The biochemistry of sulfur touches on every aspect of cellular biology, from protein structure and function to redox regulation to defense against chemical stress. Over the last decade, posttranslational modification of protein thiols by reactive oxygen or nitrogen species has emerged as a major component of signal transduction, leading to both regulatory and inflammatory responses. A variety of disease states may thus arise as a result of dysregulation in formation of oxygen- and nitrogen-containing messenger molecules or in modification of thiols. For this reason, the pathways, products, and effects of thiol, thiolate, and disulfide modification have been the subject of intense investigation. Understanding of the extent and impact of thiol modification and metabolism has advanced through observation of physiological effects, as well as by in vivo detection of sulfur-based mediators and downstream products. In PNAS, Ida et al. (1) describe a new mass spectrometric method to quantitate low molecular-weight persulfide (RSSH) and polysulfide content in cells and a proteomic/Tag-Switch assay to detect S-polythiolated protein adducts. Their findings suggest that persulfides and polysulfides are reactive species that not only must be included among sulfur-based moieties that regulate oxidative stress and redox signaling, but in fact may be major mediators in this regard.
Recently, understanding of the thiol landscape has been expanded by the inclusion of hydrogen sulfide (H2S) as a third member of the endogenous gaseous transmitter class, along with nitric oxide (NO) and carbon monoxide (CO). All three share the characteristics of membrane-permeability, higher solubility in lipids than in water [although as an acid H2S deprotonates significantly at neutral pH (2)], and identification first as toxins. Pathways for biosynthesis of H2S have been identified, and interest in its physiological effects, particularly with respect to the cardiovascular and nervous systems, has increased (3–6).
Although H2S has been proposed to be formed through the intermediacy of persulfides, Fukuto and colleagues (7) have hypothesized that H2S may generate persulfides by the known interaction with oxidized thiols (equation 1 in ref. 8). This theory suggests the possibility that persulfides and H2S may be interrelated (9).
| [1] |
The detection methods newly developed by Ida et al. (1) unexpectedly demonstrated that persulfide and polysulfide species are formed at substantially higher levels in mammalian cells, tissues, and plasma than H2S. This observation suggests that detection of H2S may actually be representative of a larger, and heretofore unrecognized, biochemistry involving persulfides.
An important step in sulfur metabolism is the conversion of homocysteine to cysteine by the cystathionine β-synthase (CBS)/cystathionine γ-lyase (CSE)-mediated transsulfuration pathway. As the primary source of cysteine, this pathway is not only critical for protein synthesis but also allows control of the levels of sulfur-containing amino acids. Cysteine is further converted through γ-glutamylcysteine synthase to glutathione (GSH), which is a critical component of cellular protection against oxidants and potentially toxic electrophiles. The CBS system is also suggested to be a major source of endogenous H2S when the substrate is cysteine rather than homocysteine (10). In fact, expression of CBS/CSE is often used as a marker for H2S biosynthesis. Ida et al. (1) ascertained that cysteine persulfide (CysSSH/CysSS−) was a significant enzymatic product of cystine (CysSSCys) modification by the CBS/CSE system and that cystine is the preferred substrate for CSE, even compared with cystathionine.
Subsequent demonstration of facile redox exchange and sulfur atom transfer between CysSSH and both low and high molecular-weight thiols (1) suggests an important role for CysSSH in alternating the thiol landscape. CysSSH is suggested to react primarily with GSH and glutathione disulfide (GSSG) to produce glutathione-based per- and polysulfide species. Glutathione persulfide (GSSH/GSS−) is reported to be as high as 100 μM in the brain. These findings show that persulfides are a major component of redox-modified thiols in vivo and open up a new aspect of sulfur biology.
Although persulfides have been long known in bacterial biology—mediating processes such as interconversion of Fe4S4 and Fe2S2 clusters—their role in mammalian systems has been less clear. Persulfide intermediates have been suggested to be formed from numerous enzymes, including rhodanse, sulfhydryl oxidase, xanthine oxidase, aldehyde oxidase, and sulfurtransferases like mercaptopyruvate sulftransferase (MPST) (11, 12). Cysteine oxidase, a nonheme iron protein, produces a persulfide intermediate in oxidation of cysteine to . Consumption of H2S by the mitochondrial enzyme sulfide quinone reductase (SQR) also forms a persulfide that can form GSS−, through sulfur atom transfer (S0 as opposed to S2− sulfhydryl). Although earlier studies have suggested an involvement of persulfides, the levels reported in Ida et al. (1) indicate that the importance of these species and their subsequent modifications in enzymatic function, cellular signaling, and stress responses must be evaluated in detail.
Persulfides should be particularly nucleophilic. As such, formation of low molecular-weight persulfides may serve as nucleophilic reductants, whereas transfer of persulfides to proteins may dramatically affect enzyme activity. Ida et al. (1) determined that formation of persulfides from modification of polysulfides by glutathione reductase led to scavenging of H2O2, and overexpression of CSE led to protection against H2O2-mediated cell death. These findings expand the understanding of the role of sulfur-based species in control of reactive oxygen species levels by metal-independent mechanisms.
