Significance
There is an unmet need for a more effective, faster-acting, and safer therapy for the life-threatening acute neurovisceral attacks that occur in the acute hepatic porphyrias. Recent studies indicate that the acute attacks are primarily caused by the neurotoxic porphyrin precursors 5-aminolevulinic acid and porphobilinogen, which accumulate as a consequence of the markedly induced expression of hepatic 5-aminolevulinic acid synthase 1 (ALAS1). Here, we demonstrate that liver-targeted small interfering RNAs specific for Alas1 are highly effective in preventing and treating the biochemically induced acute attacks in a mouse model of acute intermittent porphyria, the most common acute hepatic porphyria. These preclinical studies provide proof-of-concept for the clinical development of RNAi-mediated therapy for the acute porphyric attacks.
Keywords: RNAi therapeutics, liver-targeted siRNA, heme biosynthetic disorders
Abstract
The acute hepatic porphyrias are inherited disorders of heme biosynthesis characterized by life-threatening acute neurovisceral attacks. Factors that induce the expression of hepatic 5-aminolevulinic acid synthase 1 (ALAS1) result in the accumulation of the neurotoxic porphyrin precursors 5-aminolevulinic acid (ALA) and porphobilinogen (PBG), which recent studies indicate are primarily responsible for the acute attacks. Current treatment of these attacks involves i.v. administration of hemin, but a faster-acting, more effective, and safer therapy is needed. Here, we describe preclinical studies of liver-directed small interfering RNAs (siRNAs) targeting Alas1 (Alas1-siRNAs) in a mouse model of acute intermittent porphyria, the most common acute hepatic porphyria. A single i.v. dose of Alas1-siRNA prevented the phenobarbital-induced biochemical acute attacks for approximately 2 wk. Injection of Alas1-siRNA during an induced acute attack significantly decreased plasma ALA and PBG levels within 8 h, more rapidly and effectively than a single hemin infusion. Alas1-siRNA was well tolerated and a therapeutic dose did not cause hepatic heme deficiency. These studies provide proof-of-concept for the clinical development of RNA interference therapy for the prevention and treatment of the acute attacks of the acute hepatic porphyrias.
The acute hepatic porphyrias are caused by the deficient activities of specific enzymes in the heme biosynthetic pathway and include three autosomal dominant diseases—acute intermittent porphyria (AIP), hereditary coproporphyria (HCP), and variegate porphyria (VP)—and the autosomal recessive 5-aminolevulinic acid dehydratase deficiency porphyria (ADP) (1). These inherited metabolic disorders are characterized by life-threatening acute neurovisceral attacks that include severe abdominal pain, hypertension, tachycardia, constipation, motor weakness, seizures, and paralysis (1). Various factors, including cytochrome P450 (CYP)-inducing drugs, dieting, and hormonal changes precipitate the acute attacks by increasing the expression of hepatic 5-aminolevulinic acid synthase (ALAS1), the first and rate-limiting enzyme of the heme biosynthetic pathway (2–6). When hepatic ALAS1 is induced, the respective enzyme deficiencies create a metabolic bottleneck, thereby decreasing hepatic heme production and depleting the “free” heme pool. This leads to the loss of the negative feedback inhibition of heme on ALAS1 (7–9) and, consequently, further up-regulation of hepatic ALAS1 expression. As hydroxymethylbilane synthase (HMBS)—the third enzyme in the heme biosynthetic pathway—is less abundant in comparison with the other heme biosynthetic enzymes (10), it becomes rate-limiting when ALAS1 is markedly increased. As a result, the neurotoxic porphyrin precursors 5-aminolevulinic acid (ALA) and porphobilinogen (PBG) are overproduced in the liver and are markedly accumulated in the plasma and urine during the acute attacks (only ALA accumulates in ADP).
Currently, the standard therapy for the acute neurovisceral attacks is the i.v. administration of hemin, which provides exogenous heme for the negative feedback inhibition of ALAS1, thereby decreasing ALA and PBG production (11–13). Although patients generally respond well, its effect is slow, typically requiring three to four daily infusions to normalize the elevated plasma and urinary ALA and PBG concentrations (14). Patients who experience recurring attacks are treated prophylactically with weekly to monthly hemin infusions, which are associated with side effects such as phlebitis and iron overload (15). Thus, an alternative therapeutic approach for the acute hepatic porphyrias that is more effective, faster-acting, and safer is desirable.
Previously, the pathogenic mechanism of the acute neurovisceral attacks was unclear, particularly whether the attacks were due to the neurotoxic effects of the accumulated ALA and/or PBG or to the heme deficiency in neuronal tissues (16). However, nonporphyric liver transplant recipients who received livers from AIP patients (i.e., “domino” liver transplants) developed acute porphyric attacks despite their presumably normal neuronal heme levels, which demonstrated that the primary pathogenic mechanism is not neuronal heme deficiency but rather the effects of the neurotoxic hepatic metabolites ALA and/or PBG (17). Consistent with this finding, orthotopic liver transplantation in AIP patients and a VP patient with recurrent and incapacitating attacks resulted in the rapid normalization of ALA and PBG levels and the abrupt remission of attacks (18–20). Thus, the liver is the primary site of pathology in the acute hepatic porphyrias, and therapies designed to inhibit the induced overexpression of ALAS1 and the resulting elevation of ALA and PBG would prevent or treat the acute attacks in all four acute hepatic porphyrias.
Here, we report preclinical studies of a liver-directed RNA interference (RNAi) therapy using an i.v.-administered small interfering RNA (siRNA) specific for the Alas1 mRNA (designated Alas1-siRNA). To identify a potent Alas1-siRNA, a panel of siRNAs targeting Alas1 was initially screened for their ability to inhibit Alas1 mRNA expression in cultured hepatic cells. The most active compounds were formulated into lipid nanoparticles (LNPs) for efficient hepatic delivery (21–23) and evaluated for their ability to down-regulate hepatic Alas1 mRNA expression in wild-type mice. The selected Alas1-siRNA was then evaluated in the mouse model for AIP for its effectiveness to prevent or treat an induced acute attack. These mice have ∼30% of wild-type HMBS activity and slightly elevated baseline plasma and urinary ALA and PBG levels (24). When phenobarbital (PB), a potent inducer of ALAS1 expression, is administered, ALA and PBG are markedly increased (20- to 30-fold and 40- to 90-fold in plasma, respectively), mimicking the biochemical pathology of a human acute attack (1, 24). Prophylactic administration of Alas1-siRNA in the AIP mice completely prevented the PB-induced accumulation of plasma and urinary ALA and PBG, whereas infusion of Alas1-siRNA after induction of an acute attack rapidly and effectively decreased the markedly elevated plasma ALA and PBG.
