Significance
Although synaptic vesicle fusion plays a crucial role in neurotransmission, studies of its molecular mechanisms have relied heavily on the use of artificial liposomes reconstituted with recombinantly expressed proteins. In this study, we establish an in vitro assay using engineered yeast vacuoles bearing neuronal SNAREs for measuring neuronal SNARE-mediated membrane fusion and show that this assay provides a simple and independent means of investigating synaptic vesicle fusion mechanisms.
Abstract
Intracellular membrane fusion requires not only SNARE proteins but also other regulatory proteins such as the Rab and Sec1/Munc18 (SM) family proteins. Although neuronal SNARE proteins alone can drive the fusion between synthetic liposomes, it remains unclear whether they are also sufficient to induce the fusion of biological membranes. Here, through the use of engineered yeast vacuoles bearing neuronal SNARE proteins, we show that neuronal SNAREs can induce membrane fusion between yeast vacuoles and that this fusion does not require the function of the Rab protein Ypt7p or the SM family protein Vps33p, both of which are essential for normal yeast vacuole fusion. Although excess vacuolar SNARE proteins were also shown to mediate Rab-bypass fusion, this fusion required homotypic fusion and vacuole protein sorting complex, which bears Vps33p and was accompanied by extensive membrane lysis. We also show that this neuronal SNARE-driven vacuole fusion can be stimulated by the neuronal SM protein Munc18 and blocked by botulinum neurotoxin serotype E, a well-known inhibitor of synaptic vesicle fusion. Taken together, our results suggest that neuronal SNARE proteins are sufficient to induce biological membrane fusion, and that this new assay can be used as a simple and complementary method for investigating synaptic vesicle fusion mechanisms.
Membrane fusion mediates a variety of biological processes, such as fertilization and cell growth, hormone secretion, neurotransmission, nutrient uptake, and viral infection (1). Vesicle trafficking between organelles, a major tool for intracellular transport of materials, is also regulated by membrane fusion: Membrane fusion between transport vesicles and target compartments releases the cargo stored in the vesicles into the lumen of the compartments. To maintain the unique chemical environment of each organelle, biological membrane fusion occurs with spatiotemporal precision but without leakage of the luminal contents. This nature of biological membrane fusion may be achieved through the cooperation of various proteins, such as Rab GTPases and their effectors, SNARE [soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein (SNAP) receptor] proteins, and SNARE chaperones (2, 3). SNARE proteins bring two membrane bilayers into close proximity, which promotes the fusion of the apposed membranes (4, 5). The molecular mechanism by which SNARE proteins mediate membrane fusion has been intensively studied in synaptic vesicle fusion, which mediates neurotransmission at the synapse, whereby neurotransmitters released by presynaptic neurons are recognized by their receptors on postsynaptic neurons (6, 7). Depolarization of presynaptic nerve terminals by an action potential opens Ca2+ channels in the presynaptic membrane. Ca2+ influx into the presynaptic cell then triggers membrane fusion between the presynaptic plasma membrane and synaptic vesicles, leading to the release of neurotransmitter. Synaptic vesicle fusion is mediated by three neuronal SNARE proteins: syntaxin, SNAP25, and synaptobrevin (also referred to as VAMP). Synaptobrevin and syntaxin-1 each contain one SNARE motif, whereas SNAP25 contains two. One SNARE motif from synaptobrevin (v-SNARE) on a synaptic vesicle and three SNARE motifs provided by syntaxin-1 and SNAP25 (t-SNAREs) from the plasma membrane assemble into a tight trans-SNARE complex that brings the two membranes into close apposition. This close apposition, in turn, induces lipid bilayer merging, thus releasing neurotransmitter into the synaptic cleft.
In addition to the neuronal SNARE proteins, many other regulatory proteins, such as Munc18 and synaptotagmin, are required for synaptic vesicle fusion in vivo (8, 9). Munc18, a member of the Sec1/Munc18 (SM) protein family, seems to play a variety of roles in synaptic vesicle fusion. First, Munc18 binds to free syntaxin molecules and keeps them in a closed, inactive state, helping to prevent the formation of premature SNARE complexes (10, 11). Additionally, Munc18 interacts with the syntaxin molecule within assembled t-SNARE complexes, guiding them in a manner conducive to productive trans-SNARE complex formation, which triggers membrane fusion (12). Neuronal synaptotagmin, anchored to synaptic vesicles, functions as a calcium sensor for synaptic vesicle fusion (9, 13, 14). Although its mode of action is not yet fully defined, calcium binding to synaptotagmin triggers synaptic vesicle fusion. Despite the importance of these and many other proteins for synaptic vesicle fusion, the three neuronal SNARE proteins are thought to constitute the minimal components sufficient to drive membrane fusion: On their own, they can induce fusion between proteoliposomes carrying both syntaxin and SNAP25 and those reconstituted with synaptobrevin (15). Although these reconstitution experiments strongly support the concept that neuronal SNARE proteins suffice to induce membrane fusion, it is still unclear whether they can also induce fusion between biological membranes for the following reasons: (i) Liposome fusion, unlike biological membrane fusion, is intrinsically promiscuous: Even protein-free liposomes can fuse under certain conditions (16). (ii) Liposome fusion assays often rely on detection of lipid mixing, which can occur without content mixing. Liposome rupture (17) or clustering without fusion (18) can generate false-positive signals. (iii) Finally, because detergent is used to reconstitute SNARE proteins into liposomes, the potential presence of residual detergent, which affects the integrity of liposomes and their lipid mixing, cannot be completely excluded.
