Significance
The cellular functions of epithelia rely on their polarized architecture. Loss of their polarity is often associated with carcinoma progression and tumor metastasis. The basement membrane (BM), a specialized sheet of the extracellular matrix, secreted basally by most epithelia, plays an important role in the establishment and maintenance of epithelial cell polarity. However, the process of BM-polarized secretion is not well understood. In this study, we show that a significant decrease of the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) results in BM protein secretion on both the apical and the basal side of the epithelium. Together, our data indicate a specific role for PIP2 in the organization of epithelial architecture by restricting the deposition of BM proteins to the basal side.
Keywords: cell polarity, PTEN, PIK, Drosophila, oogenesis
Abstract
The basement membrane (BM), a specialized sheet of the extracellular matrix contacting the basal side of epithelial tissues, plays an important role in the control of the polarized structure of epithelial cells. However, little is known about how BM proteins themselves achieve a polarized distribution. Here, we identify phosphatidylinositol 4,5-bisphosphate (PIP2) as a critical regulator of the polarized secretion of BM proteins. A decrease of PIP2 levels, in particular through mutations in Phosphatidylinositol synthase (Pis) and other members of the phosphoinositide pathway, leads to the aberrant accumulation of BM components at the apical side of the cell without primarily affecting the distribution of apical and basolateral polarity proteins. In addition, PIP2 controls the apical and lateral localization of Crag (Calmodulin-binding protein related to a Rab3 GDP/GTP exchange protein), a factor specifically required to prevent aberrant apical secretion of BM. We propose that PIP2, through the control of Crag’s subcellular localization, restricts the secretion of BM proteins to the basal side.
Epithelial cells are characterized by their polarized architecture, which enables them to exert their varied functions in embryonic and adult organisms. Epithelia exhibit a profound apical–basal polarity that is manifested in the cytoplasmic and surface organization of individual cells (1–3). Loss of apical–basal cell polarity is often associated with carcinoma progression and tumor metastasis (4, 5). The establishment and maintenance of cell polarity relies on the transport of newly synthesized and recycled proteins to their correct destinations (6, 7). The lipid composition of the transport vesicles and of the plasma membrane is crucial for the establishment and maintenance of cell polarity (6–9). In particular, in 3D in vitro culture of Madin–Darby canine kidney (MDCK) cells, phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3), two phosphoinositides (PtdIns) have been shown to play critical roles in polarized vesicle trafficking by mediating the recruitment of proteins to these different domains (10, 11).
To set up a correct cell polarity, membrane asymmetry needs to be established. In cell culture, and likely during development of many tissues in multicellular organisms, this process is achieved by two external cues: one provided by the adjacent cells via cadherin-dependent adhesion and the other by interaction with the basement membrane (BM), a specialized sheet of the ECM secreted basally by the epithelial cells (12, 13). The main components of the BM are secreted glycoproteins, such as collagen IV (Coll IV), laminin, and the heparan sulfate proteoglycan perlecan (Pcan) (14), which interact with different membrane receptors, including integrin and dystroglycan (14, 15). Previous studies in model organisms and 3D culture models have shown that BM secreted by the epithelial cells at their basal side plays a role as an initial determinant in basal polarity (13, 16, 17). Moreover, Pcan, through its cellular receptor dystroglycan, is involved in the maintenance of epithelial polarity (18). Despite its important roles in the establishment and maintenance of polarity, it is not well understood how the BM achieves its own polarized accumulation on the basal side of the cells.
Recently, two new factors have been shown to be critical for the polarized deposition of BM proteins: Crag (Calmodulin-binding protein related to a Rab3 GDP/GTP exchange protein) and Rab10 (19, 20). The loss of either Crag or Rab10 leads to anomalous accumulation of BM components on both the apical as well as the basal side of epithelial cells without directly disrupting the distribution of apical or basolateral proteins (19, 20). This finding indicates that these two factors are specifically required for the restriction of the BM to the basal side and that this process is independently regulated from apical and basolateral secretion. It has been shown that Crag can act as a GTP exchange factor (GEF) for Rab10 in Drosophila (21). In addition, in Drosophila embryos, Scarface, a protease-like protein, has been shown to act as a specific regulator of laminin A-polarized deposition (22).
To further elucidate the molecular mechanism leading to the polarized secretion of BM components, we identified additional genes involved in this process using the Drosophila follicular epithelium (FE) as a model system. The FE is composed of a monolayer of somatic cells, the follicle cells (FCs), which surround the central germ-line cells during Drosophila oogenesis (Fig. 1 A and B) (23). The FE is a classical epithelium, with a distinct apical–basal polarity where the apical domain of the FCs faces the germ line and the BM is secreted at the basal side (Fig. 1 A and B). We identified Phosphatidylinositol synthase (Pis), an evolutionarily conserved enzyme involved in the synthesis of PtdIns, as a factor critical for restricted secretion of BM proteins. In addition, we show that the level of PIP2 is critical for this process. FCs with a reduced level of PIP2 allow an aberrant accumulation of BM components on both their apical and basal sides. Interestingly, a decrease of PIP2 significantly reduces the localization of Crag at the apical and lateral plasma membrane, indicating that the level of PIP2 also controls the subcellular distribution of Crag.
Fig. 1.