Results
Selected Alas1-siRNA Robustly Silenced Endogenous Alas1 mRNA Expression in Wild-Type Mice.
To identify a potent siRNA sequence, 45 Alas1-targeted siRNAs were evaluated for their ability to repress Alas1 mRNA expression in a murine BNL-C12 hepatocyte cell line. The two most active siRNAs were individually formulated into LNPs and i.v. administered to wild-type mice, and their ability to silence hepatic Alas1 mRNA expression in vivo was assessed. The most potent Alas1-siRNA (designated Alas1-siRNA1) achieved a dose-dependent knockdown of hepatic Alas1 expression 48 h after administration (Fig. S1). The highest dose (1.0 mg/kg) facilitated a robust ∼75% reduction of the endogenous hepatic Alas1 mRNA. Therefore, this dose was used for subsequent studies in the AIP mice unless otherwise specified.
Prophylactic Administration of Alas1-siRNA1 Prevented the PB-Induced Up-Regulation of Hepatic Alas1 Expression and the Resultant Accumulation of Plasma and Urinary ALA and PBG in the AIP mouse Model.
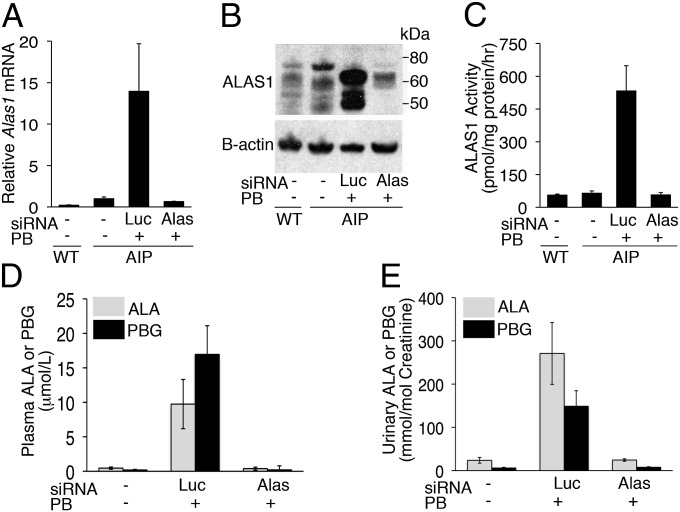
Following i.v. administration of Alas1-siRNA1, the AIP mice were challenged with three daily PB injections to investigate whether the Alas1-siRNA1 effectively prevented the PB-induced hepatic Alas1 expression. As shown in Fig. 1A, when the AIP mice were treated with a luciferase-targeted siRNA (Luc-siRNA), their hepatic Alas1 mRNA levels increased ∼14-fold over mean baseline levels 16 h after the last PB dose. In contrast, AIP mice treated with the Alas1-siRNA1 retained baseline Alas1 mRNA levels after PB induction. Consistent with these findings, hepatic ALAS1 protein levels were markedly increased following PB induction in the Luc-siRNA controls, whereas they were comparable to baseline levels in the Alas1-siRNA1–treated mice (Fig. 1B). Hepatic ALAS1 enzymatic activities were elevated ∼11-fold from mean baseline levels of 64 ± 10 to 533 ± 115 pmol/mg protein/h in the Luc-siRNA controls after PB induction. On the contrary, mice treated with the Alas1-siRNA1 had near-baseline hepatic ALAS1 activities of 57 ± 11 pmol/mg protein/h (Fig. 1C), thus indicating that these mice were protected from the PB-induced increases of hepatic Alas1 expression.
Fig. 1.
Prophylactic Alas1-siRNA1 prevents PB-induced hepatic Alas1 expression and plasma and urinary ALA and PBG accumulation. AIP mice were i.v. administered Alas1-siRNA1 or Luc-siRNA and then subjected to three daily PB injections starting the following day. Liver, plasma, and urine samples were collected ∼16 h after the last PB injection. Hepatic Alas1 (A) mRNA, (B) protein expression levels, and (C) enzymatic activities were determined. ALA and PBG concentrations were measured in the (D) plasma and (E) urine. Data from wild-type (WT) mice are also shown. In A and C–E, data are presented as mean ± SD (n = 3–8). (B) A representative Western blot image.
To evaluate whether inhibition of the PB-induced Alas1 expression abrogated the induction of the neurotoxic porphyrin precursors, plasma and urinary ALA and PBG levels were determined in a similar set of AIP mice as those used to assess hepatic Alas1 expression levels (Fig. 1 A–C). Sixteen hours after the last PB injection, mean plasma ALA and PBG concentrations in the Luc-siRNA controls were elevated ∼21- and 91-fold from baseline levels of 0.46 ± 0.16 and 0.19 ± 0.16 μmol/L to 9.7 ± 3.6 and 17 ± 4.2 μmol/L, respectively (Fig. 1D). Urinary ALA and PBG concentrations were increased ∼11- and 25-fold from baseline levels of 24 ± 6.6 and 6.1 ± 1.5 mmol/mol creatinine to 271 ± 72 and 149 ± 36 mmol/mol creatinine, respectively, consistent with previous studies (Fig. 1E) (24, 25). In contrast, mice treated with Alas1-siRNA1 had plasma ALA and PBG levels of 0.38 ± 0.22 and 0.20 ± 0.59 μmol/L and urinary ALA and PBG levels of 25 ± 2.6 and 7.7 ± 1.3 mmol/mol creatinine, respectively, all of which were comparable to baseline levels (Fig. 1 D and E). Thus, prophylactic injection of the Alas1-siRNA1 completely prevented the PB-induced up-regulation of hepatic Alas1 expression and the accumulation of plasma and urinary ALA and PBG in the AIP mice.
A Single Administration of Alas1-siRNA1 Was Protective Against the Induction of an Acute Attack for Approximately 2 Weeks.