Homotypic yeast vacuole fusion has been used to study membrane fusion mechanisms (19–21). In vitro assays using isolated yeast vacuoles have been established that measure the mixing of vacuole luminal compartments (22, 23). The fusion of isolated yeast vacuoles in vitro is mediated by evolutionarily conserved membrane fusion machinery, which involves not only SNARE proteins but also Rab and SM proteins. In this study, to address whether neuronal SNARE proteins are sufficient to induce biological membrane fusion, we engineered the budding yeast Saccharomyces cerevisiae to express neuronal SNARE proteins in vacuoles. Using these yeast vacuoles, we then show that neuronal SNARE proteins can induce vacuole fusion in a Rab- and SM protein-independent manner.
Results
Expression of Neuronal SNARE Proteins on Yeast Vacuoles.
The in vitro assay for homotypic yeast vacuole fusion has been used to study the molecular mechanisms of endomembrane fusion (1, 20). To measure fusion, vacuoles are isolated from the two yeast strains DKY6281 and BJ3505. The strain DKY6281 contains normal vacuolar proteases but lacks the major vacuolar phosphatase encoded by the PHO8 gene. The strain BJ3505 carries the wild-type PHO8 gene but lacks genes encoding the major vacuolar proteases that activate the Pho8p phosphatase. Thus, vacuoles isolated from BJ3505 or DKY6281 lack the catalytically active form of Pho8p. However, fusion between these two vacuoles allows proteases to gain access to pro-Pho8p, generating active Pho8p, which can be assayed colorimetrically. On the basis of this assay, we attempted to develop an assay for measuring vacuole membrane fusion driven by the neuronal SNARE proteins, syntaxin-1, SNAP25, and synaptobrevin-2 (Fig. 1A). To this end, we used BJ3505 and DKY6281 to genetically engineer the tester yeast strains, BJ3505 expressing the neuronal v-SNARE synaptobrevin-2 (BJ3505-Syb2) and DKY6281 expressing the neuronal t-SNAREs syntaxin-1 and SNAP25 (DKY6281-Stx1/S25). Because a subset of mammalian membrane proteins were reported to traffic to the vacuole when ectopically expressed in yeast cells (24), we used the constitutively strong ADH1 promoter to overexpress the neuronal SNARE proteins and examined whether a portion of the proteins were localized to the vacuole. As shown in Fig. 1B, all neuronal SNARE proteins expressed in yeast were detected on the vacuoles purified from BJ3505-Syb2 and DKY6281-Stx1/S25. SNAP25 lacks a transmembrane domain but is palmitoylated on its cysteine cluster for stable membrane association in neurons. Triton X-114 partitioning analysis indicates that yeast-expressed SNAP25 is also palmitoylated (Fig. S1). Because yeast vacuoles can fuse with each other via their own membrane fusion machinery, the gene encoding Nyv1p, the essential v-SNARE for homotypic vacuole fusion, was deleted from the tester strains to block normal vacuole fusion. In BJ3505-Syb2, Snc2p, the yeast ortholog of synaptobrevin-2, was also deleted because Snc2p, which is found on vacuoles (25), may replace the function of synaptobrevin-2 by interacting with its cognate t-SNAREs, syntaxin-1 and SNAP25. Because the molar ratio of SNARE proteins to lipid was shown to be a critical determinant of the rate and extent of neuronal SNARE-mediated fusion, BJ3505-Syb2 nyv1Δ snc2Δ (BJ-Syb2), and DKY6281-Stx1/S25 nyv1Δ (DKY-Stx1/S25) vacuoles were analyzed for lipid phosphorous and for each of the neuronal SNARE proteins (Table S1). Concentrations of these proteins were between 2- and 50-fold higher than those of yeast vacuolar SNARE proteins on wild-type vacuoles, but much lower than concentrations of neuronal SNARE proteins found physiologically: The molar ratio of synaptobrevin-2 to lipid (1:25,900) in BJ-Syb2 vacuoles was two orders of magnitude lower than that on purified synaptic vesicles (1:200) (26).
Fig. 1.
Neuronal SNARE proteins can support nyv1Δ vacuole fusion. (A) Assay schematic; see Results for details. BJ3505 nyv1Δ snc2Δ vacuoles overexpressing synaptobrevin-2 (BJ-Syb2) and DKY6281 nyv1Δ vacuoles overexpressing both syntaxin-1 and SNAP25 (DKY-Stx1/S25) were used to assay neuronal SNARE-mediated vacuole fusion (Methods). (B) Expression of neuronal SNARE proteins on yeast vacuoles, as revealed by immunoblotting. (C) Fusion requires synaptobrevin-2 on one vacuole and syntaxin-1/SNAP25 on the other. Vacuoles were purified from the yeast strains indicated and incubated in fusion reaction buffer without ATP at 27 °C. After 90 min, ALP activity was measured. The data represent mean ± SEM (n = 3).
Neuronal SNARE Proteins Support nyv1Δ Vacuole Fusion.