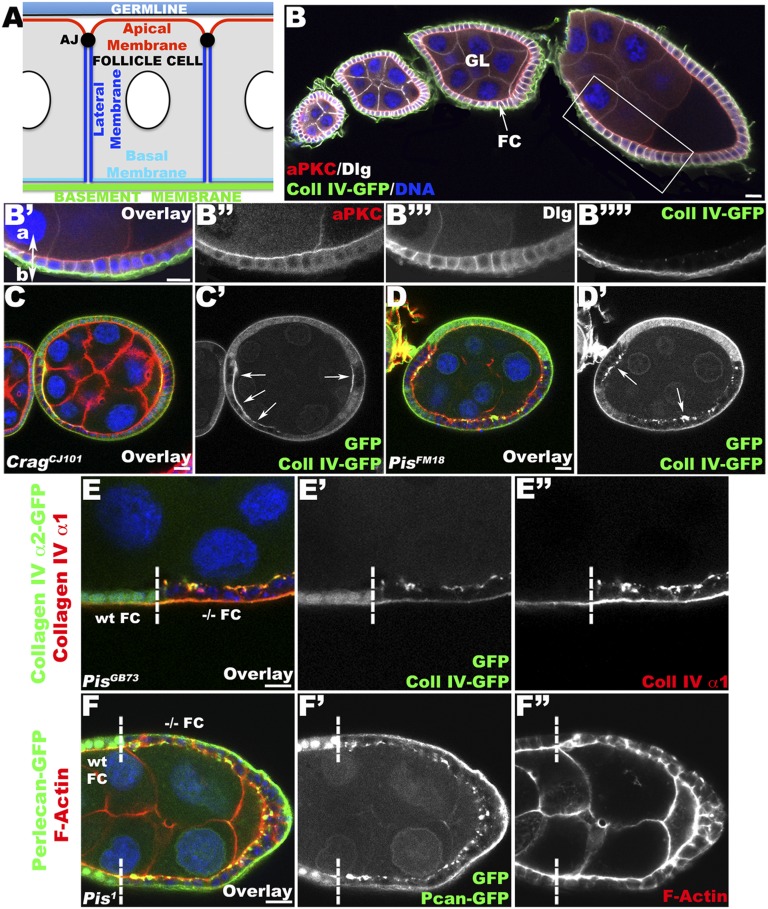
Pis mutant FCs disrupt the polarized distribution of BM proteins. (A) Schematic representation of the FE with the different polarity domains indicated. AJ, adherens junction. (B–B′′′′) Longitudinal (Lg) section through a WT ovariole containing different egg chambers (B) and through the FC layer of a WT egg chamber (magnification of B indicated with the rectangular area, B′–B′′′′) expressing Coll IV–GFP (a α2–Coll IV–GFP protein-trap; green), a major component of the BM, which accumulates at the basal side of the cell. The egg chambers are stained with aPKC (an apical domain marker; red) and Discs Large (Dlg; a lateral domain marker; white), revealing the polarized structure of the epithelium, and DNA (blue). (B′) Apical (a) and basal (b) sides of FCs are marked. (C and D) Lg section through egg chambers containing CragCJ101 (C) and PisFM18 (D) mutant FC clones expressing Coll IV–GFP (green) and stained for F-Actin (red) and DNA (blue). (C, C′) In Crag mutant FCs, marked by the absence of intracellular GFP (green), the polarized distribution of BM is disrupted, as revealed by the strong accumulation of Coll IV–GFP at the apical side of the FCs (arrows). (D, D′) The same phenotype is observed in Pis mutant FCs, where Coll IV–GFP accumulates apically in aggregates (arrows). (E and F) Lg section through the FC layer of an egg chamber containing Pis clones, marked by the absence of intracellular GFP (green; clonal boundary indicated by a dashed line; WT and −/− homozygous mutant FCs are specified), coexpressing Coll IV–GFP (green, E) or Pcan–GFP (a Pcan–GFP protein-trap; green F), and stained for α1–Coll IV (red, E, E″), F-Actin (red, F and F″), and DNA (blue). In Pis mutant FCs, Coll IV (E–E″) and Pcan (F, F′) accumulate apically, indicating that Pis is required for restriction of BM deposition to the basal side. (Bars, 10 μm.)
Results
Pis Regulates the Polarized Deposition of BM Proteins.
To find new genes specifically involved in the basal secretion of BM proteins, we performed a secondary screen of a collection of X-chromosome lethal mutations (19) using an α2–Coll IV protein trap line (Coll IV–GFP) (24) as a reporter for BM localization. Apart from the Crag alleles that had previously been described, we identified four other mutant lines affecting Pis (Fig. S1 A–F) in which we observed a mislocalization of BM proteins when FCs were homozygous mutant (Fig. 1). In addition to the mislocalization of BM proteins, we observed that these mutant FCs frequently formed multiple layers or gaps in the epithelial sheet instead of maintaining a strictly monolayered epithelium, indicating a role for the affected gene in epithelial morphogenesis (Fig. S1 B and C).
Pis is a highly conserved transmembrane protein with its catalytic domain present on the cytoplasmic side of the endoplasmic reticulum (25). It has an indispensable role in the synthesis of phosphatidylinositol (PI) (26). Pis is required for the incorporation of an inositol group in cytidine diphosphate–diacylglycerol (CDP-DAG) to produce PI (Fig. 2A). The phosphorylated derivatives of PI, known as PtdIns, are crucial regulators of calcium homeostasis, membrane trafficking, secretory pathways, and signal transduction (26). In particular, Pis has been shown to be essential for PIP2 regeneration after its hydrolysis by phospholipase C (PLC) (27).
Fig. 2.
