Abstract
Stearoyl-CoA desaturase (SCD) catalyzes the rate-limiting step in the biosynthesis of monounsaturated fatty acids. Mice with a targeted disruption of the SCD1 isoform have reduced body adiposity, increased energy expenditure, and up-regulated expression of several genes encoding enzymes of fatty acid β-oxidation in liver. The mechanisms by which SCD deficiency leads to these metabolic changes are presently unknown. Here we show that the phosphorylation and activity of AMP-activated protein kinase (AMPK), a metabolic sensor that regulates lipid metabolism during increased energy expenditure is significantly increased (≈40%, P < 0.01) in liver of SCD1 knockout mice (SCD1-/-). In parallel with the activation of AMPK, the phosphorylation of acetyl-CoA carboxylase at Ser-79 was increased and enzymatic activity was decreased (≈35%, P < 0.001), resulting in decreased intracellular levels of malonyl-CoA (≈47%, P < 0.001). An SCD1 mutation also increased AMPK phosphorylation and activity and increased acetyl-CoA carboxylase phosphorylation in leptin-deficient ob/ob mice. Lower malonyl-CoA concentrations are known to derepress carnitine palmitoyltransferase 1 (CPT1). In SCD1-/- mice, CPT1 and CPT2 activities were significantly increased (in both cases ≈60%, P < 0.001) thereby stimulating the oxidation of mitochondrial palmitoyl-CoA. Our results identify AMPK as a mediator of increased fatty acid oxidation in liver of SCD1-deficient mice.
Stearoyl-CoA desaturase (SCD) is a key enzyme involved in the biosynthesis of monounsaturated fatty acids. The preferred substrates are palmitoyl-CoA and stearoyl-CoA, which are converted to palmitoleoyl-CoA and oleoyl-CoA, respectively. These products are the most abundant monounsaturated fatty acids of phospholipids, triglycerides, cholesterol esters, and wax esters (1). Overall the expression of SCD can influence membrane fluidity, lipid metabolism, and adiposity. Increased SCD activity and alterations in the balance between saturated and monounsaturated fatty acids have been implicated in various diseases including cancer, diabetes, atherosclerosis, and obesity (2).
To investigate the physiological function of SCD, we created mice with a targeted disruption of the SCD1 gene (SCD1-/-) (3). We established that SCD1-/- mice have reduced body adiposity and increased insulin sensitivity, and are resistant to diet-induced weight gain relative to wild-type mice. The resistance to diet-induced obesity is due to increased energy expenditure (4). SCD1-/- mice also have increased fasting levels of plasma ketone bodies but reduced levels of plasma insulin and leptin. Moreover, mice with mutations in the SCD1 gene accumulate less fat in their adipose tissue and have lower levels of triglyceride and cholesterol esters in liver than wild-type animals (5). In SCD1-/- mice, the expression of several genes encoding enzymes of fatty acid oxidation are up-regulated, whereas genes encoding enzymes of lipid synthesis are down-regulated (4).
Leptin is an adipocyte-derived hormone that plays a pivotal role in regulating food intake and energy expenditure. Leptin stimulates the oxidation of fatty acids and uptake of glucose and reduces the accumulation of lipids in nonadipose tissues. Recently, leptin was found to repress RNA levels and enzymatic activity of SCD1 in liver (6). Ob/ob mice with SCD1 mutations were significantly less obese than ob/ob controls and had markedly increased energy expenditure with reduced triglyceride storage in liver (6). SCD1 is therefore a component of the novel metabolic response to leptin signaling, and SCD1 repression accounts for a significant proportion of the effects of leptin on energy expenditure and body weight. The signaling pathways that mediate the metabolic effects of leptin remain largely unknown. Leptin has been found to selectively stimulate phosphorylation and activation of the α2 catalytic subunit of AMP-activated protein kinase (AMPK), an enzyme that has been found to be a principal mediator of the effects of leptin on fatty acid oxidation in muscle (7).
AMPK is a heterotrimeric enzyme that is conserved from yeast to humans and functions as a gauge to monitor cellular energy stores. Numerous mechanisms of AMPK action on lipid and carbohydrate metabolism have been proposed (8–10). AMPK regulates the expression of genes of lipid synthesis by modulating the activities of transcription factors and coactivators (11). Phosphorylation of p300 by AMPK inhibits its interaction with several nuclear receptors, i.e., peroxisome proliferator-activated receptors and thyroid hormone, retinoic acid, and retinoid X receptors, resulting in either activation or repression of gene expression (12). Phosphorylation of carbohydrate-response-element-binding protein by AMPK blocks DNA binding and mediates inhibition of glucose-induced gene transcription (13). AMPK activation also suppresses the expression of SREBP-1c, a key transcription factor regulating genes of fatty acid and triglyceride biosynthesis (14). In addition to regulating gene transcription, AMPK regulates (by phosphorylation/dephosphorylation) the activity of enzymes of fatty acid and cholesterol synthesis (15–18).