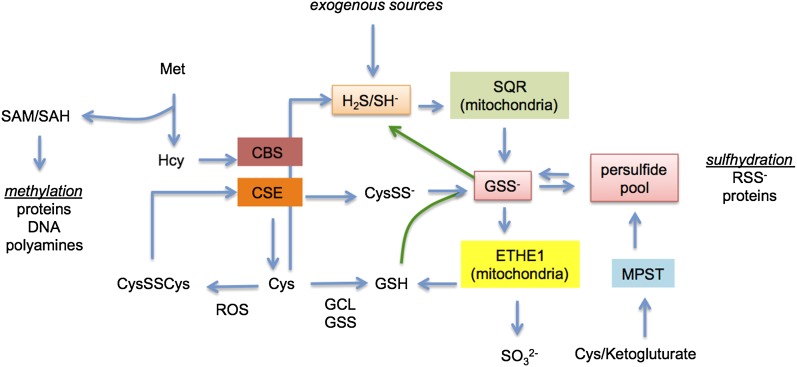
The analysis by Ida et al. (1) indicates that H2S-forming enzymes may also contribute to the persulfide pool and that there is a biological and chemical relationship between H2S and persulfides (Fig. 1). The physiological concentration of H2S is 10–30 nM in most tissues (13). Because H2S is a mitochondrial poison, H2S levels are controlled through consumption by SQR (H2S KM ∼ µM) to form a protein persulfide intermediate that generates GSS−. Cellular consumption of H2S is robust at 40 pmol⋅mg⋅s, suggesting the importance of consumption to homeostasis. This finding also suggests that high levels of H2S, from endogenous sources, the microbiome, diet, environmental exposure, or H2S-producing compounds, will be converted into the persulfide pool.
Fig. 1.
Enzymes and biochemistry involved in persulfide and H2S cross-talk. Exogenous sources include the microbiome, diet, environmental exposure, or H2S-producing compounds. Abbreviations: Cys, cysteine; CysSS−, cysteine persulfide; CysSSCys, cystine; CBS, cystathionine β-synthase; CSE, cystathionine γ lyase; ETHE1, persulfide dioxygenase; GCL, γ-glutamylcysteine synthetase or glutamate cysteine ligase; GSH, glutathione; GSS−, glutathione persulfide; GSS, glutathione synthetase; Hcy, homocysteine; Met, methionine; MPST, 3-mercaptopyruvate sulfurtransferase; RSS−, persulfide; ROS, reactive oxygen species; SAH, S-adenosyl homocysteine; SAM, S-adenosyl methionine; SQR, sulfide quinone reductase.
In particular, it should be considered that although H2S can react with disulfides, this
The article by Ida et al. indicates both crosstalk between H2S and persulfides and a mechanism to enhance the reactivity of H2S.
may be slow compared with cellular consumption by SQR. Thus, H2S levels may be primarily controlled enzymatically. In turn, GSS− is consumed by persulfide dioxygenase (ETHE1), a nonheme iron and O2-dependent enzyme to produce and GSH, suggesting that oxygen levels influence GSS− levels. Thus, numerous enzymes may participate in regulation of the balance between thiols, persulfides, and H2S (3, 14, 15). The low KM of SQR for H2S and higher KM of ETHE1 for GSS− indicates that persulfides should be the predominant species. In principle, it would seem that upon cytoplasmic generation of H2S by CBS/CSE and cysteine aminotransferase/MPST, H2S would be rapidly converted to GSS− by mitochondrial enzymes. Direct oxidation of CysSSCys by CBS/CSE to CysSS− and sulfur atom transfer to GSS−, as described by Ida et al. (1), presents another important mechanism to form persulfides under increased oxidative conditions. In fact, GSS− appears to be the quintessential mediator of sulfur atom transfer in the cell and is the persulfide adduct in highest quantity. Taking these data together, we find that the biological equilibrium between persulfides and H2S may favor persulfides by at least several orders of magnitude.
In summary, the article by Ida et al. (1) presents a new perspective of the redox landscape in biology. This analysis uncovers a new, and likely critical, modifier of redox- and thiol-based signaling and metabolism and suggests that the underlying mechanisms must be refined. Persulfides may be the kinetic factors that direct electrophiles and oxidants to specific sites, in part by generating low pKa targets for thiols that were otherwise kinetically unreactive. Furthermore, detection of persulfides expands the impact of CBS, CSE, and MST on thiol metabolism. Given the potential for detection of H2S to represent a larger pool of sulfur-based compounds, the article by Ida et al. (1) indicates both cross-talk between H2S and persulfides and a mechanism to enhance the reactivity of H2S. Sulfur atom transfer from low molecular-weight thiols to protein thiols would dramatically change thiol signaling and the metabolic landscape in cells. In a sense, persulfide protein adducts may provide a steering mechanism in thiol-based cellular signaling.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
See companion article on page 7606.
References
- 1.Ida T, et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci USA. 2014;111:7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 3.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 5.Coletta C, Szabo C. Potential role of hydrogen sulfide in the pathogenesis of vascular dysfunction in septic shock. Curr Vasc Pharmacol. 2013;11(2):208–221. [PubMed] [Google Scholar]
- 6.Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113(1):14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francoleon NE, Carrington SJ, Fukuto JM. The reaction of H2S with oxidized thiols: Generation of persulfides and implications to H2S biology. Arch Biochem Biophys. 2011;516(2):146–153. doi: 10.1016/j.abb.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Rao GS, Gorin G. Reaction of cystine with sodium sulfide in sodium hydroxide solution. J Org Chem. 1959;24(6):749–753. [Google Scholar]
- 9.Jackson MI, et al. The effects of nitroxyl (HNO) on H2O2 metabolism and possible mechanisms of HNO signaling. Arch Biochem Biophys. 2013;538(2):120–129. doi: 10.1016/j.abb.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26(3):243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 11.Mueller EG. Trafficking in persulfides: Delivering sulfur in biosynthetic pathways. Nat Chem Biol. 2006;2(4):185–194. doi: 10.1038/nchembio779. [DOI] [PubMed] [Google Scholar]
- 12.Jackson MR, Melideo SL, Jorns MS. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. 2012;51(34):6804–6815. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 13.Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 14.Kabil O, Motl N, Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbapap.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26(1):13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]