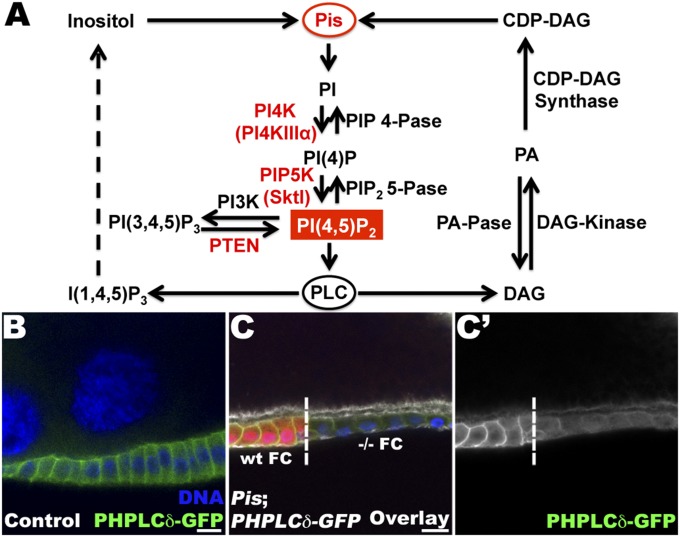
Pis and PIP2 pathway. (A) Schematic representation of the PtdIns metabolic cycle. Pis plays a critical role catalyzing the incorporation of inositol and CDP-DAG to produce PI, the backbone of all PtdIns. Some of the PtdIns’ metabolic steps and their respective products are shown with the specific Phosphoinositide kinase (PIK) and PtdIns phosphatase implicated. (B) Lg section through the FE expressing the PIP2 reporter, Ubi–PH–PLCδ–GFP and stained for GFP (green), F-actin (white), and DNA (blue). In WT FCs, PIP2 is localized at the plasma membrane on the apical and lateral domains. (C, C′) Lg section through FCs containing PisFM18 mutant cells, expressing Ubi–PH–PLCδ–GFP. Staining for GFP (green), F-actin (white), and DNA (blue) reveals that the PIP2 reporter is absent from the membrane of Pis mutant cells, which are marked by the absence of the RFP clonal marker, in contrast to the WT cells. The clonal boundary is indicated with a dashed line. WT and −/− homozygous FCs are specified. (Bar, 10 μm.)
To confirm our initial observation that BM proteins are mislocalized in Pis mutant cells, we visualized the distribution of two main components of the BM membrane: Coll IV (α1 and α2 chains of Coll IV) and Pcan. In wild-type (WT) FC, Coll IV and Pcan are localized in vesicles and secreted exclusively to the basal side of the cell (Fig. 1 and ref. 18). Pis mutant FCs accumulate α1 and α2 Coll IV apically (Fig. 1E). We obtained a previously described null allele of Pis (Pis1) (27) and confirmed that the abnormal apical accumulation of BM proteins, including Coll IV (α1 and α2 chains) and Pcan–GFP (28) (Fig. 1F), is also observed in FCs mutant for this null allele. Although, Crag and Pis mutant cells showed the same apical mislocalization of BM proteins, we observed some differences in their phenotypes. BM components accumulated in a uniform, compact extracellular layer in Crag mutant FCs (Fig. 1C) and in extracellular aggregates in Pis mutant FCs (Fig. 1 D–F). By 3D reconstruction of Pis or Crag mutant FE, we found, however, that in both cases Coll IV is secreted apically to the outside of the cells (Fig. S2 and Movies S1 and S2). Together, these data indicate that we have identified Pis as a regulator of polarized BM deposition in the FE.
PIP2 Levels Are a Crucial Determinant for the Polar Secretion of BM Proteins.
Pis has been shown to be involved in PI production, and therefore in the regulation of all PtdIns levels (26). Among the various forms of PtdIns, PIP2 can mediate distinct biological activities, including cell polarity, secretion, vesicular trafficking, and cell adhesion (8–11, 29). We therefore investigated whether, among all of the various PtdIns, PIP2 levels might be specifically involved in the regulation of polarized BM deposition. Consequently, we assayed the intracellular levels and distribution of PIP2 in Pis mutant FCs using the PIP2 reporter, Ubi–PH–PLCδ–GFP, which has been shown to specifically recognize PIP2 (30). As in mammalian epithelial cells, PIP2 is localized to the plasma membrane of WT FCs and shows a distinct polarized distribution (Fig. 2B). In the Drosophila FE, PIP2, as revealed by the PIP2 reporter, is clearly detected at the apical and lateral domains of the FCs (Fig. 2B). No specific accumulation of PIP2 is detectable at the basal side of the cell using this PIP2 reporter and taking the diffuse cytoplasmic staining into account. In Pis mutant FCs of late-stage egg chambers, we observed a clear decrease in intracellular PIP2 on apical and lateral sides, confirming a critical role of Pis in the regulation of PIP2 levels (Fig. 2C).