Phosphorylation of acetyl-CoA carboxylase (ACC) at Ser-79 by AMPK leads to inhibition of ACC activity and decreased malonyl-CoA content. Malonyl-CoA is required for fatty acid biosynthesis and also inhibits the mitochondrial carnityl palmitoyltransferase shuttle system, the rate-limiting step in the import and oxidation of fatty acids in mitochondria (19). ACC catalyzes a pivotal step in fuel metabolism as it links fatty acid and carbohydrate metabolism through the shared intermediate acetyl-CoA. ACC2 knockout mice show increased fatty acid oxidation in muscle, heart, and liver and are lean (20). Thus, we hypothesized that AMPK acting through the inhibition of ACC would be one mechanism accounting for increased fatty acid oxidation in the livers of SCD1-deficient mice. Indeed, we found that the phosphorylation and activity of AMPK are increased in SCD1-/- mice and we show that phosphorylation and inactivation of ACC results in decreased levels of malonyl-CoA and activation of carnitine palmitoyltransferase 1 (CPT1) leading to increased palmitate oxidation in liver mitochondria of SCD1-/- mice.
Materials and Methods
Animals. The generation of SCD1-/- and abJ/abJ;ob/ob mice has been described (3, 6). Twelve-week-old prebred homozygous (SCD1-/-) and wild-type (SCD1+/+) male mice on a 129SV background were used. The abJ/abJ;ob/ob and abJ/ab+;ob/ob control mice on a C57BL/6 background were 16 weeks old. Mice were maintained on a 12-h dark/light cycle and fed a normal nonpurified diet (5008 test diet, PMI Nutrition International, Richmond, IN). The breeding of these animals was in accordance with the protocols approved by the Animal Care Research Committees of the University of Wisconsin and The Rockefeller University Laboratory Animal Research Center.
Materials. l-[3H]carnitine, [14C]sodium bicarbonate, [γ-32P]ATP, [14C]acetyl-CoA, [14C]palmitic acid, and [14C]palmitoyl-CoA were purchased from American Radiolabeled Chemicals (St. Louis). SAMS peptide and anti-phospho-ACC antibody were obtained from Upstate (Waltham, MA). Anti-ACC1, anti-ACC2, and anti-CPT1 antibodies were from Alpha Diagnostic (San Antonio, TX). Other chemicals were purchased from Sigma or Fisher Scientific.
Preparation of Mitochondria and Measurement of Mitochondrial Fatty Acid Oxidation. Mitochondria were isolated essentially as described by Vance (21). The mixture used to measure mitochondrial fatty acid oxidation contained, in a final volume of 2.5 ml, 2 ml of modified Krebs-Henseleit buffer (pH 7.4), 0.2 mM [14C]palmitoyl-CoA bound to 7.2 mg/ml BSA, 4 mM ATP, 0.5 mM l-carnitine, 0.05 mM CoA, 2 mM DTT, and 0.5 ml of suspended mitochondria (5 mg protein/ml). Palmitoyl-CoA oxidation was determined in the presence and absence of 2 mM KCN, and the cyanide-sensitive part of the oxidation was taken as mitochondrial oxidation (22). Labeled CO2 was trapped in 10 M KOH. An aliquot of the neutralized extract was analyzed for the [14C] content of the ketone bodies (23), and another aliquot was adjusted to pH 4 with 3 M acetate buffer and extracted twice with petroleum ether to remove tracers of [14C]palmitoyl-CoA, and the aqueous phase was then counted (acid-soluble labeled oxidation products) (23).
Measurement of AMPK Activity. Livers from SCD1-/- and SCD1+/+, abJ/abJ;ob/ob, and abJ/ab+;ob/ob mice were snapfrozen. AMPK isolation was performed as described by Kudo et al. (24). The AMPK activity in the PEG 8000 fraction was assessed as described (25). To 50 μl of reaction mixture (40 mM Hepes, pH 7.0/200 μM SAMS peptide/200 μM AMP/80 mM NaCl/0.8 mM EDTA/0.8 mM dithiothreitol/8% glycerol/200 μM [γ-32P]ATP) 10 μl of PEG 8000 fraction was added. The mixture was incubated for 30 min at 30°C. Forty-microliter aliquots were then removed and spotted onto P81 Whatman filters, which were then washed three times with 1% H3PO4 and once with acetone, air dried, and counted by using a standard scintillation procedure.
Western Blot Analysis and ACC Activity Assay. Fifty micrograms of purified protein obtained as described above or isolated mitochondria was loaded onto a 9% SDS/PAGE gel. The separated proteins were transferred to nitrocellulose membranes that were blotted by using antibodies against phosphopeptides based on the amino acid sequence surrounding Thr-172 of the α subunit of human AMPK and Ser-79 of rat ACC. Protein levels of α1 and α2 AMPK, and ACC1, ACC2, and CPT1, were determined by using specific antibodies. Anti-AMPK α1 and α2 and anti-AMPK-pT172 antibodies were obtained as described (26, 27). The proteins were visualized by using ECL (Amersham Biosciences) as described by the manufacturer and quantified by densitometry. ACC activity in the 6% PEG 8000 fraction was determined by using the [14C]bicarbonate fixation assay as described (28).