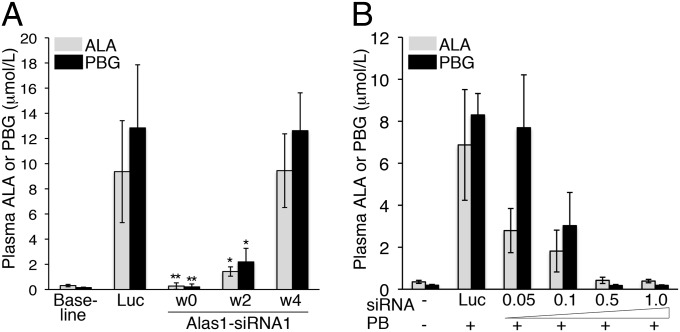
The durability of a single prophylactic dose of Alas1-siRNA1 to prevent the biochemical induction of an acute attack was assessed. As demonstrated above, the AIP mice were completely protected from the PB-induced increases of plasma ALA and PBG when the Alas1-siRNA1 was injected and then immediately challenged with three daily doses of PB (week 0; Fig. 2A). When subjected to three daily PB injections 2 wk later, mean plasma ALA and PBG increased ∼5- and ∼15-fold over mean baseline levels to 1.4 ± 0.37 and 2.2 ± 1.1 μmol/L, respectively. Importantly, these levels were significantly lower (P < 0.005) compared with the mean plasma ALA and PBG concentrations in the Luc-siRNA controls, which were elevated ∼30- and ∼86-fold over mean baseline levels to 9.4 ± 4.1 and 12.9 ± 5.0 μmol/L, respectively. By the fourth week, daily PB injections resulted in similarly increased plasma ALA and PBG levels in both the Alas1-siRNA1– and Luc-siRNA–treated AIP mice (Fig. 2A). Thus, a single Alas1-siRNA1 injection provided significant protection against the induction of an acute attack for approximately 2 wk.
Fig. 2.
Preventive effect of a single Alas1-siRNA1 dose is durable and dose-dependent. (A) AIP mice were i.v. administered the Alas1-siRNA1 or Luc-siRNA and then subjected to three daily PB injections at weeks (w) 0, 2, and 4. Plasmas were collected ∼16 h after the last PB injection, and the ALA and PBG levels were determined. Data shown are means ± SDs (n = 4–7). *P < 0.005 versus Luc controls and **P < 0.0005 versus Luc controls, Student t test. (B) AIP mice were i.v. administered 1.0 mg/kg Luc-siRNA or 0.05, 0.1, 0.5, or 1.0 mg/kg of the Alas1-siRNA1 and then challenged with three daily PB injections. Plasma ALA and PBG levels were determined and are shown as mean ± SD (n = 3–6).
Alas1-siRNA1–Mediated Suppression of Plasma ALA and PBG Was Dose Responsive.
To investigate the dose–response relationship between Alas1-siRNA1 and its ability to prevent the induction of an acute attack, AIP mice were administered varying doses of the Alas1-siRNA1 (0.05, 0.1, 0.5, and 1.0 mg/kg) and then challenged with three daily PB injections. A clear dose–response effect was observed, as shown in Fig. 2B. When AIP mice were treated with 0.05 mg/kg of the Alas1-siRNA1, the highest levels of plasma ALA and PBG were observed: ∼8- and ∼40-fold elevated over mean baseline values, respectively. These levels were only slightly lower than those following injection of the Luc-siRNA, which increased plasma ALA and PBG levels to ∼20- and ∼44-fold over mean baseline levels, respectively. AIP mice treated with 0.1 mg/kg Alas1-siRNA1 had moderately elevated levels of plasma ALA and PBG, whereas those treated with 0.5 and 1.0 mg/kg retained baseline plasma ALA and PBG levels (Fig. 2B).
Alas1-siRNA1 Rapidly Reduced Plasma ALA and PBG Levels During an Ongoing Acute Attack.
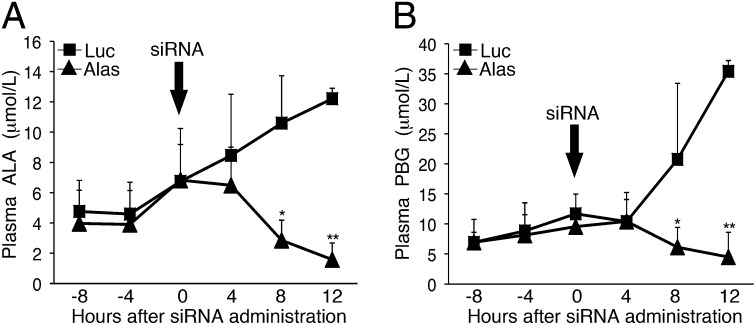
AIP mice were administered three daily PB doses to induce a biochemical acute attack with markedly elevated plasma ALA and PBG concentrations. Then the mice were injected with the Alas1-siRNA1 or the Luc-siRNA to evaluate the effectiveness and rapidity of Alas1-siRNA1 to reduce plasma porphyrin precursor levels. As shown in Fig. 3, the plasma ALA and PBG levels continually increased in the mice injected with the Luc-siRNA, whereas in the Alas1-siRNA1–treated mice they were markedly and significantly decreased within 8 h of administration. At 8 h postinjection, mean plasma ALA and PBG in the Alas1-siRNA1–treated mice were 2.9 ± 1.3 and 6.1 ± 3.3 μmol/L, respectively, ∼70% lower than those in the Luc-siRNA controls (P < 0.05; Fig. 3 A and B). These levels further decreased at 12 h postadministration, at which time plasma ALA and PBG levels were 1.6 ± 1.1 and 4.5 ± 4.1 μmol/L, respectively, ∼87% lower than those in the Luc-siRNA controls (P < 0.00005; Fig. 3 A and B).
Fig. 3.
Alas1-siRNA1 rapidly decreases plasma ALA and PBG concentrations during an ongoing acute attack. AIP mice were biochemically induced with three daily PB injections (110, 130, and 150 mg/kg) and then treated with i.v. ALAS1-siRNA1 (n = 4) or Luc-siRNA (n = 3) ∼12 h after the last PB dose. Plasmas were collected every 4 h, and (A) ALA and (B) PBG levels were monitored. Data represent mean ± SD. *P < 0.05 and **P < 0.00005, Student t test.
A Single Dose of Alas1-siRNA1 Reduced Plasma ALA and PBG Levels More Rapidly and Effectively than a Single Hemin Infusion.