To examine whether the neuronal SNARE proteins expressed on the vacuole can support the fusion of Nyv1p-deficient vacuoles, vacuoles were isolated from BJ-Syb2 and DKY-Stx1/S25 and mixed together. After 90-min incubation at 27 °C in the absence of ATP, the activity of mature Pho8p was measured (Fig. 1A). As shown in Fig. 1C (black bars) and Fig. S2, incubation of BJ-Syb2 vacuoles with DKY-Stx1/S25 vacuoles at 27 °C (Fig. 1C, bar 8), but not on ice (bar 4), resulted in mature Pho8p activity, indicating that the neuronal SNARE proteins support the fusion of Nyv1p-deficient vacuoles. This fusion signal was not diminished by inhibitory antibodies against the vacuolar t-SNARE Vam3p (bar 12), which is essential for vacuole fusion, but was completely blocked by the addition of the cytoplasmic domain of synaptobrevin-2 (Syb2-CD; bar 16), which competes with vacuole-bound synaptobrevin-2 for the vacuolar syntaxin-1 and SNAP25 (15), or by a phosphorylated phosphatidylinositol ligand, MARCKS effector domain (MED; bar 20) (27). By contrast, no fusion was observed between BJ3505 nyv1Δ snc2Δ and DKY6281 nyv1Δ vacuoles, between BJ-Syb2 and DKY6281 nyv1Δ vacuoles, or between BJ3505 nyv1Δ snc2Δ and DKY-Stx1/S25 vacuoles (Fig. 1C, bars 5–7). Furthermore, neither DKY-Stx1 nor DKY-S25 vacuoles supported fusion with BJ-Syb2 vacuoles (Fig. S3), suggesting that both neuronal t-SNAREs are required for fusion and that vacuolar t-SNAREs cannot substitute for neuronal t-SNAREs. Thus, these results indicate that the fusion was mediated by trans interaction between the neuronal t-SNAREs from DKY-Stx1/S25 vacuoles and the neuronal v-SNARE from BJ-Syb2 vacuoles, but not by interaction between endogenous SNARE proteins on the vacuole or between the neuronal SNARE proteins from one vacuole and yeast vacuolar SNARE proteins from another (Fig. 1C, compare bars 5–8).
Neuronal SNARE-Driven Vacuole Fusion Bypasses the Requirement for Ypt7p and Vps33p.
Homotypic yeast vacuole fusion involves priming, docking, and bilayer fusion/compartment mixing. During priming, Sec18p (the yeast ortholog of mammalian NSF) and its cochaperone Sec17p (the yeast ortholog of mammalian α-SNAP) disassemble cis-SNARE complexes into individual SNARE proteins (28). Vacuole docking requires the Ypt7p GTPase and its effector complex, homotypic fusion and vacuole protein sorting complex (HOPS). HOPS is a heterohexameric complex of Vps11, Vps16, Vps18, Vps33, Vps39, and Vps41 subunits that binds selectively to GTP-bound Ypt7p (29). Because Ypt7p and the HOPS subunit Vps33p, a member of the SM protein family, are prerequisites for the assembly of trans-SNARE complexes between apposed vacuoles and subsequent vacuolar membrane fusion (30), they may also be required for neuronal SNARE-driven vacuole fusion. To test this possibility, we used two Ypt7p inhibitors: an inhibitory antibody specific to Ypt7p and GDI/Gyp1-46p, which efficiently inactivates and removes Ypt7p from vacuolar membranes (31, 32), and an anti-Vps33p antibody (29). Although these inhibitors completely prevented homotypic yeast vacuole fusion as reported (2) (Fig. 2A, gray bars), neuronal SNARE-driven vacuole fusion was completely resistant to these inhibitors (black bars), strongly suggesting that the neuronal SNARE-driven vacuolar membrane fusion occurs independently of the Ypt7p/Vps33p-mediated docking step. Although neuronal SNARE-driven vacuole fusion did not require a Rab-mediated docking step, it was anticipated that physical contact between vacuoles would still be required for fusion. As expected, 20-fold dilution of fusion reactions markedly inhibited fusion (Fig. 2B and Fig. S4).
Fig. 2.
Neuronal SNARE-mediated vacuole fusion does not require the proteins essential for normal yeast vacuole fusion and can occur without massive content leakage. (A) Neuronal SNARE-mediated vacuole fusion was not prevented by inhibitors that completely block normal yeast vacuole fusion. Vacuoles were purified from yeast strains indicated. BJ3505 vacuoles and DKY6281 vacuoles (gray bars) were mixed and incubated in fusion reaction buffer with 1 mM ATP at 27 °C for 90 min in the absence or presence of the inhibitors shown (see SI Methods for all inhibitor concentrations). Likewise, BJ-Syb2 vacuoles and DKY-Stx1/S25 vacuoles (black bars) were mixed and incubated in fusion reaction buffer (without ATP) at 27 °C for 90 min in the absence or presence of the inhibitors. After 90 min, ALP activity was measured, and fusion values (%) were normalized to reactions that were performed without inhibitor at 27 °C (BJ3505/DKY6281, 3.33 ± 0.49 U; BJ-Syb2/DKY-Stx1/S25, 0.9 ± 0.082 U). Because neuronal SNARE-mediated vacuole fusion reactions were performed in the absence of ATP, Sec18p (yeast NSF) could not be active and, thus, disassembly of SNARE complexes would not occur. (B) Neuronal SNARE-mediated vacuole fusion occurs independently of Rab function, but requires physical interactions between vacuoles. BJ-Syb2 and DKY-Stx1/S25 vacuoles were mixed and incubated in fusion reaction buffer (without ATP) at 27 °C for 90 min in the absence or presence of the reagents indicated. For 20-fold dilution, samples were diluted with reaction buffer. After 90 min, all samples were chilled on ice and adjusted to the same volume with reaction buffer. The vacuoles were reisolated by centrifugation, resuspended in 30 μL of ice-cold reaction buffer, and assayed for fusion. (C) Neuronal SNARE-driven vacuole fusion can occur without massive content leakage. Vacuoles from BJ3505 and DKY6281 (bars 1–4) or BJ-Syb2-GFP and DKY-Stx1/S25 (bars 5–16) were used to assay vacuole fusion and lysis. Fusion (black bars) and percent GFP release (gray bars) were measured after 90-min incubation at 27 °C. The data represent mean ± SEM (n = 3).
Neuronal SNAREs Can Induce Biological Membrane Fusion Without Massive Content Leakage.
