int J Epidemiol. 2009;38(1):217–231. doi: 10.1093/ije/dyn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamakoshi K, et al. The metabolic syndrome is associated with elevated circulating C-reactive protein in healthy reference range, a systemic low-grade inflammatory state. Int J Obes Relat Metab Disord. 2003;27(4):443–449. doi: 10.1038/sj.ijo.0802260. [DOI] [PubMed] [Google Scholar]
- 20.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 22.Reijntjes A, et al. Costs and benefits of bullying in the context of the peer group: A three wave longitudinal analysis. J Abnorm Child Psychol. 2013;41(8):1217–1229. doi: 10.1007/s10802-013-9759-3. [DOI] [PubMed] [Google Scholar]
- 23.Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T. Association of markers of systemic inflammation, C reactive protein, serum amyloid A, and fibrinogen, with socioeconomic status. J Epidemiol Community Health. 2003;57(9):730–733. doi: 10.1136/jech.57.9.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loucks EB, et al. Association of educational level with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2006;163(7):622–628. doi: 10.1093/aje/kwj076. [DOI] [PubMed] [Google Scholar]
- 25.Koster A, et al. Health ABC Study Association of inflammatory markers with socioeconomic status. J Gerontol A Biol Sci Med Sci. 2006;61(3):284–290. doi: 10.1093/gerona/61.3.284. [DOI] [PubMed] [Google Scholar]
- 26.Shanahan L, et al. Sex-differentiated changes in C-reactive protein from ages 9 to 21: The contributions of BMI and physical/sexual maturation. Psychoneuroendocrinology. 2013;38(10):2209–2217. doi: 10.1016/j.psyneuen.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danese A, et al. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65(4):409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finkelhor D, Ormrod RK, Turner HA. Polyvictimization and trauma in a national longitudinal cohort. Dev Psychopathol. 2007;19(1):149–166. doi: 10.1017/S0954579407070083. [DOI] [PubMed] [Google Scholar]
- 30.Radford L, Corral S, Bradley C, Fisher HL. The prevalence and impact of child maltreatment and other types of victimization in the UK: Findings from a population survey of caregivers, children and young people and young adults. Child Abuse Negl. 2013;37(10):801–813. doi: 10.1016/j.chiabu.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Knack JM, Jensen-Campbell LA, Baum A. Worse than sticks and stones? Bullying is associated with altered HPA axis functioning and poorer health. Brain Cogn. 2011;77(2):183–190. doi: 10.1016/j.bandc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Hansen ÅM, et al. Bullying at work, health outcomes, and physiological stress response. J Psychosom Res. 2006;60(1):63–72. doi: 10.1016/j.jpsychores.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 33.Rudolph KD, Troop-Gordon W, Granger DA. Peer victimization and aggression: Moderation by individual differences in salivary cortisol and alpha-amylase. J Abnorm Child Psychol. 2010;38(6):843–856. doi: 10.1007/s10802-010-9412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouellet-Morin I, et al. Increased serotonin transporter gene (SERT) DNA methylation is associated with bullying victimization and blunted cortisol response to stress in childhood: A longitudinal study of discordant monozygotic twins. Psychol Med. 2013;43(9):1813–1823. doi: 10.1017/S0033291712002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouellet-Morin I, et al. A discordant monozygotic twin design shows blunted cortisol reactivity among bullied children. J Am Acad Child Adolesc Psychiatry. 2011;50(6):574–582, e3. doi: 10.1016/j.jaac.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouellet-Morin I, et al. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol Psychiatry. 2011;70(11):1016–1023. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton LD, Newman ML, Delville CL, Delville Y. Physiological stress response of young adults exposed to bullying during adolescence. Physiol Behav. 2008;95(5):617–624. doi: 10.1016/j.physbeh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Juvonen J, Graham S, Schuster MA. Bullying among young adolescents: The strong, the weak, and the troubled. Pediatrics. 2003;112(6 Pt 1):1231–1237. doi: 10.1542/peds.112.6.1231. [DOI] [PubMed] [Google Scholar]