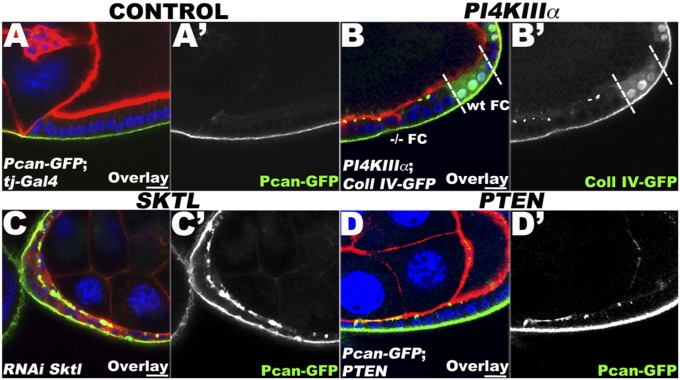
To determine whether the BM mislocalization phenotype observed in Pis mutant cells is due to the decrease of PIP2, we examined the role of other enzymes implicated in PtdIns production in BM-polarized deposition. Downstream of Pis, the production of PIP2 requires two different steps, which are catalyzed by two distinct classes of PI kinases (Fig. 2A) (26). First, PI is phosphorylated by PI4K to generate PI4P, which is then phosphorylated by PIP5K to produce PIP2. In the same original screen of X-linked lethal mutations, we also isolated PIP4K (PI4KIIIα) mutants and previously showed that cells mutant for this gene exhibit a loss of the PIP2 reporter from the apical plasma membrane, thereby indicating a role of PI4KIIIα in the distribution of PIP2 (31). Here, we tested these mutations for mislocalization of BM proteins and found that FCs mutant for PI4KIIIα show an apical accumulation of Coll IV (Fig. 3B). Similarly, we found that when skittles (sktl), a Drosophila PIP5K, is knocked down using RNAi in FCs, the BM protein Pcan accumulates at the apical side (Fig. 3C; compare with Fig. 3A). Together, these data suggest that the loss of PIP2 at the plasma membrane resulting from the mutations in PI4KIIIa and sktl is the crucial factor for the abnormal secretion of BM proteins on the apical side of the epithelial cells.
Fig. 3.
PIP2 levels control polarized BM deposition. (A, A′) Lg section through WT FCs (tj–Gal4) expressing Pcan–GFP (green), stained for F-Actin (red) and DNA (blue). (B, B′) Lg section through the FC layer of an egg chamber containing PI4KIIIαGS27 clones, marked by the absence of intracellular GFP, coexpressing Coll IV–GFP (green) and stained for F-Actin (red) and DNA (blue). Clonal boundaries are indicated by a dashed line. WT and −/− homozygous FCs are specified. In PI4KIIIα mutant FCs, BM proteins accumulate apically in aggregates. (C, C′) Lg section through FCs expressing Pcan–GFP (green), stained for F-Actin (red) and DNA (blue), and expressing an RNAi construct against sktl (PIP5K) using tj–Gal4. The knockdown of PIP5K leads to the apical localization of Pcan–GFP. (D, D′) Lg section through the FC layer of an egg chamber containing PTENC494 clones, marked by the absence of intracellular GFP, coexpressing Pcan–GFP (green) and stained for F-Actin (red) and DNA (blue). In PTEN mutant FCs, Pcan–GFP accumulates apically in aggregates. Together, these data indicate that PIP2 controls the polarized secretion of BM proteins. (Bars, 10 μm.)
To further confirm that PIP2 is involved in BM polarity, we perturbed its production independently of the Pis/PI4K/PIP5K pathway by using mutants in Phosphatase and Tensin homolog on Chromosome 10 (PTEN) (Fig. 3D and Fig. S3) as well an RNAi line (Fig. S4). PTEN is a phosphatase that catalyzes the conversion of PIP3 to PIP2 (26). In FCs mutant for the loss-of-function allele PTENC494, we again observed apically localized Pcan in distinct aggregates (Fig. 3D), indicating that in the absence of PTEN, BM proteins are also not correctly restricted to the basal side. We also isolated a separate mutation in PTEN (JH59) in a screen on chromosome 2L and observed the same apical mislocalization phenotype in homozygous mutant FCs (Fig. S3). In addition, we were able to reproduce the same phenotype using an RNAi knockdown against PTEN (Fig. S4). The severity of the BM mislocalization observed in PTEN mutant cells, using two different mutants and one RNAi line, was less pronounced than in Pis mutant cells (Fig. 3D and Figs. S3 and S4; compare with Fig. 1). Mutations in PTEN are, in fact, not expected to block the production of PIP2 as severely, as long as Pis and the PI kinase pathways are still functioning (see pathway in Fig. 2A). Together, therefore, our data clearly indicate that PIP2 levels are critical in the polarized deposition of BM proteins in the FE.
Loss of PIP2 Does Not Lead to a General Loss of Polarity in the FE.
Although, to our knowledge, this is the first report indicating that PIP2 has a crucial role in the polarized secretion of BM proteins, PIP2 has been previously shown to be important for the establishment of cell polarity in mammalian MDCK cells (10, 11). PTEN has also been shown to localize early to the apical domain, and its activity is required for the segregation of PIP2 and PIP3 (10). Furthermore, recent studies have established that the ratio of PTEN and PIP2/PIP3 is important for apical polarity in the Drosophila embryo (32). It therefore seemed possible that the BM mislocalization phenotype we observed could be due to a more general perturbation of cell polarity rather than to a specific effect on BM secretion.