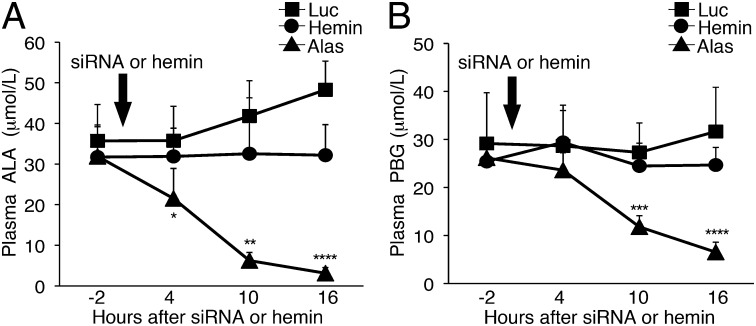
To directly compare the effectiveness of single doses of Alas1-siRNA1 (2.0 mg/kg) and hemin (4.0 mg/kg) to reduce the biochemically induced levels of plasma ALA and PBG, the AIP mice were injected with Alas1-siRNA1, hemin, or Luc-siRNA (2.0 mg/kg). To maximize the induction of ALA and PBG, the biochemical induction was performed using three daily doses of PB and 3,5-diethoxycarbonyl-1,4-dihydro-2,4,6-trimethylpyridine (DDC), an inhibitor of the last enzyme in the heme biosynthetic pathway, ferrochelatase. Plasma ALA and PBG concentrations measured 2 h before siRNA or hemin treatment (i.e., ∼6 h after the last PB/DDC dose) were markedly elevated to 32–36 and 25–30 μmol/L (∼90- to 100-fold and 150- to 170-fold over mean baseline levels), respectively (Fig. 4 A and B). Administration of Alas1-siRNA1 rapidly decreased the mean plasma ALA levels from 32 μmol/L to 22, 6.2, and 3.1 μmol/L (corresponding to decreases of 32, 80, and 90%) at 4, 10, and 16 h postinjection, respectively (Fig. 4A). Mean plasma PBG concentrations significantly decreased from 26 to 12 and 6.5 μmol/L (corresponding to decreases of 55 and 75%) at 10 and 16 h postinjection (Fig. 4B), respectively. In contrast, a single hemin administration resulted in essentially unchanged plasma ALA and PBG concentrations at 4, 10, and 16 h postinjection (Fig. 4 A and B). AIP mice that were administered the Luc-siRNA, on the contrary, further increased their plasma ALA and PBG concentrations. In particular, plasma ALA concentrations increased from pretreatment levels of 36 to 42 and 48 μmol/L (corresponding to increases of 17 and 35%) at 10 and 16 h postinjection, respectively (Fig. 4A). PBG levels also increased slightly from 29 to 32 μmol/L (10% increase) at 16 h (Fig. 4B).
Fig. 4.
Alas1-siRNA1 reduces biochemically induced plasma ALA and PBG levels more rapidly and effectively than hemin. Biochemical attacks were induced in the AIP mice by administration of three daily doses of PB (90, 100, and 110 mg/kg) and DDC (20 mg/kg). About 8 h following the last PB/DDC dose, the mice were administered a single i.v. dose of Alas1-siRNA1 (2.0 mg/kg; n = 7), Luc-siRNA (2.0 mg/kg; n = 4), or hemin (4.0 mg/kg; n = 5). Plasma (A) ALA and (B) PBG levels were monitored every 6 h and are presented as mean ± SD. *P < 0.05 vs. hemin, **P < 0.005 vs. hemin, ***P < 0.0005 vs. hemin, and ****P < 0.00005 vs. hemin, Student t test.
Alas1-siRNA1 Protects Against the Neuromotor Decline Elicited by Repeated Induction of Acute Attacks.
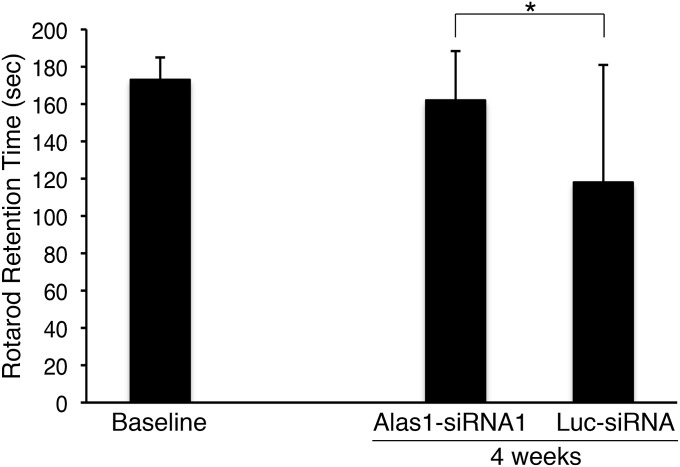
Recent studies have demonstrated that repeated induction of the biochemical attacks in the AIP mice result in neuromotor impairment (26). Therefore, to assess the impact of Alas1-siRNA1 treatment on neuromotor function by rotarod studies, AIP mice were administered Alas1-siRNA1 or Luc-siRNA (both at 2.0 mg/kg) every 1–2 wk and subjected to three daily PB/DDC inductions every week. After four weekly PB/DDC inductions, the Alas1-siRNA1–treated mice were able to stay on the rotarod for an average time of 162 ± 26 s during a 3-min test, whereas the Luc-siRNA controls achieved only 118 ± 63 s (P = 0.0042; Fig. 5). It should be noted that neuromotor decline was not consistently induced in the Luc-siRNA controls using PB/DDC, and rotarod performances at 4 wk varied greatly among the individual mice (range: 12–180 s).
Fig. 5.
Alas1-siRNA1 treatment protects against induced neuromotor decline. AIP mice were administered Alas1-siRNA1 (n = 20) or Luc-siRNA (n = 16; both at 2.0 mg/kg) every 1–2 wk and subjected each week to three daily PB (100, 110, and 120 mg/kg/d) and DDC (25 mg/kg) inductions for 4 wk. Neuromotor function was evaluated on a rotarod apparatus rotating at 16 rpm for a maximal time of 180 s. Rotarod testing was performed ∼30 h after the last PB/DDC dose. Data represent mean ± SD. *P = 0.0042, Student t test.
Alas1-siRNA1 Was Well Tolerated at High Doses and Did Not Alter Hepatic Heme Status.