Yeast vacuolar SNARE proteins overexpressed on vacuoles support Rab-bypass fusion but, at the same time, drive membrane lysis, thus resulting in massive content leakage (2). Similarly, in neuronal SNARE-mediated liposome fusion assays, the protein-to-lipid ratios required for lipid mixing often leads to liposome leakiness (17). The content leakiness of membrane-enclosed compartments containing excess SNARE proteins may be attributed to the tendency of the transmembrane regions of neuronal SNARE proteins to destabilize lipid bilayers (33). Although the neuronal SNARE proteins on vacuoles are significantly less abundant than those on the proteoliposomes used in most studies (17, 34), these proteins are 2- to 48-fold more abundant than yeast SNARE proteins found on native vacuoles (Table S1) (35). Thus, to test whether neuronal SNARE-mediated vacuole fusion is accompanied by content leakage, we genetically targeted green fluorescent protein (GFP) to the vacuolar lumen of the yeast strain BJ-Syb2, generating BJ-Syb2-GFP. By incubating BJ-Syb2-GFP vacuoles with DKY-Stx1/S25 vacuoles in the fusion reaction described above, we were able to assay for fusion and lysis by measuring Pho8p activity and the level of extravacuolar GFP, respectively (2). Consistent with previous observations (2), some lysis occurred during vacuole isolation (Fig. 2C, bar 6), and a low rate of further lysis occurred during incubation at 27 °C (bar 8). Interestingly, however, this lysis did not depend on fusion because it still occurred when fusion was completely inhibited by Syb2-CD (compare bars 8 and 10). By contrast, when fusion was stimulated by the addition of 5 μM his6-SNAP25, some fusion-dependent lysis occurred (compare bars 14 and 16), consistent with the previous finding that the addition of Vam7p, a vacuolar t-SNARE, promoted fusion-dependent lysis during normal vacuole fusion (2). Thus, these results suggest that neuronal SNARE proteins can induce biological membrane fusion at the SNARE protein level that would not lead to massive membrane rupture.
Botulinum Neurotoxin Inhibits Neuronal SNARE-Mediated Vacuole Fusion.
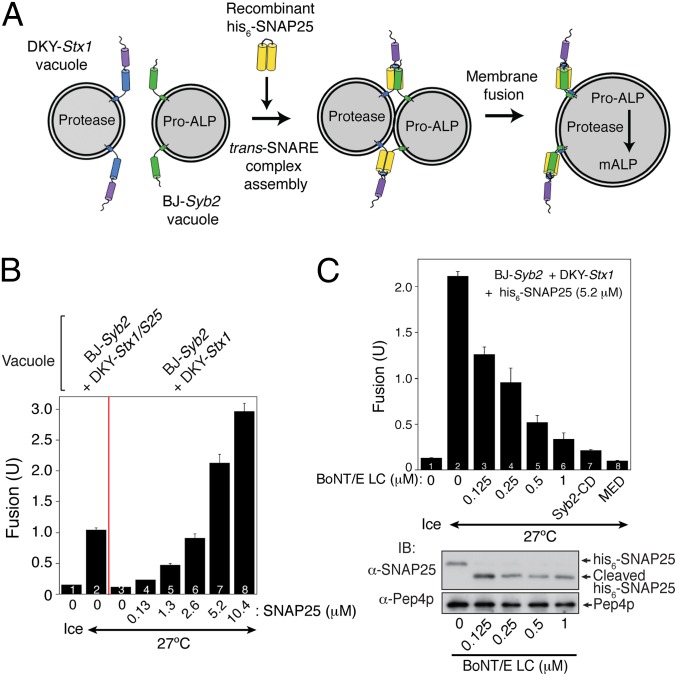
Because SNAP25, unlike syntaxin-1 or synaptobrevin-2, lacks a transmembrane domain, it can be expressed in Escherichia coli, purified, and added directly to fusion reactions containing syntaxin-1-bearing vacuoles (DKY-Stx1) and synaptobrevin-2-bearing vacuoles (BJ-Syb2) (Fig. 3A). As shown in Fig. 3B, no fusion was observed between DKY-Stx1 vacuoles and BJ-Syb2 vacuoles without the addition of recombinant his6-SNAP25 (bar 3). Upon addition of his6-SNAP25, however, fusion signals were observed and increased in proportion to the concentration of his6-SNAP25 (Fig. 3B, bars 4–8). To confirm that his6-SNAP25–triggered vacuole fusion is mediated by the t-SNARE function of SNAP25, we examined whether botulinum neurotoxin serotype E light chain (BoNT/E LC), which removes the last 29 aa of unassembled SNAP25 molecules (Fig. S5A) (36), blocked the fusion (Fig. 3C). Increasing concentrations of BoNT/E LC efficiently prevented his6-SNAP25–triggered fusion between DKY-Stx1 and BJ-Syb2 vacuoles, but had little effect on normal vacuole fusion (Fig. S5B), indicating that the fusion inhibition by BoNT/E LC was specific. These data suggest that fully zippered trans-SNARE complexes are required for neuronal SNARE-mediated vacuole fusion, just as was observed in vivo (37), and for neuronal SNARE-triggered liposome fusion (36, 38).
Fig. 3.
Botulinum neurotoxin inhibits neuronal SNARE-mediated vacuole fusion. (A) Schematic representation of recombinant his6-SNAP25–triggered fusion between vacuoles from BJ-Syb2 and DKY-Stx1. (B) Vacuoles were isolated from BJ-Syb2 and DKY-Stx1, mixed, and incubated at 27 °C in the presence of the indicated concentrations of his6-SNAP25. (C) BoNT/E light chain blocks his6-SNAP25-triggered fusion. Vacuoles isolated from BJ-Syb2 and DKY-Stx1 were mixed and incubated at 27 °C in the presence of his6-SNAP25 with the indicated concentrations of BoNT/E light chain. After 90 min of incubation, ALP activity was measured, and the cleavage of his6-SNAP25 by BoNT/E light chain was analyzed by immunoblotting. The vacuolar protease Pep4p was used as a loading control. The data represent mean ± SEM (n = 3).