Determination of Malonyl-CoA Content. Malonyl-CoA levels in liver were measured according to the methods from McGarry et al. (29). One milligram of frozen tissue was homogenized in 5 ml of 6% HClO4. Extracts were neutralized to pH 6.0 with KOH. The reaction mixture was contained, in a volume of 1 ml, 0.2 M potassium phosphate buffer (pH 7.0), 2.5 mM DTT, 2 mM EDTA, 0.2 mM NADPH, 1 mg BSA, 0.68 μM [3H]acetyl-CoA, and 0.1 ml of liver extract. Reactions were initiated by the addition of 100 μg of highly purified fatty acid synthetase and were incubated at 37°C for 2 h. The reactions were terminated by addition of 25 μl of 70% HClO4. The reaction products were extracted with petroleum ether and assayed for radioactivity in a liquid scintillation counter.
Measurement of CPT1 and CPT2 Activities. The CPT1 assay was performed as described by Bremer (30). Briefly, 200 μg of mitochondrial protein was added to the assay medium containing 20 mM Hepes (pH 7.3), 75 mM KCl, 2 mM KCN, 1% fat-free BSA, 70 μM palmitoyl-CoA, 0.25 mM l-[3H]carnitine, with or without 10, 30, 50, or 100 μM malonyl-CoA. Samples were incubated at 37°C for 3 min. The reaction was stopped by the addition of 0.5 ml of 4 M ice-cold perchloric acid. Mitochondria were centrifuged, the pellet was washed with 500 μl of 2 mM perchloric acid, and centrifugation was repeated. The resulting pellet was then resuspended in 800 μl of ddH2O and extracted with 600 μl of butanol. Three hundred microliters of the butanol-phase was counted by liquid scintillation. CPT2 activity was measured in Tween 20 extracts of whole mitochondria according to Kolodziej et al. (31). Purified mitochondria (1–2 mg of protein) were treated with Tween 20 (3 μl of detergent per mg of protein) for 40 min at 0°C, with two periods (10 s) of sonication. CPT2 activity in the supernatant was assayed in the same manner as CPT1 activity described above. The enzyme was not sensitive to malonyl-CoA.
Incorporation of [14C]palmitic Acid into Liver Lipids. Ten microcurie (Ci; 1 Ci = 37 GBq) of [14C]palmitic acid suspended in albumin was administered into the tail vein. Ten minutes after administration of the label, the liver was extracted and homogenized. Lipids were extracted (32) and assayed for radioactivity in a liquid scintillation counter.
Protein Determination. Protein concentration was measured by the Bradford method with BSA as a standard.
Statistical Analyses. Results were analyzed by using the Student's t test. A difference of P < 0.05 was considered significant. Values are presented as means ± SD (n = 5 mice per group in the case of SCD1+/+ and SCD1-/- mice, and n ≥ 3 in the case of abJ/abJ;ob/ob and abJ/ab+;ob/ob mice).
Results
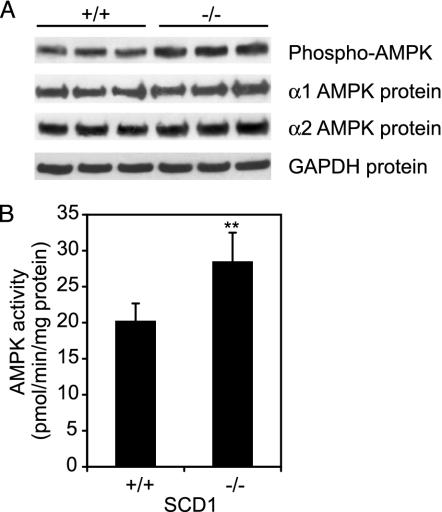
AMPK Is Activated in SCD1-/- Mice. One of the mechanisms that could account for the increased energy expenditure in SCD1-deficient mice is the activation of AMPK. AMPK phosphorylation was increased 52% in SCD1-/- mice relative to the wild-type controls (Fig. 1A). We then assayed AMPK activity by SAMS peptide phosphorylation in the presence of saturating (200 μM) concentrations of ATP. AMPK activity was increased 40% in SCD1-/- mice relative to the wild-type mice (P < 0.01) (Fig. 1B). The total protein levels of α1-AMPK and α2-AMPK were increased 20% and 15%, respectively, suggesting increased gene expression, but the level of GAPDH used as a loading control was not significantly altered (Fig. 1 A).
Fig. 1.