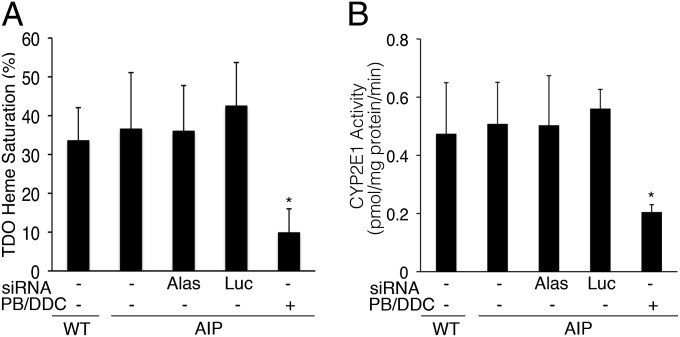
AIP mice i.v.-injected with 1.0, 2.0, or 4.0 mg/kg of Alas1-siRNA1 appeared healthy over a 2-wk period and had normal liver alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels at 1 wk postadministration (Table S1). Of note, these doses represent a range of two- to eightfold over the minimum dose (0.5 mg/kg) required to completely prevent the induction of a biochemical acute attack (Fig. 2B). As ALAS1 catalyzes the first and rate-limiting step of the heme biosynthetic pathway, a potential safety concern was whether the Alas1-siRNA1 would decrease the hepatic “free” heme pool to levels low enough to interfere with hemoprotein function. Thus, hepatic heme status was assessed in the AIP mice treated with the Alas1-siRNA1 (1.0 mg/kg) by determining heme saturation of hepatic tryptophan 2,3-dioxygenase (TDO). TDO is a heme-dependent liver enzyme required for tryptophan metabolism and has previously been shown to be sensitive to decreased heme availability (27, 28). At 24 h after administration of the Alas1-siRNA1, heme saturation of TDO remained at baseline levels of ∼35%, comparable to levels in the wild-type mice (Fig. 6A). This was in contrast to the ∼10% heme saturation in the AIP mice treated with three daily injections of PB/DDC. When the same set of mice was evaluated for hepatic CYP2E1 catalytic activity as a measure of hemoprotein function, untreated and Alas1-siRNA1–treated AIP mice had comparable mean CYP2E1 activities: 0.51 ± 0.14 and 0.50 ± 0.14 pmol/mg protein/min, respectively (Fig. 6B). These levels were similar to the mean baseline level in the wild-type mice: 0.47 ± 0.18 pmol/mg protein/min.
Fig. 6.
Alas1-siRNA1 administration does not alter hepatic heme status or CYP2E1 activity. AIP mice were administered a single 1.0 mg/kg i.v. dose of Alas1-siRNA1 or Luc-siRNA, and the livers were isolated ∼24 h later to assess (A) heme saturation of TDO and (B) CYP2E1 activity. Data are shown as mean ± SD (n = 4–6). *P < 0.01, ANOVA.
Discussion
RNAi therapeutics are an emerging class of drugs that offer the potential to inhibit the expression of virtually any gene of interest. This therapeutic strategy takes advantage of a naturally occurring regulatory process known as RNAi, in which double-stranded RNAs are cleaved into short fragments called siRNAs, which then bind to mRNAs in a sequence-specific manner and trigger the degradation of their transcripts (29, 30). During the past decade, efforts have focused on developing methods for efficient and safe delivery of siRNAs to various tissues, particularly the liver. Methods for hepatic delivery involve various encapsulation techniques such as LNPs or the conjugation of a ligand for uptake by liver-specific receptors [e.g., N-acetylgalactosamine (GalNAc) for the hepatocyte asialoglycoprotein receptor (21–23)]. Currently, liver-directed RNAi therapies are being evaluated in clinical trials for the treatment of a broad range of disorders, including transthyretin (TTR) amyloidosis (31), hepatitis C (32), liver cancers (33), and familial hypercholesterolemia (34, 35). Recent phase I and II studies for TTR amyloidosis have demonstrated safe, robust, and durable silencing of TTR (31), clinically validating the safety and efficacy of LNP-based siRNA approaches.
Here, we describe preclinical studies of LNP-encapsulated siRNAs directed against Alas1 for the prevention and treatment of the acute neurovisceral attacks that occur in the four acute hepatic porphyrias AIP, VP, HCP, and ADP. RNAi is an attractive therapeutic approach for these disorders, as it is now evident that the main site of pathology of the acute hepatic porphyrias is the liver. That hemin, the current treatment, exerts its therapeutic effect by providing exogenous heme for the negative feedback inhibition of hepatic ALAS1 validates the use of siRNAs to repress hepatic ALAS1 expression for the prevention and treatment of the acute attacks. Although hemin is effective, it is slow to take effect and is associated with various side effects such as phlebitis, thrombosis, and iron overload (15). Recently, orthotopic liver transplantation has successfully treated several acute hepatic porphyria patients with recurrent and uncontrollable attacks (18–20), but it is associated with high morbidity and limited organ availability and should be reserved as a last-resort treatment. Thus, there is a clear need for a more effective, faster-acting, and safer therapy.
To this end, efforts were directed to develop a potent Alas1-siRNA therapy by evaluating a panel of siRNAs for their ability to inhibit hepatic Alas1 expression in vitro and in vivo (Fig. S1). The selected Alas1-siRNA1 was evaluated in an AIP mouse model for its ability to prevent or treat a biochemically induced acute attack. Prophylactic administration of Alas1-siRNA1 completely prevented the PB-induced accumulation of plasma and urinary ALA and PBG and thus fully protected the mice from induction of an acute attack (Fig. 1 D and E). The protective effect was indeed mediated through the inhibition of hepatic Alas1 mRNA expression (Fig. 1 A–C). The ability of Alas1-siRNA1 to prevent an acute attack was dose-dependent (Fig. 2B) and durable (Fig. 2A). In fact, a single prophylactic dose of Alas1-siRNA1 provided significant protection against the induction of an acute attack for ∼2 wk (Fig. 2A). This may represent a major advantage over prophylactic hemin, which is infused as often as twice a week in patients with frequent attacks. When Alas1-siRNA1 was administered during an ongoing acute attack, it significantly decreased plasma ALA and PBG levels within 8 h (Fig. 3 A and B), more rapidly and effectively than a single standard dose of hemin (Fig. 4 A and B). The Alas1-siRNA1 decreased ALA more rapidly than it did PBG (Figs. 3 and 4), consistent with the fact that ALA is the immediate downstream metabolite of ALAS1. Importantly, preventing the repeated accumulation of ALA and PBG by administrating Alas1-siRNA1 protected the AIP mice from the induced neuromotor deterioration as assessed by rotarod studies (Fig. 5). This finding further supports that the neurological dysfunction in the acute hepatic porphyrias is primarily caused by the toxicity of ALA and PBG and suggests that Alas1-siRNA1 administration will result in the prevention or improvement of the acute neurovisceral symptoms.
There were no safety issues with the Alas1-siRNA1, and liver function was normal at doses eightfold higher (4.0 mg/kg) than the minimum dose (0.5 mg/kg) required to completely prevent the induction of a biochemical acute attack (Table S1). Moreover, administration of Alas1-siRNA1 did not alter heme saturation of TDO (Fig. 6A), thereby indicating that Alas1-siRNA1 did not cause hepatic heme deficiency even at a dose that was twofold higher (1.0 mg/kg) than the minimum required to prevent a biochemical acute attack. Consistent with this finding, 1.0 mg/kg Alas1-siRNA1 administration did not alter the hepatic activity of a representative hemoprotein, CYP2E1 (Fig. 6B).