Munc18 Can Stimulate Neuronal SNARE-Mediated Vacuole Fusion.
Synaptic vesicle fusion requires not only the neuronal SNARE proteins but also a variety of regulatory proteins, such as Munc18. To examine whether Munc18 can stimulate neuronal SNARE-mediated vacuole fusion, we attempted to coexpress Munc18 with the neuronal t-SNARE proteins syntaxin-1 and SNAP25 by generating the yeast strain DKY-Stx1/S25/M18, which expresses Munc18. Vacuolar localization of Munc18 seemed to require syntaxin-1 to be present on the vacuole because Munc18 was not detected in vacuoles lacking syntaxin-1 (Fig. S6). Interestingly, coexpression of Munc18 with syntaxin-1 and SNAP25 in yeast markedly enhanced syntaxin-1 levels on the vacuole (Fig. 4A). This enhancement is likely to be because Munc18 was recruited to the vacuole by interacting with syntaxin-1, and this interaction may protect syntaxin-1 from degradation by vacuolar proteases. Despite this increased level of syntaxin-1 in the presence of Munc18, DKY-Stx1/S25/M18 vacuoles fused less efficiently with BJ-Syb2 than DKY-Stx1/S25, indicating that Munc18 plays an inhibitory role in neuronal SNARE-mediated vacuole fusion (Fig. 4B). One plausible scenario is that syntaxin-1, when coexpressed with Munc18, preferentially binds to Munc18 over SNAP25 because of its higher affinity for the former (39, 40). Under these conditions, Munc18 is likely to keep syntaxin-1 in a closed, inactive conformation that is unable to participate in the trans-SNARE complex formation that supports membrane fusion (10, 11), as reported in studies using proteoliposomes (41). Because Munc18 is a soluble protein, it can be readily expressed and purified as a recombinant protein from E. coli. To test whether Munc18 plays a stimulatory role in neuronal SNARE-driven vacuole fusion by interacting with preformed syntaxin-1/SNAP25 heterodimers, recombinant his6-Munc18 was generated and directly added to fusion reactions containing DKY-Stx1/S25 and BJ-Syb2 vacuoles. Consistent with the results from previous experiments using liposomes reconstituted with syntaxin-1 and SNAP25 (42, 43), his6-Munc18 moderately enhanced neuronal SNARE-mediated vacuole fusion (Fig. 4C, compare bars 2 and 5), indicative of its role as a positive regulator of neuronal SNARE-mediated membrane fusion. This Munc18-mediated fusion enhancement was even more dramatic in his6-SNAP25-triggered fusion between DKY-Stx1 vacuoles and BJ-Syb2 vacuoles. As shown in Fig. 4D, although 1 μM his6-SNAP25 induced only a marginal amount of fusion in the absence of his6-Munc18 (bar 2), addition of his6-Munc18 markedly enhanced fusion (compare bars 2 and 4). The comparable amounts of syntaxin-1 on DKY-Stx1/S25 and DKY-Stx1 vacuoles (Fig. S7) suggest that the configuration of neuronal t-SNARE proteins is critical for fusion stimulation by Munc18.
Fig. 4.
Munc18 can stimulate neuronal SNARE-mediated vacuole fusion. (A) Immunoblotting analysis of purified vacuoles: Asterisk indicates a degradation product of Munc18. (B) Munc18 coexpressed with neuronal t-SNAREs in yeast inhibits neuronal SNARE-mediated vacuole fusion. Fusion reactions containing BJ-Syb2 vacuoles and either DKY-Stx1/S25 or DKY-Stx1/S25/M18 were incubated at 27 °C. At the times indicated, each reaction was placed on ice. ALP activity was assayed after 90 min as a measure of fusion. (C) Recombinant his6-Munc18 moderately stimulates fusion between BJ-Syb2 and DKY-Stx1/S25 vacuoles. Vacuoles isolated from BJ-Syb2 and DKY-Stx1/S25 were mixed and incubated at 27 °C in the absence or presence of his6-Munc18. (D) Munc18 enhances his6-SNAP25–triggered fusion between BJ-Syb2 and DKY-Stx1 vacuoles. BJ-Syb2 vacuoles and DKY-Stx1 vacuoles were mixed and incubated in the presence of 1 μM his6-SNAP25 with the indicated concentrations of his6-Munc18. (E) The polarity of the synaptobrevin-2 C terminus may not play a critical role in membrane fusion. BJ-Syb2 (wild-type or mutant) vacuoles and DKY-Stx1/S25 vacuoles were mixed and incubated at 27 °C in the absence or presence of Syb2-CD. After 90-min incubation, ALP activity was measured. (F) The expression levels of synaptobrevin-2 (wild-type or mutant) on the vacuoles used in E were analyzed by immunoblotting and quantification of band intensities. The data represent mean ± SEM (n = 3), and the blot shown is a representative image from one of three independent experiments.
Addition of Polar Amino Acids to the C Terminus of Synaptobrevin-2 Has Little Effect on Neuronal SNARE-Driven Vacuole Fusion.