AMPK is activated in SCD1-/- mice. (A) Phospho-AMPK and α1 and α2 AMPK protein subunits were quantified by using the Western blot technique. (B) The AMPK activity in PEG 8000 fraction, with SAMS peptide as substrate, was assessed as described in Materials and Methods. **, P < 0.01 vs. control.
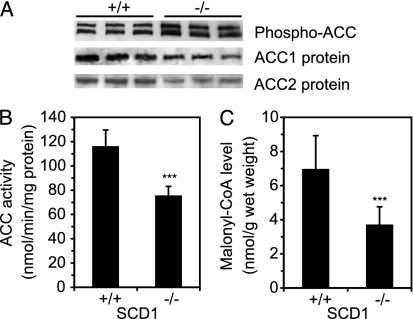
To determine whether AMPK activation mediates increased fatty acid oxidation in the setting of SCD1 deficiency, we examined the ACC Ser-79 phosphorylation and enzymatic activity. The level of ACC1 and ACC2 Ser-79 phosphorylation was 62% higher (P < 0.01) in SCD1-/- mice relative to SCD1+/+ mice (Fig. 2A). ACC1 and ACC2 protein levels were decreased, reflecting a decrease in ACC mRNA (M.M. and J.M.N., unpublished data) consistent with reduced levels of mRNAs of enzymes encoding genes of lipid biosynthesis previously observed in SCD1-/- mice (4). ACC activity decreased by 35% in SCD1-/- mice relative to SCD1+/+ controls (75.03 and 115.61 nmol/min/mg protein, respectively; P < 0.001) (Fig. 2B).
Fig. 2.
Phospho-ACC and ACC protein level (A), ACC activity (B), and malonyl-CoA concentration (C) in liver of SCD1+/+ and SCD1-/- mice. Phospho-ACC [visible are the two known ACC isoforms, ACC1 (265 kDa) and ACC2 (280 kDa)], ACC1, and ACC2 proteins were quantified by Western blotting. ACC activity in the 6% PEG 8000 fraction was determined by using the [14C]bicarbonate fixation assay. Reaction was conducted for 2 min in the presence of 10 mM citrate and stopped by adding 10% perchloric acid. Malonyl-CoA level was measured radiochemically according to McGarry et al. (29). Highly purified fatty acid synthetase from chicken was used. ***, P < 0.001 vs. controls.
To determine whether decreased ACC activity leads to reduced malonyl-CoA concentrations, we assayed malonyl-CoA levels in the acid soluble liver extract by measuring the incorporation of [3H]acetyl-CoA into palmitic acid in the presence of NADPH and highly purified fatty acid synthetase. The malonyl-CoA content decreased ≈50% (P < 0.001) in the SCD1-/- livers relative to SCD1+/+ controls (Fig. 2C).
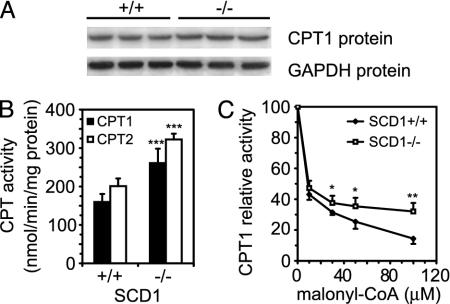
Increased Mitochondrial Fatty Acid Oxidation in Mice with Disruptions in SCD1. A fall in malonyl-CoA would be expected to result in increased activities of CPT1 and CPT2, enzymes responsible for activated fatty acid transport into mitochondria for β-oxidation. Although CPT1 protein level was slightly increased (18%, P < 0.05) (Fig. 3A), CPT1 activity was 63% higher in SCD1-/- mice relative to wild-type controls (260.76 nmol/min/mg of protein in SCD1-/- and 159.68 nmol/min/mg of protein in SCD1+/+ mice; P < 0.001) (Fig. 3B). The lower Km value for enzyme purified from liver of SCD1-/- mice (35.1 ± 1.4 μM carnitine) relative to that from wild-type mice (50.9 ± 4.5 μM of carnitine; P < 0.001) is consistent with increased CPT1 activity. CPT2 activity was also higher in SCD1-/- mice relative to wild-type controls (322.43 nmol/min/mg of protein and 200.96 nmol/min/mg of protein, respectively; P < 0.001) (Fig. 3B). During increased fatty acid oxidation, hepatic CPT1 becomes less sensitive to inhibition by malonyl-CoA. The CPT1 activity isolated from livers of SCD1-/- mice was less sensitive to inhibition by malonyl-CoA than the enzyme from wild-type mice (86% and 68% of inhibition in presence of 100 μM malonyl-CoA, respectively; P < 0.01) (Fig. 3C).
Fig. 3.
CPT1 protein level (A), CPT1 and CPT2 activities (B), and effect of malonyl-CoA on CPT1 activity (C) in mitochondria of SCD1+/+ and SCD1-/- mice. CPT1 protein level was measured by the Western blot technique. Intact mitochondria were separated according to Vance (21), and CPT1 and CPT2 activities were measured radiochemically by using l-[3H]carnitine. CPT2 activity was measured in Tween 20 extracts of mitochondria as described in Materials and Methods. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 vs. controls.