- 39.Jansen DE, Veenstra R, Ormel J, Verhulst FC, Reijneveld SA. Early risk factors for being a bully, victim, or bully/victim in late elementary and early secondary education. The longitudinal TRAILS study. BMC Public Health. 2011;11(1):440. doi: 10.1186/1471-2458-11-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 41.Costello EJ, et al. The Great Smoky Mountains Study of Youth. Goals, design, methods, and the prevalence of DSM-III-R disorders. Arch Gen Psychiatry. 1996;53(12):1129–1136. doi: 10.1001/archpsyc.1996.01830120067012. [DOI] [PubMed] [Google Scholar]
- 42.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 43.Worthman CM, Stallings JF. Hormone measures in finger-prick blood spot samples: New field methods for reproductive endocrinology. Am J Phys Anthropol. 1997;104(1):1–21. doi: 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 44.Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 2001;131(5):1631S–1636S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- 45.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: The Chicago health, aging, and social relations study. Psychosom Med. 2006;68(3):376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 46.Williams SR, McDade TW. The use of dried blood spot sampling in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i131–i136. doi: 10.1093/geronb/gbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA) J Am Acad Child Adolesc Psychiatry. 2000;39(1):39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Schreier A, et al. Prospective study of peer victimization in childhood and psychotic symptoms in a nonclinical population at age 12 years. Arch Gen Psychiatry. 2009;66(5):527–536. doi: 10.1001/archgenpsychiatry.2009.23. [DOI] [PubMed] [Google Scholar]
- 49.Rønning JA, et al. Cross-informant agreement about bullying and victimization among eight-year-olds: Whose information best predicts psychiatric caseness 10-15 years later? Soc Psychiatry Psychiatr Epidemiol. 2009;44(1):15–22. doi: 10.1007/s00127-008-0395-0. [DOI] [PubMed] [Google Scholar]
- 50.Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: Implications of cross-informant correlations for situational specificity. Psychol Bull. 1987;101(2):213–232. [PubMed] [Google Scholar]
- 51.McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50(3):652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- 52.McDade TW, et al. Epstein-Barr virus antibodies in whole blood spots: A minimally invasive method for assessing an aspect of cell-mediated immunity. Psychosom Med. 2000;62:560–568. doi: 10.1097/00006842-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 53.McDade TW, et al. Predictors of C-reactive protein in Tsimane’ 2 to 15 year-olds in lowland Bolivia. Am J Phys Anthropol. 2005;128(4):906–913. doi: 10.1002/ajpa.20222. [DOI] [PubMed] [Google Scholar]
- 54.Pearson TA, et al. Centers for Disease Control and Prevention American Heart Association Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 55.Woodward M, Rumley A, Lowe GDO, Tunstall-Pedoe H. C-reactive protein: Associations with haematological variables, cardiovascular risk factors and prevalent cardiovascular disease. Br J Haematol. 2003;122(1):135–141. doi: 10.1046/j.1365-2141.2003.04387.x. [DOI] [PubMed] [Google Scholar]
- 56.Elovainio M, et al. Cardiovascular Risk in Young Finns Study Depressive symptoms and C-reactive protein: The Cardiovascular Risk in Young Finns Study. Psychol Med. 2006;36(6):797–805. doi: 10.1017/S0033291706007574. [DOI] [PubMed] [Google Scholar]
- 57.Gimeno D, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun. 2009;23(7):936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ascher BH, Farmer EMZ, Burns BJ, Angold A. The Child and Adolescent Services Assessment (CASA): Description and psychometrics. J Emot Behav Disord. 1996;4(1):12–20. [Google Scholar]
- 60.Copeland W, Shanahan L, Costello EJ, Angold A. Cumulative prevalence of psychiatric disorders by young adulthood: A prospective cohort analysis from the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2011;50(3):252–261. doi: 10.1016/j.jaac.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.