Thus, these preclinical studies of RNAi therapy in the AIP mouse model demonstrated that Alas1-siRNA1 was well tolerated and highly effective in both preventing and treating the induced biochemical attacks. Extending this RNAi approach to human patients will require identification of a potent Alas1-siRNA that recognizes the human ALAS1 sequence. A GalNAc-conjugate–based approach instead of using LNPs for hepatic delivery would be particularly advantageous, as this would permit s.c. administration. Appropriate toxicology studies in animal models will be required before advancing to phase I clinical trials to determine safety and efficacy of the approach in humans.
Materials and Methods
Design, Synthesis, and Screening of Murine Alas1 Liver-Targeted siRNAs.
siRNAs specific for murine Alas1 were designed using the reference nucleotide sequence NM_020559.2 (National Center for Biotechnology Information Reference Sequence). To identify Alas1-siRNAs that are highly specific and do not recognize off-target genes, the 19-mer candidate sequences were subjected to a homology search against the RefSeq mRNA database. The number of mismatches in the seed region, nonseed region, and at the cleavage site were determined relative to all other transcripts in the database. Ranking of the off-target potential was used to select the top 45 specific duplexes for synthesis and evaluation. Single-stranded RNAs were synthesized at Alnylam Pharmaceuticals on controlled pore glass using standard phosphoroamidites obtained from Proligo Biochemie. Ion exchange HPLC was used for deprotection and purification of the crude oligonucleotides. Yield and concentration were determined by UV absorption at 260 nm. siRNA duplexes were generated by annealing equimolar amounts of complementary strands in a water bath at 90 °C for 3 min followed by cooling at room temperature for several hours. For screening, siRNAs were reverse-transfected into mouse Bnl-C12 cells cultured in minimum essential media (Life Technologies) using RNAiMax (Life Technologies). Twenty-four hours posttransfection, Alas1 mRNA levels were determined relative to a housekeeping control using the Quantigene 2.0 branched DNA assay according to the manufacturer’s instructions (Panomics). Dose–response studies of the best candidates from the primary screens were performed to identify sequences for scale-up synthesis and in vivo testing.
siRNA Formulation in LNPs and Validation in Wild-Type Mice.
The siRNAs were formulated into LNPs using a spontaneous vesicle formation procedure as previously described (36). The component molar ratio of the particles was ∼50/10/38.5/1.5 (ionizable lipid DLin-KC2-DMA:disteroylphosphatidylcholine:cholesterol:PEG2000-dimyristylglycerol) with a final lipid:siRNA ratio of ∼12:1. The average particle size was determined to be ∼40–70 nM by dynamic light scattering (Zetasizer Nana ZS). The siRNA entrapment efficiency was determined to be >95%. Alas1-siRNA LNP formulations were administered to female C57BL/6 mice (40–60 d old) via tail-vein injection at a volume of 0.01 mL/g body weight. All studies were approved by the Alnylam Pharmaceuticals Institutional Animal Care and Use Committee consistent with local, state, and federal regulations. Hepatic Alas1 mRNA levels were quantified in mouse tissue and normalized against the expression of glyceraldehyde 3-phosphate dehydrogenase 48 h postdose using the Quantigene 2.0 assay (Panomics).
Preclinical Studies in AIP Mouse Model.
Animal procedures were reviewed and approved by the Mount Sinai Institutional Animal Care and Use Committee. The previously described T1/T2 AIP mice (24) were bred and housed in a barrier facility at the Icahn School of Medicine at Mount Sinai. PB induction was performed as previously described (24), except the mice were induced for 3 consecutive days instead of 4 d, and the dose was increased to 110, 120, and 130 mg/kg/d unless otherwise specified. For combined PB/DDC inductions, DDC (20–25 mg/kg) was resuspended in corn oil and administered by oral gavage, and the daily PB doses were reduced to 90, 100, and 110 mg/kg/day or 100, 110, and 120 mg/kg/day as indicated. Hemin (Panhematin) was reconstituted in a 25% human albumin solution immediately before use in amber tubes. For rotarod performance analyses, mice were trained for 2 d (three trials per day, 180 s maximum) and then tested at a rotation speed of 16 rpm (three trials per day, 180 s maximum). Mice that failed to achieve a mean rotarod retention time of at least 148 s at baseline were excluded from the study. Overnight urines (16 h fractions) were collected in metabolic cages and blood samples were collected via the facial-vein technique. Mice were killed at the indicated times by perfusion with PBS via the left ventricle under ketamine/xylazine anesthesia unless specified otherwise. Livers were harvested, snap-frozen in liquid nitrogen, and stored in the dark at −80 °C until use.
siRNA or hemin was administered to male AIP mice (40–120 d old) via tail-vein injections. The siRNA and hemin formulations were diluted accordingly so that the mice consistently received a volume of 0.01 mL/g weight.
Plasma and Urinary ALA and PBG Quantification.
ALA and PBG were extracted and purified from plasma and urine samples using a solid-phase extraction column followed by butylation with 3 N hydrochloric acid in butanol. ALA and PBG esters were then separated on a reverse-phase C8 column using a gradient program and analyzed on an Agilent 6460 tandem mass spectrometer as previously described (37). Urinary ALA and PBG were expressed as millimoles/mole creatinine. Urinary creatinine was measured using a colorimetric assay based on the picric acid method (38).
RNA Extraction and Quantitative Real-Time PCR.
Total RNA (1 μg) was isolated from liver tissues using TRIzol reagent (Life Technologies) and reverse-transcribed with SuperScript II (Life Technologies). Real-time PCR was performed using predesigned Taqman assays (Life Technologies) for Alas1 and β-actin, and transcript levels were quantitated with an ABI Prism 7900 sequence detection system. Relative Alas1 transcript levels were determined by the comparative Ct method using β-actin as an internal control. Experiments were performed in triplicates.
Western Blot Analysis.