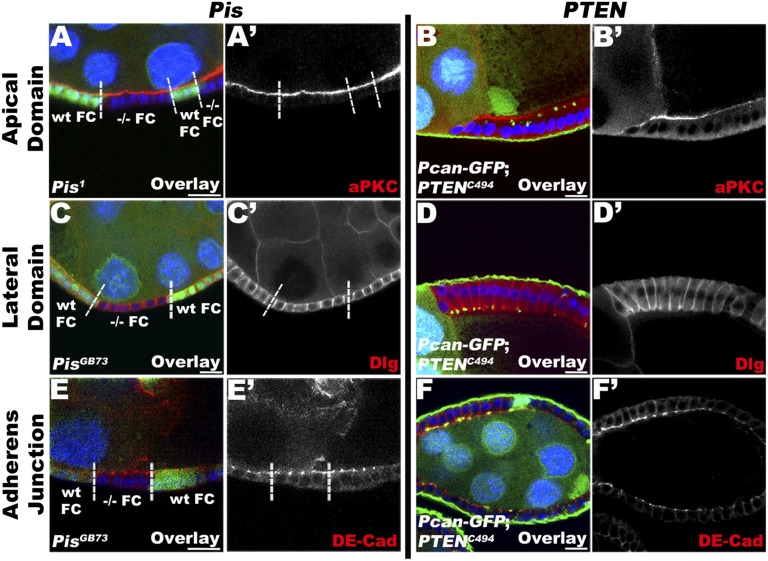
To examine whether the aberrant mislocalization of BM proteins in Pis and PTEN mutant FCs is specific or whether it is due to a global loss of epithelial cell polarity, we analyzed the distribution of other major apical, basolateral, and adherens junctional markers. All of the examined mutant genotypes produced occasional multilayered epithelia. We therefore focused our analysis on mutant FCs that maintain normal monolayer organization, to avoid analyzing secondary effects due to an eventual loss of epithelial architecture caused by the mislocalized BM and its subsequent effects on cell polarity possibly via integrin signaling (Fig. 4 and Figs. S3 and S4). We used atypical protein kinase C (aPKC) and Crumbs (Fig. S5A for Crumbs) for the apical domain; Discs Large (Dlg) and Fasciclin II (Fig. S5B) for the lateral domain; and, finally, DE–cadherin (DE-Cad) and Armadillo (Fig. S5C) for the junction complex. Using these different markers, we did not observe any extension or diminution of the different domains. We therefore conclude that reducing the levels of PIP2 does not directly alter the polarized trafficking of these apical and lateral polarity proteins. Instead, our data suggest that the exclusion of BM components from the apical side of the FE is particularly sensitive to a reduction in PIP2.
Fig. 4.
Normal apical–basal polarity is maintained in FCs depleted of PIP2. Lg section through the FC layer of egg chambers stained for markers of epithelial polarity, such as aPKC (apical domain marker; A and B), Dlg (lateral domain marker; C and D), and DE-Cad (adherens junction marker; E and F), and stained for DNA (blue). Egg chambers containing Pis (A, C, and E) or PTEN (B, D, and F) mutant clones, marked by the absence of intracellular GFP; clonal boundaries are indicated by a dashed line. WT and −/− homozygous FCs are specified. Pis and PTEN mutant FCs do not show any difference in the distribution of the apical marker aPKC (A, A′ and B, B′, red), the lateral marker Dlg (C, C′ and D, D′, red), or the adherens junction marker DE-Cad (E, E′ and F, F′, red). Note that in PTEN mutant FCs, Pcan–GFP accumulates apically. Together, these data indicate that disrupting the function of components involved in PIP2 production has no direct effect on the maintenance of polarity domains. (Bars, 10 μm.)
PIP2 Levels Are Required for the Proper Localization of Crag at the Plasma Membrane.
To determine whether there is an effect of the decrease of PIP2 on known factors involved in BM polarized secretion, we analyzed the distributions of Crag and Rab10 in Pis mutant FCs.
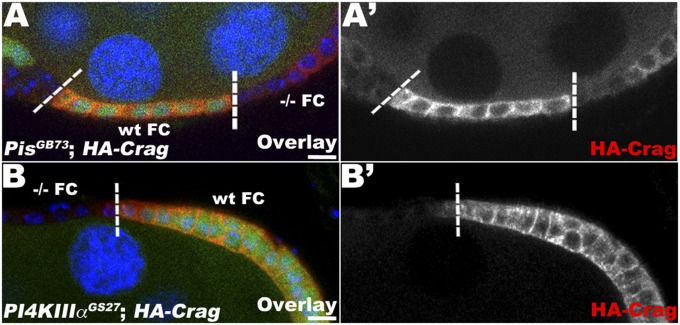
In FCs, Crag localizes principally at the plasma membrane and also colocalizes in intracellular puncta that overlap with Rab11 and Rab5 endosomal compartments (19). Because antibodies are no longer available to detect endogenous Crag, we used a full-length HA–Crag construct as a readout for Crag localization. This construct has been shown to serve as a faithful marker of endogenous Crag (19). Using this construct, we observed that in WT FCs, HA–Crag localized at the plasma membrane and had a cytoplasmic localization (Fig. 5, WT FC). However, in Pis mutant FCs, Crag no longer accumulated at the plasma membrane, but instead showed only a diffuse cytoplasmic localization (Fig. 5A). In Pis mutant FCs, Crag levels were also reduced overall compared with WT FCs. It should be noted that this phenotype is not fully penetrant, and some Pis mutant FCs still showed normal Crag localization. However, larger clones in late egg chambers showed a higher tendency for loss of Crag at the plasma membrane, suggesting that cells need to be severely depleted of PIP2 to lose Crag from the plasma membrane. Similarly, in FCs mutant for PI4KIIIα, an enzyme required for PIP2 production downstream of Pis, Crag was lost from the plasma membrane, and its overall level was reduced (Fig. 5B).
Fig. 5.
PIP2 levels control the plasma membrane localization of Crag. Lg section through the FC layer of an egg chamber containing PisGB73 (A) and PI4KIIIαGS27 clones (B), marked by the absence of intracellular GFP, coexpressing HA–Crag (UAS HA–Crag, red) and stained for DNA (blue); clonal boundaries are indicated by dashed lines. WT and −/− homozygous FCs are specified. In Pis (A, A′) and PI4KIIIα (B, B′) mutant cells, HA–Crag loses its plasma membrane localization, and its levels are reduced overall, suggesting that PIP2 levels are required for targeting or maintaining Crag at the membrane. (Bars, 10 μm.)