A recent study by Lindau and coworkers suggested that neuronal SNARE complexes pull the C terminus of synaptobrevin-2 deep into the synaptic vesicle membrane, which then disrupts vesicular membrane continuity, leading to fusion pore formation; this conclusion was based on the observation that the addition of polar amino acids to the synaptobrevin-2 C terminus drastically inhibited exocytosis in chromaffin cells and that this inhibition correlated strongly with the polarity of the amino acids (44). However, this earlier study did not directly test whether the added amino acids influenced membrane fusion per se. To clarify this issue using the neuronal SNARE-driven vacuole fusion system, the strains BJ-Syb2-QQ (double glutamine), BJ-Syb2-EE (double glutamic acid), BJ-Syb2-GG (double glycine), and BJ-Syb2-VV (double valine) were generated. Vacuoles isolated from these strains were incubated with DKY-Stx1/S25 vacuoles. After incubation at 27 °C for 90 min, fusion was assayed (Fig. 4E). Although the expression of the synaptobrevin-2 mutants was significantly lower than that of the wild type (Fig. 4F), they supported fusion comparably to the wild type. These results suggest that the polarity of the synaptobrevin C-terminal amino acids is not critical for membrane fusion per se. Furthermore, our data suggest that the neuronal SNARE-driven vacuole fusion assay complements proteoliposome-based assays and offers a simple and independent method for studying synaptic vesicle fusion mechanisms.
Discussion
Although neuronal SNARE proteins are sufficient to drive liposome fusion (15), synaptic vesicle fusion in vivo requires other protein factors, such as Munc18. It was thus unclear whether neuronal SNARE proteins would be sufficient to drive biological membrane fusion. By using cell fusion, which is mediated by flipped SNARE proteins expressed at the cell surface, Rothman and coworkers showed that neuronal SNARE proteins can fuse cells (45). However, the authors used only mutant forms of neuronal SNARE proteins to prevent unnatural glycosylation and disulfide bond formation on the flipped neuronal SNAREs. More importantly, the extracellular leaflet of the plasma membrane differs significantly in chemical composition to the other leaflet, which is normally involved in SNARE-mediated membrane fusion events, including synaptic vesicle fusion. Moreover, because cultured cells can spontaneously fuse with one another under certain conditions (46), one cannot exclude the possibility that flipped SNARE proteins only facilitated the cell fusion process, rather than driving it, and that cell fusion was instead mediated by other cell surface proteins. Finally, the authors did not examine whether the flipped SNARE-mediated cell fusion was accompanied by cell rupture or content leakage.
Compared with reconstitution studies using neuronal SNARE-containing liposomes, the neuronal SNARE-driven vacuole fusion system described here offers several advantages. First, whereas the in vitro fusion of SNARE proteoliposomes is likely to be influenced by the quality and purity of recombinant SNARE proteins made by E. coli, vacuolar expression of neuronal SNARE proteins can be easily achieved by simple introduction of the corresponding genes into yeast cells. Thus, once yeast strains that express neuronal SNARE proteins are established, vacuoles bearing SNARE proteins of nearly identical quality can be consistently prepared for reproducible experiments. As demonstrated in Fig. 4E, the effect of various SNARE mutations on membrane fusion can also be readily investigated without expressing and purifying recombinant proteins carrying the mutations, which is a major source of variability in proteoliposome-based fusion assays. Given the successful expression of neuronal SNARE proteins on yeast vacuoles achieved in this study, it is likely that other mammalian SNARE proteins or even other fusogenic proteins can be similarly expressed on yeast vacuoles, thereby permitting assessment of the mechanisms by which they regulate membrane fusion. Second, proteoliposomes, which are prepared in the presence of detergents, are intrinsically unstable, whereas isolated yeast vacuoles are likely to be stably sealed compartments. Thus, vacuolar membrane fusion may better represent a biologically relevant process than synthetic liposome fusion does.
According to our estimation of the density of neuronal SNARE proteins on vacuoles (Table S1), the lipid/protein ratios of vacuolar synaptobrevin-2, syntaxin-1, and SNAP25 are 2.59 × 104, 4.96 × 104, and 16.0 × 104, respectively. Proteoliposomes with neuronal SNAREs at these low densities are unlikely to support fusion (17, 34). This difference may be explained by a critical role of neutral lipids with small head groups, such as phosphatidylethanolamine, diacylglycerol, and cholesterol, in biological membrane fusion (35). These lipids, which are present in biological membranes but rarely included in the phosphatidylcholine/phosphatidylserine proteoliposomes used in most studies, tend to form a nonbilayer structure, which is essential for the ensuing lipid rearrangements that constitute membrane fusion (35).
Normal vacuole fusion requires the Rab GTPase Ypt7p and its effector, the HOPS complex (47, 48). The major role of Ypt7p is thought to be the recruitment of the HOPS complex to the vacuolar membrane (49), and Ypt7p and the vacuole-bound HOPS complex play an essential role in vacuole docking, during which vacuoles cluster together (48, 50). Thus, it is possible that neuronal SNARE-mediated vacuole fusion requires the preceding Ypt7p/HOPS-mediated docking step. Surprisingly, however, Ypt7p/HOPS-mediated vacuole clustering seems to contribute little to the fusion event because the fusion signal was completely resistant to docking inhibitors (Fig. 2A, bars 5–7). Nonetheless, Ypt7p/HOPS-mediated vacuole docking is likely to occur in neuronal SNARE-mediated vacuole fusion because the vacuoles contain all of the proteins required (50). These results suggest that although the Ypt7p/HOPS-mediated docking step precedes SNARE-mediated membrane fusion, the docking step does not stimulate fusion, presumably because Ypt7p and the HOPS complex cannot communicate with the exogenous SNARE proteins. Thus, the two steps may not simply occur in a linear manner as previously thought; instead, a complicated cross-talk between the two steps may exist to ensure that a membrane docking step mediated by a Rab protein specifically supports a membrane fusion step performed by an appropriate set of SNARE proteins.