Liver samples were homogenized in chilled lysis buffer [20 mM Hepes, 150 mM sodium chloride, pH 7.4, 0.2% IPEGAL, and protease inhibitor mixture (Roche)], and protein concentrations were determined using the DC protein assay kit (Bio-Rad). For each sample, protein (35 μg) was separated by 10% SDS-PAGE and transferred to a polyvinylidene membrane. The membranes were blocked with 5% nonfat dry milk in PBS with 0.2% Tween-20 for 1 h at room temperature, incubated with anti-ALAS1 antibody (1:5,000 dilution; Fitzgerald Industries International) overnight at 4 °C, and then incubated with HRP-conjugated anti-rabbit IgG antibodies (1:10,000 dilution; Sigma Aldrich) for 1 h at room temperature. Signals were detected using the enhanced chemiluminescence Supersignal West Pico Detection Kit (Thermo Scientific). The membranes were reprobed with anti–β-actin/HRP antibody (Abcam) to normalize loading amounts.
ALAS1 Enzyme Activity Assay.
Hepatic tissue (∼100 mg) was added to 4 vol of 0.25 M sucrose and homogenized on ice. The protein concentration for each sample was determined and then adjusted to 12 mg/mL in 0.125 M sucrose on ice. Succinyl acetone was added to each sample to a final concentration of 50 μM to inhibit ALA-dehydratase activity. A 25-μL sample was diluted 1:1 with assay buffer for a final concentration of 50 mM glycine, 100 μM succinyl-CoA, 80 μM pyridoxal phosphate, 50 μM succinyl acetone, and 25 mM potassium phosphate (pH 7.4). The mixture was incubated at 37 °C for 30 min and then diluted with 450 μL ice-cold water. Samples were then derivitized with 150 μL of water, 37% formaldehyde, ethanol, and acetyl acetone (107:5:15:23). The mixture was vortexed and incubated at 100 °C for 5 min. Samples were kept on ice in the dark until processing for separation. The derivitized peak corresponding to ALA was separated by ultra performance liquid chromatography (UPLC) in a Waters Acquity UPLC system on a BEH C18 1.7-μM, 2.1 × 100-mm column. The fluorescence detector was set at 370 nm excitation and 460 nm emission. The solvent system was 0.2% formic acid in water (A) and neat methanol (B) with a flow rate of 0.3 mL/min in a 12-min run with a gradient optimized for peak separation. Experiments were performed in triplicates.
Hepatic TDO and CYP2E1 Activity Assays.
Hepatic heme status was assessed by the activity of TDO in mouse liver homogenates. TDO activity was determined by measuring the formation of kynurenine from l-tryptophan in either the absence (holoenzyme activity) or the presence (total enzyme activity) of added hemin, as previously described (39). In brief, retrograde hepatic perfusion was performed on anesthetized mice using ice-cold 0.15 M potassium chloride (KCl) with inflow via the inferior vena cava and outflow via the portal vein. Perfused livers were isolated, homogenized in 1:2 (wt/vol) chilled lysis buffer [0.15 M KCl, 0.2 M sodium phosphate, pH 7.0, and EDTA-free protease inhibitor mixture (Roche)] and centrifuged at 21,000 × g for 15 min at 4 °C. The supernatant was incubated with l-tryptophan in a final volume of 750 μL (2.5 mM l-tryptophan, 0.075 M KCl, 0.1 M sodium phosphate, pH 7.0) at 37 °C. For measurements of total enzyme activity, a final concentration of 2 μM hematin was added at the beginning of the reaction. Enzyme reactions were stopped at 90 and 120 min by addition of 300 μL of 0.9 M trichloroacetic acid. Mixtures were vortexed and centrifuged at 21,000 × g for 10 min at 4 °C. Supernatant (208 μL) was added to 125 μL of 0.6 M NaOH, and kynurenine concentrations were determined by measuring absorbance at 365 nm using a Hewlett Packard 845x UV-Visible spectrophotometer. TDO activity was calculated using an extinction coefficient of 4.54 × 103 M−1⋅cm−1, and percentage of heme saturation was calculated by the following formula: (holoenzyme TDO activity)/(total TDO activity) × 100. The final percentage of heme saturation was calculated by averaging the values determined at 90 and 120 min.
Microsomal CYP2E1 activity was determined as previously described (40). CYP2E1 activity was calculated using an extinction coefficient of 9.53 × 105 M−1⋅cm−1 and expressed as picomoles/milligram protein/minute.
Statistical Analysis.
Statistical analyses were performed by unpaired, one-tailed Student t test or one-way ANOVA as indicated. Differences of P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Hector Bergonia (University of Utah School of Medicine) and Jingyu Huang (Icahn School of Medicine at Mount Sinai) for their excellent technical assistance in determining ALAS1 activity and ALA/PBG concentrations, respectively, and Arthur Cederbaum and Defeng Wu (Icahn School of Medicine at Mount Sinai) for their help with the CYP2E1 activity assay. We also thank the following individuals at Alnylam Pharmaceuticals: Brian Bettencourt, Satya Kuchimanchi, Klaus Charisse, and Stuart Milstein for the design, synthesis, and screening of siRNAs; Varun Kumar for formulation of lipid nanoparticles; and Rachel Meyers for advice. This work was supported in part by a grant from Alnylam Pharmaceuticals and by National Institutes of Health (NIH) Grants K01 DK087971 (to M.Y.) and U54 DK083909 (for The Porphyrias Consortium). The latter is a part of the NIH Rare Disease Clinical Research Network, supported through collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science and the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Conflict of interest statement: A.L., K.F., and W.Q. are employees and shareholders of Alnylam Pharmaceuticals; and M.Y. and R.J.D. are consultants to Alnylam Pharmaceuticals. The authors have filed a patent covering RNAi therapies for the treatment of porphyrias.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406228111/-/DCSupplemental.