Another regulator of BM-polarized deposition is Rab10. It has been shown that Crag can act as a GEF for Rab10 (20, 21). In addition, Crag physically interacts with Rab10 and is critical for the correct localization of Rab10 to the basal side of the FE (20). Because we found that Pis activity is important for the subcellular localization of Crag, we similarly assayed the localization of Rab10 in Pis mutant FCs. However, we could not detect any differences in Rab10 localization between Pis mutant and WT cells (Fig. S5D), suggesting that PIP2 levels are not crucial for the observed Rab10 accumulation in the cytoplasm and at the basal side of the epithelial cells. It seems that the decrease and loss of Crag from the apical and lateral membranes is not sufficient to affect the basal accumulation of Rab10. Together, our data suggest that PIP2 levels are essential for proper polarized BM deposition, possibly through the regulation of the subcellular localization of Crag, in epithelial cells.
Discussion
The polarized organization of epithelial cells is normally established and maintained by external cues (12). In particular, it has been shown that the BM can direct the orientation of the apico-basal axis of epithelial cells (15–17, 33, 34). In addition, incubating MDCK cells with BM proteins such as collagen or laminin leads to polarity reversal (13, 35, 36). Despite its important roles, little is known about the mechanism by which BM proteins are specifically secreted at the basal side of the cell. Recently, two regulators of the process, Crag and Rab10, were shown to be critical in the polarized accumulation of BM proteins by preventing aberrant apical secretion (19, 20). Here, we report a critical role for PIP2 in the control of the polarized secretion of BM components such as Coll IV and Pcan, which, to our knowledge, has not been previously recognized.
In Pis mutant FCs, Pcan and Coll IV accumulated on both sides of the epithelium. Similarly, when we affected the function of different enzymes implicated in PIP2 production downstream of Pis, such as PI4K and PIP5K, we also observed BM proteins to accumulate apically. The same phenotype was also observed in cells mutant for PTEN, which encodes an enzyme required for the production of PIP2 from PIP3. These results indicate that affecting the production of PIP2 in different ways leads to the mislocalization of BM components. In contrast, the distributions of other polarity proteins to the apical, basolateral, and junctional domains were not immediately affected in epithelial cells that showed a clear decrease of intracellular PIP2.
Although FCs with a decrease of PIP2 showed a clear apical mislocalization of BM proteins, the observed accumulation was patchier and less coherent than in Crag mutant FCs. This somewhat weaker phenotype can be explained by the fact that there are multiple ways to produce PIP2 in the cells, and it therefore may take a longer time after the cells become mutant for PIP2 levels to be sufficiently exhausted before a mislocalization of BM components occurs.
PIP2 has been shown to be important in the establishment and maintenance of epithelial polarity. In particular, the ratio of PIP2/PIP3 was found to be critical for the establishment and maintenance of the apical and lateral domains (10, 11). In nonpolarized MDCK cells, PIP2 and PIP3 are evenly distributed along the plasma membrane. However, in early stages of the polarization, PIP2 becomes concentrated at the apical domain, whereas PIP3 remains more abundant at the basolateral domain (10). This process is initiated by PTEN, which localizes to the apical domain and mediates the segregation of PIP2 to the apical domain and PIP3 to the basolateral surface, thus recruiting apical determinants to the apical domain. In Drosophila, PIP2 has also been implicated in the establishment of the apical domain and the recruitment of apical polarity proteins (32). Here, we show that higher levels of PIP2 are not required primarily to maintain polarity domains, but instead control the polarized secretion of BM proteins. In addition, we found that PIP2 is required for the proper localization of Crag to the apical and lateral domains of the plasma membrane, further suggesting that the control of the polarized secretion of BM proteins by PIP2 is mediated in part by the regulation of Crag localization. It seems likely that, similar to the establishment and maintenance of the apical domain, where PIP2 interacts with different polarity-promoting proteins, PIP2, possibly indirectly, interacts apically with Crag, a component necessary to prevent apical accumulation of BM components. In addition, the decrease in intracellular PIP2 levels had no visible effect on the cytoplasmic localization of Rab10, despite the fact that Crag has been reported to be required for the proper distribution of Rab10 (20). This result can be explained best by the fact that Crag accumulates both at the apical and lateral membrane and in intracellular compartments (19). The loss of PIP2 affects the overall level of Crag and, in particular, strongly reduces Crag localization to the membrane, but presumably still supports sufficient levels of intracellular Crag to maintain the subcellular localization of Rab10 in basal regions of the cell.
A recent study suggested that basal secretion of the BM proteins could in part be explained by the localization of the respective mRNAs to a basal compartment within the FCs and the resulting proteins being targeted to basally localized Golgi clusters (20). However, our results show that this localization cannot be the determining factor for the exclusion of the BM proteins from the apical side. In the absence of either Crag or Rab10, or with low levels of PIP2, the BM proteins are still secreted on the basal side, but in addition, they now also accumulate apically. It has been shown that the FCs themselves produce the BM proteins found on their basal side (37). Therefore, Crag and Pis are not required for the basal secretion of BM proteins, but specifically for the prevention of the abnormal apical accumulation. The mutant phenotypes also show that localization of the respective mRNAs and targeting of the proteins to basal Golgi compartments are not sufficient to prevent abnormal secretion of these proteins on the apical side of the cell. Instead, the phenotypes that we report show that exocytic vesicles containing BM protein must be able to reach the apical side and deliver their cargo in this location. It requires the activity of Crag and sufficient levels of PIP2 to block this apical path of secretion. Although targeting secretion toward the basal side may certainly facilitate the basal accumulation of the BM proteins, the negative role of PIP2 and of Crag are the important factors in assuring that BM proteins are normally only secreted on the basal side. In summary, our results show that PIP2, Crag, and Rab10 are primarily involved in the blocking of apical secretion of BM proteins and not in their basal targeting.