In this study, we show that neuronal SNARE proteins can fully support membrane fusion between the vacuoles of the budding yeast Saccharomyces cerevisiae. To our knowledge, this is the first experimental demonstration that an endomembrane fusion event in one species can be mediated by a set of SNARE proteins that function in an evolutionarily distant species, thus strongly supporting the idea that endomembrane fusion machinery is highly conserved throughout evolution. Despite some mechanistic similarities between yeast vacuole fusion and synaptic vesicle fusion events, synaptic vesicle fusion employs several unique players, such as synaptotagmin and complexin, to achieve its extremely high speed and calcium ion dependency. Further studies will therefore be required to establish neuronal SNARE-mediated vacuole fusion, which is strictly regulated by these proteins and by calcium ions.
Methods
Plasmids, yeast strains, and reagents used for this work are described in SI Methods.
Vacuole Isolation and in Vitro Vacuole Fusion Assay.
Vacuoles were isolated as described (23). Standard in vitro fusion reactions (30 μL), for native vacuole fusion, contained 3 μg of vacuoles lacking the proteases Pep4p and Prb1p (BJ3505 or its derivatives), 3 μg of vacuoles from cells without Pho8p (DKY6281 or its derivatives), reaction buffer (125 mM KCl, 5 mM MgCl2, 10 mM Pipes-KOH at pH 6.8, and 200 mM sorbitol), 264 nM purified Pbi2p (IB2), 10 μM CoA, 1 mM ATP, 1 mg/mL creatine kinase, and 29 mM creatine phosphate. After 90 min of incubation at 27 °C, fusion was measured by assaying ALP. For neuronal SNARE-mediated vacuole fusion, standard 30-μL fusion reactions contained 3 μg of BJ-Syb2 vacuoles, 3 μg of DKY-Stx1/S25 vacuoles, reaction buffer, 264 nM purified IB2, and 10 μM CoA. After a 90-min incubation at 27 °C in the absence of ATP, the activity of ALP was measured for fusion. Fusion units (U) are µmol of p-nitrophenylate formed min−1⋅µg−1 pep4∆ vacuole.
GFP Release Assay.
GFP release assays were performed with vacuoles containing luminal GFP isolated from BJ3505-GFP or BJ-Syb2-GFP, and vacuoles isolated from DKY6281 or DKY-Stx1/S25, as described (2). Standard vacuole fusion reactions were used for the GFP release assay, and each reaction was performed on a 3× scale (90 µL). After 90 min, 30-µL aliquots were transferred to a prechilled tube and kept on ice until being assayed for Pho8p activity (fusion). For GFP detection, 30-µL aliquots were placed in a prechilled tube containing 30 µL of PS buffer (10 mM Pipes⋅KOH, pH 6.8 and 200 mM sorbitol) with 125 mM KCl.
Supplementary Material
Acknowledgments
We thank Drs. Nicolas Buchler (Duke University) and Frederick Cross (Rockefeller University) for kindly providing the plasmid p405TDH3 and Dr. Christopher Stroupe (University of Virginia) for his advice on measurement of vacuole lipid levels. This work was supported by the Bio-Imaging Research Center at Gwangju Institute of Science and Technology and by Science Research Center of Excellence Program of Korea Ministry of Science, ICT & Future Planning/National Research Foundation of Korea Grant 2007-0056157.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400036111/-/DCSupplemental.
References
- 1.Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003;112(4):519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 2.Starai VJ, Jun Y, Wickner W. Excess vacuolar SNAREs drive lysis and Rab bypass fusion. Proc Natl Acad Sci USA. 2007;104(34):13551–13558. doi: 10.1073/pnas.0704741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Südhof TC. Membrane fusion as a team effort. Proc Natl Acad Sci USA. 2007;104(34):13541–13542. doi: 10.1073/pnas.0706168104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNew JA, Weber T, Engelman DM, Söllner TH, Rothman JE. The length of the flexible SNAREpin juxtamembrane region is a critical determinant of SNARE-dependent fusion. Mol Cell. 1999;4(3):415–421. doi: 10.1016/s1097-2765(00)80343-3. [DOI] [PubMed] [Google Scholar]
- 5.Li F, et al. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat Struct Mol Biol. 2007;14(10):890–896. doi: 10.1038/nsmb1310. [DOI] [PubMed] [Google Scholar]
- 6.Südhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol. 2011;3(12):3. doi: 10.1101/cshperspect.a005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490(7419):201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hata Y, Slaughter CA, Südhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366(6453):347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 9.Geppert M, et al. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79(4):717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 10.Dulubova I, et al. A conformational switch in syntaxin during exocytosis: Role of munc18. EMBO J. 1999;18(16):4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404(6776):355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 12.Rodkey TL, Liu S, Barry M, McNew JA. Munc18a scaffolds SNARE assembly to promote membrane fusion. Mol Biol Cell. 2008;19(12):5422–5434. doi: 10.1091/mbc.E08-05-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brose N, Petrenko AG, Südhof TC, Jahn R. Synaptotagmin: A calcium sensor on the synaptic vesicle surface. Science. 1992;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 14.Bhalla A, Chicka MC, Tucker WC, Chapman ER. Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat Struct Mol Biol. 2006;13(4):323–330. doi: 10.1038/nsmb1076. [DOI] [PubMed] [Google Scholar]
- 15.Weber T, et al. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92(6):759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 16.Gad AE, Broza R, Eytan GD. Calcium-induced fusion of proteoliposomes and protein-free liposomes. Effect of their phosphatidylethanolamine content on the structure of fused vesicles. Biochim Biophys Acta. 1979;556(2):181–195. doi: 10.1016/0005-2736(79)90041-5. [DOI] [PubMed] [Google Scholar]