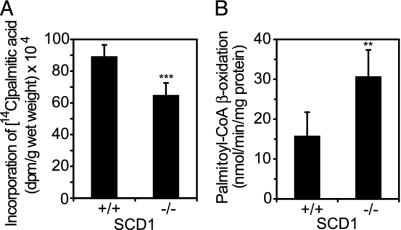
To determine whether SCD1 deficiency increased fatty acid utilization, we injected [14C]palmitate into the tail vein and assayed its incorporation into lipids. As shown in Fig. 4A, [14C]palmitate incorporation into storage lipids was decreased in SCD1-/- mice compared to SCD1+/+ controls. However, the rate of β-oxidation, as assayed by the oxidation of [14C]palmitoyl-CoA in liver mitochondria, was >2-fold (P < 0.01) higher in SCD1-/- mice compared to wild-type controls (Fig. 4B).
Fig. 4.
Incorporation of [14C]palmitic acid into lipids (A) and palmitoyl-CoA oxidation in mitochondria of SCD1+/+ and SCD1-/- mice (B). Ten microcurie of [14C]palmitic acid was administered in the tail vein. Ten minutes after administration of the label, liver was taken and homogenized. Then lipids were extracted and assayed for radioactivity in a liquid scintillation counter. Oxidation of palmitoyl-CoA was measured in isolated mitochondria and presented as the sum of labeled CO2 released during oxidation, [14C] content in the ketone bodies fraction, and acid-soluble labeled oxidation products as described in Materials and Methods. **, P < 0.01; and ***, P < 0.001 vs. controls.
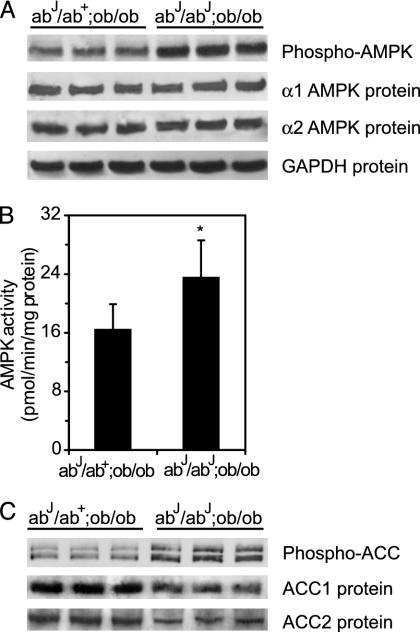
SCD1 Deficiency Increases AMPK Activity in Ob/ob Leptin-Deficient Mice. Leptin has been shown to increase fatty acid oxidation in muscle both directly and indirectly via nervous stimulation from the brain, and in both cases this is associated with activation of the α2 catalytic subunit of AMPK (7). To explore the effects of SCD1 deficiency on AMPK-mediated fatty acid oxidation in leptin-deficient mice, ob/ob mice were intercrossed with abJ/abJ mice, which have a natural mutation in the SCD1 gene to generate the abJ/abJ;ob/ob mice (6). These double-mutant mice showed a significant increase in AMPK phosphorylation (≈2-fold; Fig. 5A) and activity (≈44%, P < 0.05; Fig. 5B), and an increase in ACC phosphorylation at Ser-79 (≈2-fold; Fig. 5C) and decrease in ACC1 and ACC2 protein levels (Fig. 5C) relative to abJ/ab+;ob/ob controls. The ACC activity was also decreased in abJ/abJ;ob/ob mice (data not shown).
Fig. 5.
SCD1 deficiency increases AMPK phosphorylation (A) and activity (B) and ACC phosphorylation (C) in ob/ob mice. Phospho-AMPK and α1 and α2 AMPK protein subunits in liver of ob/ob (abJ/ab+;ob/ob) and double-mutant (abJ/abJ;ob/ob) mice were assayed by using the Western blot technique. AMPK activity was assayed as described in Materials and Methods. Phospho-ACC1 (265 kDa) and ACC2 (280 kDa) isoforms and ACC1 and ACC2 protein levels were assayed by Western blotting. *, P < 0.05 vs. control.