Although loss of PIP2 and Crag from the FCs, in general, does not result in an overall loss of cell polarity, we occasionally observe more severe phenotypes in mutant epithelia with multilayering or gaps (see also ref. 19). In addition, at later stages of egg chamber development, the mutant cells often die, and such egg chambers do not form normal eggs. Because the more general epithelial abnormalities are usually associated with later stages and larger mutant clones, it is possible that the abnormal secretion of BM proteins to the apical side may eventually lead to such general epithelial defects. These abnormalities may well correspond to the observed loss of cell polarity in mammalian epithelial cells, when they are treated with exogenous collagen or laminin (13, 35, 36). Alternatively, it is also possible that the loss of PIP2 has other, more direct consequences on the long-term maintenance of the epithelial polarity complexes without involving Crag. Nevertheless, Crag appears to be particularly sensitive to the loss of PIP2, because it is lost from the membranes, whereas other polarity proteins are still maintained at apparently normal levels.
Our results allow us to propose a model in which PIP2 plays a previously unidentified, essential role in the polarized secretion of BM proteins. In epithelial cells, PIP2, which assumes high levels at the apical and lateral plasma membrane, prevents the secretion of BM components possibly via direct or indirect recruitment of Crag and other factors to those domains. Crag prevents the abnormal accumulation of BM proteins at these sites through an as-yet-unknown mechanism. In Crag mutants, BM proteins are unselectively secreted to both the basal and the apical side of the epithelial cells. In mutants in the PtdIns pathway, where the intracellular level of PIP2 is low, Crag is no longer localized properly at the plasma membrane, and its overall levels are reduced. This process, in turn, also leads to the loss of polarized secretion of BM proteins and an unselective accumulation of BM proteins at both sides of the cells. In summary, we have discovered a crucial role for PIP2 in regulating cell polarity by controlling the polarized accumulation of BM proteins, which is ultimately an essential component in the establishment or maintenance of cell polarity.
Materials and Methods
Fly Stocks and Genetics.
Duplication lines used for mapping were obtained from the Bloomington Stock Center. The following Drosophila stocks were used: CragCJ101 (19); PisGB73, PisFM18, PisFJ78, and PisFV4 (this study); Pis1 and hs-Pis (27) (a gift from C. Montell, University of California, Santa Barbara, CA); PTENJH59 (this study); PTENC494 (38) (a gift from J. A. Brill, University of Toronto, Toronto), PHPLCδ–GFP [PIP2 probe (30)]; UAS–mCD8–RFP (Bloomington); tj–Gal4 (a gift from D. Bilder, University of California, Berkeley, CA); UAS–RNAi–Sktl (TRIP line JF02796; Bloomington); UAS–RNAi–PTEN (TRIP line JF01987; Bloomington); UAS–dicer 2 (Bloomington); UAS–HA–CragA (19); and UASp–YFP–Rab10 (39). The protein trap lines Vkg–GFP [CC00791 (24)] and Pcan–GFP [ZCL1700 (28)] were obtained from Flytrap. FC clones for Pis, PTEN, PI4KIIIα, and Crag were induced by using the Flipase/FRT method (19).
Mapping and Rescue of Pis Mutations.
Mapping and sequencing of PisGB73 and PisFM18 were performed as described (19). PisFJ78 and PisFV4 were not sequenced, but give equally strong phenotypes. Rescue experiments were performed by subjecting PisGB73; hs–Pis and PisFM18; hs–Pis flies to daily heat-shock treatments for 1 h from the embryonic to the adult stage.
Immunofluorescence Stainings.
Ovaries were dissected and immunostained as described (19). Ovaries, mounted in Aquapolymount (Polysciences), were visualized by using a Zeiss LSM510 or Nikon A1 laser-scanning confocal microscope. The following primary antibodies were used: mouse anti-Coll IV [6G7; 1:10 (40)], rabbit anti-aPKC (1:1,000; Santa Cruz), rat anti–DE-Cad [DCAD2; 1:20; Developmental Studies Hybridoma Bank (DSHB)], mouse anti-Dlg (4F3; 1:100; DSHB), rat anti-HA (3F10; 1:50; Roche), rabbit anti-GFP (AB3080P; 1:500; Millipore), mouse anti-Crb (cq4; 1/5; DSHB), mouse anti-FasII (1D4; 1/1000; DSHB), and mouse anti-Arm (N2 7A1; 1/50; DSHB). Secondary antibodies used were Alexa Fluor 488-, 568-, and 647-conjugated (1:400; Life Technologies). Alexa Fluor 546 Phalloidin (1:500; Life Technologies) and Alexa Fluor 647 Phalloidin (1:50; Life Technologies) were used to stain F-actin. DNA was stained by using Hoechst (10 μg/mL; Life Technologies).
Supplementary Material
Acknowledgments
We thank Natalie Denef and Yan Yan for conducting the original mutant screen that led to the isolation of the Pis mutations and Elena Domanitskaya and Yi Sun for conducting the original mutant screen that led to the isolation of the PTEN mutation. We thank Craig Montell, Julie Brill, the Developmental Studies Hybridoma Bank, and the Bloomington stock center for providing flies and antibodies; Joe Goodhouse and Gary Laevsky for advice with confocal microscopy; and members of the T.S. and E. Wieschaus laboratories for feedback and advice. We also thank Wei Li, Shawn Little, Julie Merkle, and Jean Schwarzbauer for helpful comments on manuscript. This work was supported by the Howard Hughes Medical Institute and U.S. Public Health Service Grant R01 GM077620.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407351111/-/DCSupplemental.