References
- 1.Anderson KE, Sassa S, Bishop DF, Desnick RJ. Disorders of heme biosynthesis: X-linked sideroblastic anemia and the porphyrias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 2961–3062. [Google Scholar]
- 2.Granick S. Induction of the synthesis of delta-aminolevulinic acid synthetase in liver parenchyma cells in culture by chemical that induce acute porphyria. J Biol Chem. 1963;238:2247–2249. [PubMed] [Google Scholar]
- 3.Handschin C, et al. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell. 2005;122(4):505–515. doi: 10.1016/j.cell.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 4.Miller LK, Kappas A. The effect of progesterone on activities of delta-aminolevulinic acid synthetase and delta-aminolevulinic acid dehydratase in estrogen-primed avian oviduct. Gen Comp Endocrinol. 1974;22(2):238–244. doi: 10.1016/0016-6480(74)90114-2. [DOI] [PubMed] [Google Scholar]
- 5.Sassa S, Bradlow HL, Kappas A. Steroid induction of delta-aminolevulinic acid synthase and porphyrins in liver. Structure-activity studies and the permissive effects of hormones on the induction process. J Biol Chem. 1979;254(20):10011–10020. [PubMed] [Google Scholar]
- 6.de Matteis F. Disturbances of liver porphyrin metabolism caused by drugs. Pharmacol Rev. 1967;19(4):523–557. [PubMed] [Google Scholar]
- 7.Yamamoto M, Kure S, Engel JD, Hiraga K. Structure, turnover, and heme-mediated suppression of the level of mRNA encoding rat liver delta-aminolevulinate synthase. J Biol Chem. 1988;263(31):15973–15979. [PubMed] [Google Scholar]
- 8.Drew PD, Ades IZ. Regulation of the stability of chicken embryo liver delta-aminolevulinate synthase mRNA by hemin. Biochem Biophys Res Commun. 1989;162(1):102–107. doi: 10.1016/0006-291x(89)91968-2. [DOI] [PubMed] [Google Scholar]
- 9.Lathrop JT, Timko MP. Regulation by heme of mitochondrial protein transport through a conserved amino acid motif. Science. 1993;259(5094):522–525. doi: 10.1126/science.8424176. [DOI] [PubMed] [Google Scholar]
- 10.Bishop DF, Desnick RJ. Assays of the heme biosynthetic enzymes. Preface. Enzyme. 1982;28(2-3):91–93. [PubMed] [Google Scholar]
- 11.Anderson KE, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142(6):439–450. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]
- 12.Mustajoki P, Tenhunen R, Tokola O, Gothoni G. Haem arginate in the treatment of acute hepatic porphyrias. Br Med J (Clin Res Ed) 1986;293(6546):538–539. doi: 10.1136/bmj.293.6546.538-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonkowsky HL, et al. Repression of the overproduction of porphyrin precursors in acute intermittent porphyria by intravenous infusions of hematin. Proc Natl Acad Sci USA. 1971;68(11):2725–2729. doi: 10.1073/pnas.68.11.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustajoki P, Nordmann Y. Early administration of heme arginate for acute porphyric attacks. Arch Intern Med. 1993;153(17):2004–2008. [PubMed] [Google Scholar]
- 15.Goetsch CA, Bissell DM. Instability of hematin used in the treatment of acute hepatic porphyria. N Engl J Med. 1986;315(4):235–238. doi: 10.1056/NEJM198607243150406. [DOI] [PubMed] [Google Scholar]
- 16.Meyer UA, Schuurmans MM, Lindberg RL. Acute porphyrias: Pathogenesis of neurological manifestations. Semin Liver Dis. 1998;18(1):43–52. doi: 10.1055/s-2007-1007139. [DOI] [PubMed] [Google Scholar]
- 17.Dowman JK, Gunson BK, Bramhall S, Badminton MN, Newsome PN. Liver transplantation from donors with acute intermittent porphyria. Ann Intern Med. 2011;154(8):571–572. doi: 10.7326/0003-4819-154-8-201104190-00015. [DOI] [PubMed] [Google Scholar]
- 18.Seth AK, Badminton MN, Mirza D, Russell S, Elias E. Liver transplantation for porphyria: Who, when, and how? Liver Transpl. 2007;13(9):1219–1227. doi: 10.1002/lt.21261. [DOI] [PubMed] [Google Scholar]
- 19.Soonawalla ZF, et al. Liver transplantation as a cure for acute intermittent porphyria. Lancet. 2004;363(9410):705–706. doi: 10.1016/S0140-6736(04)15646-8. [DOI] [PubMed] [Google Scholar]
- 20.Stojeba N, et al. Recovery from a variegate porphyria by a liver transplantation. Liver Transpl. 2004;10(7):935–938. doi: 10.1002/lt.20136. [DOI] [PubMed] [Google Scholar]
- 21.Akinc A, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18(7):1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank-Kamenetsky M, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA. 2008;105(33):11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmermann TS, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441(7089):111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 24.Lindberg RL, et al. Porphobilinogen deaminase deficiency in mice causes a neuropathy resembling that of human hepatic porphyria. Nat Genet. 1996;12(2):195–199. doi: 10.1038/ng0296-195. [DOI] [PubMed] [Google Scholar]
- 25.Johansson A, Nowak G, Möller C, Blomberg P, Harper P. Adenoviral-mediated expression of porphobilinogen deaminase in liver restores the metabolic defect in a mouse model of acute intermittent porphyria. Mol Ther. 2004;10(2):337–343. doi: 10.1016/j.ymthe.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Unzu C, et al. Sustained enzymatic correction by rAAV-mediated liver gene therapy protects against induced motor neuropathy in acute porphyria mice. Mol Ther. 2011;19(2):243–250. doi: 10.1038/mt.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cebrián ME, Albores A, Connelly JC, Bridges JW. Assessment of arsenic effects on cytosolic heme status using tryptophan pyrrolase as an index. J Biochem Toxicol. 1988;3:77–86. doi: 10.1002/jbt.2570030203. [DOI] [PubMed] [Google Scholar]
- 28.Badawy AA. Tryptophan pyrrolase, the regulatory free haem and hepatic porphyrias. Early depletion of haem by clinical and experimental exacerbators of porphyria. Biochem J. 1978;172(3):487–494. doi: 10.1042/bj1720487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 30.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 31.Coelho T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369(9):819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 32.Janssen HL, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 33.Tabernero J, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 34.Cully M. Dyslipidaemia: RNAi targeting PCSK9 decreases lipid levels in a human trial. Nat Rev Cardiol. 2013;10(12):682. doi: 10.1038/nrcardio.2013.163. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald K, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: A randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383(9911):60–68. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayaraman M, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl. 2012;51(34):8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, et al. A LC-MS/MS method for the specific, sensitive, and simultaneous quantification of 5-aminolevulinic acid and porphobilinogen. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(24):2389–2396. doi: 10.1016/j.jchromb.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamb E, Newman DJ, Price CP. Kidney function tests. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Textbook of Clinical Chemistry. 4th Ed. Philadelphia: Saunders; 2006. pp. 798–801. [Google Scholar]
- 39.Feigelson P, Greengard O. The activation and induction of tryptophan pyrrolase during experimental porphyria and by amino-triazole. Biochim Biophys Acta. 1961;52:509–516. doi: 10.1016/0006-3002(61)90409-7. [DOI] [PubMed] [Google Scholar]
- 40.Wu D, Cederbaum AI. Development and properties of HepG2 cells that constitutively express CYP2E1. Methods Mol Biol. 2008;447:137–150. doi: 10.1007/978-1-59745-242-7_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.