Discussion
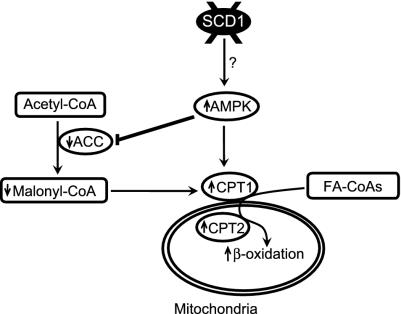
We have previously shown that loss of SCD1 function activates genes of fatty acid oxidation and reduces expression of genes that encode enzymes of fatty acid synthesis in liver (4). The signals induced by SCD1 deficiency leading to increased fatty acid oxidation were not known. However, in the present study we found that SCD1 deficiency activates AMPK, an enzyme that functions as a fuel gauge to monitor the status of cellular energy. We found that activated AMPK increased ACC phosphorylation, a well known mechanism for ACC inactivation, thereby resulting in reduced malonyl-CoA levels. The reduction in malonyl-CoA levels derepresses CPT1 leading to an increase in the transport of fatty acids into mitochondria and their subsequent oxidation. These observations, shown schematically in Fig. 6, begin to provide the mechanisms for increased fatty acid oxidation in SCD1-/- animals.
Fig. 6.
Changes in lipid metabolism due to activation of AMPK in liver of mice with disruption of SCD1 gene: a proposed model. Activation of AMPK decreases ACC activity that in turn decreases malonyl-CoA level in liver. A drop in malonyl-CoA content activates CPT1 activity and increases transport of fatty acids into mitochondria for β-oxidation. AMPK can also phosphorylate a cytosolic protein that activates CPT1 (33).?, Mechanism of AMPK activation by SCD1 deficiency is unknown.
The mitochondrial carnitine system plays a crucial role in β-oxidation. CPT1 catalyzes the esterification of long-chain acyl-CoAs to l-carnitine for transport into mitochondria for fatty acid oxidation. The acyl-carnitine conjugate is converted back to the acyl-CoA ester inside mitochondria by CPT2 (19). SCD1-/- mice have significantly increased CPT1 and CPT2 activities and decreased Km value for CPT1. Also, CPT1 gene expression is up-regulated (4) and protein level is slightly increased (Fig. 3A) in these animals relative to wild-type controls. Malonyl-CoA is an allosteric inhibitor of CPT1. During energy excess, the increased malonyl-CoA inhibits CPT1 activity, preventing the oxidation of newly formed fatty acids bound for energy storage. On the other hand, during starvation and exercise malonyl-CoA levels fall to permit the oxidation of fatty acids for energy production (34). Malonyl-CoA levels are decreased ≈2-fold in SCD1-/- liver resulting in CPT1 activation. The CPT1 from livers of SCD1-/- mice was also found to be less sensitive to inhibition by malonyl-CoA than the enzyme from wild-type mice. Decreased sensitivity to CPT1 inhibition by malonyl-CoA has been observed during increased fatty acid oxidation (19) and is consistent with increased fatty acid oxidation in livers of SCD1-/- mice.
Malonyl-CoA formation is mainly regulated by changes in the activity of the ACC and malonyl-CoA decarboxylase. ACC activity is controlled by various mechanisms including changes in degree of polymerization, allosteric regulation by citrate and glutamate, and covalent modification by phosphorylation/dephosphorylation (35). Although several protein kinases can phosphorylate ACC, it is currently accepted that in intact hepatocytes and in the liver in vivo this phosphorylation, which leads to inactivation of the enzyme, is mainly mediated by AMPK (36, 37). AMPK inactivates ACC by phosphorylation at Ser-79 (38). We found higher ACC phosphorylation at Ser-79 position in liver extracts from SCD1-/- mice, even when the ACC1 and ACC2 protein levels were decreased. Phosphorylated ACC can be reactivated by a glutamate-dependent type 2A protein phosphatase that is known to dephosphorylate a synthetic peptide encompassing the Ser-79 phosphorylation site for AMPK in ACC (39). In liver, the activation state of ACC will result from a balance between the activities of AMPK and the protein phosphatase.
Insulin promotes rapid activation of ACC by dephosphorylation (35, 40), whereas glucagon has the opposite effect (37, 41, 42). SCD1-/- mice have lower levels of plasma insulin (4, 43) and increased glucagon levels (S. H. Lee and J.M.N., unpublished data). Together with activation of AMPK, these hormonal changes could support higher ACC phosphorylation (decreasing its activity) and therefore increased fatty acid oxidation in livers of SCD1-/- mice. The other possible mechanism is that saturated fatty acyl CoAs that build up in SCD1 deficiency allosterically inhibit ACC, thus reducing cellular levels of malonyl-CoA. A decrease in the cellular levels of malonyl-CoA would de-repress fatty acid oxidation. The findings in the SCD1-/- mice are therefore similar to those observed in mice lacking ACC2, which also have increased fatty acid oxidation in liver, muscle, and heart (20) and, like SCD1-/- mice, are lean and resistant to diet-induced obesity. However, it is not known whether ACC2-deficient mice also have increased AMPK activity.