References
- 1.Thompson BJ. Cell polarity: Models and mechanisms from yeast, worms and flies. Development. 2013;140(1):13–21. doi: 10.1242/dev.083634. [DOI] [PubMed] [Google Scholar]
- 2.Müller HA, Bossinger O. Molecular networks controlling epithelial cell polarity in development. Mech Dev. 2003;120(11):1231–1256. doi: 10.1016/j.mod.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Roignot J, Peng X, Mostov K. Polarity in mammalian epithelial morphogenesis. Cold Spring Harb Perspect Biol. 2013;5(2):5. doi: 10.1101/cshperspect.a013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellenbroek SI, Iden S, Collard JG. Cell polarity proteins and cancer. Semin Cancer Biol. 2012;22(3):208–215. doi: 10.1016/j.semcancer.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Boulan E, Kreitzer G, Müsch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6(3):233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 7.Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol. 2012;14(12):1235–1243. doi: 10.1038/ncb2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene. 2008;27(41):5464–5476. doi: 10.1038/onc.2008.243. [DOI] [PubMed] [Google Scholar]
- 9.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Belmonte F, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128(2):383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gassama-Diagne A, et al. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol. 2006;8(9):963–970. doi: 10.1038/ncb1461. [DOI] [PubMed] [Google Scholar]
- 12.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422(6933):766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu W, et al. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16(2):433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yurchenco PD. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3(2):3. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22(7):521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Edgar D, Fässler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell. 2003;4(5):613–624. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: Insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3(7):531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- 18.Schneider M, et al. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133(19):3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denef N, Chen Y, Weeks SD, Barcelo G, Schüpbach T. Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell. 2008;14(3):354–364. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerner DW, et al. A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis. Dev Cell. 2013;24(2):159–168. doi: 10.1016/j.devcel.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong B, et al. Crag is a GEF for Rab11 required for rhodopsin trafficking and maintenance of adult photoreceptor cells. PLoS Biol. 2012;10(12):e1001438. doi: 10.1371/journal.pbio.1001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorrosal G, Pérez L, Herranz H, Milán M. Scarface, a secreted serine protease-like protein, regulates polarized localization of laminin A at the basement membrane of the Drosophila embryo. EMBO Rep. 2010;11(5):373–379. doi: 10.1038/embor.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horne-Badovinac S, Bilder D. Mass transit: Epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn. 2005;232(3):559–574. doi: 10.1002/dvdy.20286. [DOI] [PubMed] [Google Scholar]
- 24.Buszczak M, et al. The carnegie protein trap library: A versatile tool for Drosophila developmental studies. Genetics. 2007;175(3):1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YJ, Guzman-Hernandez ML, Balla T. A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes. Dev Cell. 2011;21(5):813–824. doi: 10.1016/j.devcel.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maffucci T. An introduction to phosphoinositides. Curr Top Microbiol Immunol. 2012;362:1–42. doi: 10.1007/978-94-007-5025-8_1. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Montell C. A phosphoinositide synthase required for a sustained light response. J Neurosci. 2006;26(49):12816–12825. doi: 10.1523/JNEUROSCI.3673-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci USA. 2001;98(26):15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schill NJ, Anderson RA. Out, in and back again: PtdIns(4,5)P(2) regulates cadherin trafficking in epithelial morphogenesis. Biochem J. 2009;418(2):247–260. doi: 10.1042/BJ20081844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gervais L, Claret S, Januschke J, Roth S, Guichet A. PIP5K-dependent production of PIP2 sustains microtubule organization to establish polarized transport in the Drosophila oocyte. Development. 2008;135(23):3829–3838. doi: 10.1242/dev.029009. [DOI] [PubMed] [Google Scholar]
- 31.Yan Y, Denef N, Tang C, Schüpbach T. Drosophila PI4KIIIalpha is required in follicle cells for oocyte polarization and Hippo signaling. Development. 2011;138(9):1697–1703. doi: 10.1242/dev.059279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chartier FJ, Hardy EJ, Laprise P. Crumbs controls epithelial integrity by inhibiting Rac1 and PI3K. J Cell Sci. 2011;124(Pt 20):3393–3398. doi: 10.1242/jcs.092601. [DOI] [PubMed] [Google Scholar]
- 33.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen JP, Reddy SS, Priess JR. Laminin is required to orient epithelial polarity in the C. elegans pharynx. Development. 2012;139(11):2050–2060. doi: 10.1242/dev.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Brien LE, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3(9):831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 36.Zuk A, Matlin KS. Apical beta 1 integrin in polarized MDCK cells mediates tubulocyst formation in response to type I collagen overlay. J Cell Sci. 1996;109(Pt 7):1875–1889. doi: 10.1242/jcs.109.7.1875. [DOI] [PubMed] [Google Scholar]
- 37.Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331(6020):1071–1074. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang H, et al. PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development. 1999;126(23):5365–5372. doi: 10.1242/dev.126.23.5365. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, et al. Thirty-one flavors of Drosophila rab proteins. Genetics. 2007;176(2):1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray MA, Fessler LI, Palka J. Changing distributions of extracellular matrix components during early wing morphogenesis in Drosophila. Dev Biol. 1995;168(1):150–165. doi: 10.1006/dbio.1995.1068. [DOI] [PubMed] [Google Scholar]