- 17.Dennison SM, Bowen ME, Brunger AT, Lentz BR. Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys J. 2006;90(5):1661–1675. doi: 10.1529/biophysj.105.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Seven AB, Su L, Jiang Q-X, Rizo J. Membrane bridging and hemifusion by denaturated Munc18. PLoS ONE. 2011;6(7):e22012. doi: 10.1371/journal.pone.0022012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wickner W. Yeast vacuoles and membrane fusion pathways. EMBO J. 2002;21(6):1241–1247. doi: 10.1093/emboj/21.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wickner W. Membrane fusion: Five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- 21.Ostrowicz CW, Meiringer CTA, Ungermann C. Yeast vacuole fusion: A model system for eukaryotic endomembrane dynamics. Autophagy. 2008;4(1):5–19. doi: 10.4161/auto.5054. [DOI] [PubMed] [Google Scholar]
- 22.Jun Y, Wickner W. Assays of vacuole fusion resolve the stages of docking, lipid mixing, and content mixing. Proc Natl Acad Sci USA. 2007;104(32):13010–13015. doi: 10.1073/pnas.0700970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994;126(1):87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sander P, et al. Heterologous expression of the human D2S dopamine receptor in protease-deficient Saccharomyces cerevisiae strains. Eur J Biochem. 1994;226(2):697–705. doi: 10.1111/j.1432-1033.1994.tb20098.x. [DOI] [PubMed] [Google Scholar]
- 25.Jun Y, et al. Reversible, cooperative reactions of yeast vacuole docking. EMBO J. 2006;25(22):5260–5269. doi: 10.1038/sj.emboj.7601413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127(4):831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W. Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol. 2004;167(6):1087–1098. doi: 10.1083/jcb.200409068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85(1):83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 29.Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97(17):9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins KM, Wickner WT. Trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc Natl Acad Sci USA. 2007;104(21):8755–8760. doi: 10.1073/pnas.0702290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eitzen G, Will E, Gallwitz D, Haas A, Wickner W. Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J. 2000;19(24):6713–6720. doi: 10.1093/emboj/19.24.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert S, Will E, Gallwitz D. Identification of the catalytic domains and their functionally critical arginine residues of two yeast GTPase-activating proteins specific for Ypt/Rab transport GTPases. EMBO J. 1999;18(19):5216–5225. doi: 10.1093/emboj/18.19.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langosch D, et al. Peptide mimics of SNARE transmembrane segments drive membrane fusion depending on their conformational plasticity. J Mol Biol. 2001;311(4):709–721. doi: 10.1006/jmbi.2001.4889. [DOI] [PubMed] [Google Scholar]
- 34.Ji H, et al. Protein determinants of SNARE-mediated lipid mixing. Biophys J. 2010;99(2):553–560. doi: 10.1016/j.bpj.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zick M, Stroupe C, Orr A, Douville D, Wickner WT. Membranes linked by trans-SNARE complexes require lipids prone to non-bilayer structure for progression to fusion. Elife. 2014;3:e01879. doi: 10.7554/eLife.01879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuette CG, et al. Determinants of liposome fusion mediated by synaptic SNARE proteins. Proc Natl Acad Sci USA. 2004;101(9):2858–2863. doi: 10.1073/pnas.0400044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keller JE, Neale EA. The role of the synaptic protein snap-25 in the potency of botulinum neurotoxin type A. J Biol Chem. 2001;276(16):13476–13482. doi: 10.1074/jbc.M010992200. [DOI] [PubMed] [Google Scholar]
- 38.Melia TJ, et al. Regulation of membrane fusion by the membrane-proximal coil of the t-SNARE during zippering of SNAREpins. J Cell Biol. 2002;158(5):929–940. doi: 10.1083/jcb.200112081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pevsner J, et al. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13(2):353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 40.Rickman C, Meunier FA, Binz T, Davletov B. High affinity interaction of syntaxin and SNAP-25 on the plasma membrane is abolished by botulinum toxin E. J Biol Chem. 2004;279(1):644–651. doi: 10.1074/jbc.M310879200. [DOI] [PubMed] [Google Scholar]
- 41.Ma C, Su L, Seven AB, Xu Y, Rizo J. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science. 2013;339(6118):421–425. doi: 10.1126/science.1230473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathore SS, et al. Syntaxin N-terminal peptide motif is an initiation factor for the assembly of the SNARE-Sec1/Munc18 membrane fusion complex. Proc Natl Acad Sci USA. 2010;107(52):22399–22406. doi: 10.1073/pnas.1012997108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128(1):183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 44.Ngatchou AN, et al. Role of the synaptobrevin C terminus in fusion pore formation. Proc Natl Acad Sci USA. 2010;107(43):18463–18468. doi: 10.1073/pnas.1006727107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu C, et al. Fusion of cells by flipped SNAREs. Science. 2003;300(5626):1745–1749. doi: 10.1126/science.1084909. [DOI] [PubMed] [Google Scholar]
- 46.Mortensen K, Lichtenberg J, Thomsen PD, Larsson L-I. Spontaneous fusion between cancer cells and endothelial cells. Cell Mol Life Sci. 2004;61(16):2125–2131. doi: 10.1007/s00018-004-4200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J Cell Biol. 1997;136(2):307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25(8):1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hickey CM, Stroupe C, Wickner W. The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association. J Biol Chem. 2009;284(24):16118–16125. doi: 10.1074/jbc.M109.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stroupe C, Hickey CM, Mima J, Burfeind AS, Wickner W. Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components. Proc Natl Acad Sci USA. 2009;106(42):17626–17633. doi: 10.1073/pnas.0903801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.