The mechanism of AMPK activation by SCD1 deficiency is unknown. AMPK activity depends on the AMP/ATP ratio that is changed during states of high energy expenditure as well as by numerous stress conditions that inhibit ATP synthesis (8, 10, 18, 36). During exercise AMPK coordinately regulates ACC, malonyl-CoA decarboxylase, and glycerol-3-phosphate acyltransferase (GPAT) activity in liver, adipose tissue, and (except for GPAT) in muscle (18). We found that after exhausting exercise, SCD activity in wild-type mice liver dramatically decreased (data not shown). Thus, SCD deficiency could be involved in AMPK activation and regulation of lipid metabolism during exercise. We established that SCD1-/- mice are more active and have higher energy expenditure than wild-type mice (4). It is possible that these metabolic changes due to increased ATP consumption cause an increase in AMP concentration leading to AMPK activation. Leptin may also have effects on the AMPK system. It has been shown that leptin directly and indirectly activates AMPK in skeletal muscle (7) and white adipocytes (44), although not in heart (45). The effects of leptin on AMPK in liver are not fully known. SCD1 gene expression is repressed by leptin in liver and SCD1 deficiency has been shown to mimic the metabolic effects of leptin in ob/ob mice (6). However, the activation of AMPK in liver of SCD1-/- mice seems to be leptin-independent because increased AMPK phosphorylation and enzymatic activity and increased ACC phosphorylation were still observed in the livers of abJ/abJ;ob/ob doublemutant mice (Fig. 5). These observations indicate that SCD1 expression is involved in regulation of AMPK function in mouse liver. Studies are underway to determine the effects of SCD deficiency in other metabolically important tissues.
In summary, the results presented here show that SCD1 deficiency leads to activation of AMPK. As shown schematically in Fig. 6, activation of AMPK leads to decreased ACC, thus causing a drop in the malonyl-CoA level in liver. Decreased malonyl-CoA content activates CPT1 activity and increases transport of fatty acids into mitochondria for their β-oxidation. High SCD1 expression favors fat storage, whereas low SCD expression leads to fat burning or leanness (46). SCD1 therefore appears to be an important metabolic control point and is emerging as a promising therapeutic target that could be used in the treatment of obesity, diabetes, and other metabolic diseases.
Acknowledgments
We thank Dr. Salih J. Wakil (Baylor College of Medicine, Houston) for providing purified fatty acid synthetase. This work was supported by National Institute of Health Grants RO1-DK62388 (to J.M.N.), RO1-DK41096 (to J.M.F.), and MSTP GM07739 (to P.C.), American Heart Association Postdoctoral Fellowship 0420051Z (to A.D.), Wellcome Trust Grant 065565 (to D.G.H.), and European Commission Grant QLG1-CT-2001-01488 (to D.G.H.).
Abbreviations: ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; CPT, carnitine palmitoyltransferase; SCD, stearoyl-CoA desaturase.
References
- 1.Enoch, H. G., Catala, A. & Strittmatter, P. (1976) J. Biol. Chem. 251, 5095-5103. [PubMed] [Google Scholar]
- 2.Ntambi, J. M. (1999) J. Lipid Res. 40, 1549-1558. [PubMed] [Google Scholar]
- 3.Miyazaki, M., Man, W. C. & Ntambi, J. M. (2001) J. Nutr. 131, 2260-2268. [DOI] [PubMed] [Google Scholar]
- 4.Ntambi, J. M., Miyazaki, M., Stoehr, J. P., Lan, H., Kendziorski, C. M., Yandell, B. S., Song, Y., Cohen, P., Friedman, J. P. & Attie, A. D. (2002) Proc. Natl. Acad. Sci. USA 99, 11482-11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazaki, M., Kim, Y. C., Gray-Keller, M. P., Attie, A. D. & Ntambi, J. M. (2000) J. Biol. Chem. 275, 30132-30138. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, P., Miyazaki, M., Socci, N. D., Hagge-Greenberg, A., Liedtke, W., Soukas, A. A., Sharma, R., Hudgins, L. C., Ntambi, J. M. & Friedman, J. M. (2002) Science 297, 240-243. [DOI] [PubMed] [Google Scholar]
- 7.Minokoshi, Y., Kim, Y. B., Peroni, O. D., Fryer, L. G. D., Müller, C., Carling, D. & Kahn, B. B. (2002) Nature 415, 339-343. [DOI] [PubMed] [Google Scholar]
- 8.Hardie, D. G., Scott, J. W., Pan, D. A. & Hudson, E. R. (2003) FEBS Lett. 546, 113-120. [DOI] [PubMed] [Google Scholar]
- 9.Ferre, P., Azzout-Marniche, D. & Foufelle, F. (2003) Biochem. Soc. Trans. 31, 220-223. [DOI] [PubMed] [Google Scholar]
- 10.Kemp, B. E., Stapleton, D., Campbell, D. J., Chen, Z. P., Murthy, S., Walter, M., Gupta, A., Adams, J. J., Katsis, F., Van Denderen, B., et al. (2003) Biochem. Soc. Trans. 31, 162-168. [DOI] [PubMed] [Google Scholar]
- 11.Woods, A., Azzout-Marniche, D., Foretz, M., Stein, S. C., Lemarchand, P., Ferre, P., Foufelle, F. & Carling, D. (2000) Mol. Cell. Biol. 20, 6704-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang, W., Hong, Y. H., Shen, X. Q., Frankowski, C., Camp, H. S. & Left, T. (2001) J. Biol. Chem. 276, 38341-38344. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi, T., Osatomi, K., Yamashita, H., Kabashima, T. & Uyeda, K. (2002) J. Biol. Chem. 277, 3829-3835. [DOI] [PubMed] [Google Scholar]
- 14.Zhou, G., Myers, R., Li, Y., Chen, Y., Fenyk-Melody, J., Wu, M., Ventre, J., Doebber T., Fujii, N., Musi, N., et al. (2001) J. Clin. Invest. 108, 1167-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke, P. R. & Hardie, D. G. (1990) EMBO J. 9, 2439-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies, S. P., Carling, D., Munday, M. R. & Hardie, D. G. (1992) Eur. J. Biochem. 203, 615-623. [DOI] [PubMed] [Google Scholar]
- 17.Muoio, D. M., Seefeld, K., Witters, L. A. & Coleman, R. A. (1999) Biochem. J. 338, 783-791. [PMC free article] [PubMed] [Google Scholar]
- 18.Park, H., Kaushik, V. K., Constant, S., Prentki, M., Przybytkowski, E., Ruderman, N. B. & Saha, A. K. (2002) J. Biol. Chem. 277, 32571-32577. [DOI] [PubMed] [Google Scholar]
- 19.Kerner, J. & Hoppel, C. (2000) Biochim. Biophys. Acta 1486, 1-17. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Elheiga, L., Matzuk, M. M., Abo-Hashema, K. A. & Wakil, S. J. (2001) Science 291, 2613-2616. [DOI] [PubMed] [Google Scholar]
- 21.Vance, J. E. (1990) J. Biol. Chem. 265, 7248-7256. [PubMed] [Google Scholar]
- 22.Mannaerts, G. P., Debeer, L. J., Thomas, J. & De Schepper, P. J. (1979) J. Biol. Chem. 11, 4585-4595. [PubMed] [Google Scholar]
- 23.Mannaerts, G. P., Thomas, J., Deeber, L. J., McGarry, J. D. & Foster, D. W. (1978) Biochim. Biophys. Acta 529, 201-211. [DOI] [PubMed] [Google Scholar]
- 24.Kudo, N., Barr, A. J., Barr, R. L., Desai, S. & Lopaschuk, G. D. (1995) J. Biol. Chem. 270, 17513-17520. [DOI] [PubMed] [Google Scholar]
- 25.Sakoda, H., Ogihara, T., Anai, M., Fujishiro, M., Ono, H., Onishi, Y., Katagiri, H., Abe, M., Fukushima, Y., Shojima, N., et al. (2002) Am. J. Physiol. 282, E1239-E1244. [DOI] [PubMed] [Google Scholar]
- 26.Woods, A., Salt, I., Scott, J., Hardie, D. G. & Carling, D. (1996) FEBS Lett. 397, 347-351. [DOI] [PubMed] [Google Scholar]
- 27.Sugden, C., Crawford, R. M., Halford, N. G. & Hardie, D. G. (1999) Plant J. 19, 433-439. [DOI] [PubMed] [Google Scholar]
- 28.Witters, L. A. & Kemp, B. E. (1992) J. Biol. Chem. 267, 2864-2867. [PubMed] [Google Scholar]
- 29.McGarry, J. D., Stark, M. J. & Foster, D. W. (1978) J. Biol. Chem. 253, 8291-8293. [PubMed] [Google Scholar]
- 30.Bremer, J. (1981) Biochim. Biophys. Acta 665, 628-631. [DOI] [PubMed] [Google Scholar]
- 31.Kolodziej, M. P., Crilly, P. J., Corstorphine, C. G. & Zammit, V. A. (1992) Biochem. J. 281, 415-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bligh, E. G. & Dyer, W. J. (1959) Can. J. Biol. Chem. Physiol. 37, 911-917. [DOI] [PubMed] [Google Scholar]
- 33.Velasco, G., Gomez del Pulgar, T., Carling, D. & Guzman, M. (1998) FEBS Lett. 439, 317-320. [DOI] [PubMed] [Google Scholar]
- 34.Thupari, J. N., Landree, L. E., Ronnet, G. V. & Kuhajda, F. P. (2002) Proc. Natl. Acad. Sci. USA 99, 9498-9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witters, L. A., Watts, T. D., Daniels, D. L. & Evans, J. L. (1988) Proc. Natl. Acad. Sci. USA 85, 5473-5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardie, D. G. (1992) Biochim. Biophys. Acta 1123, 231-238. [DOI] [PubMed] [Google Scholar]
- 37.Woods, A., Munday, M. R., Scott, J., Yang, X., Carlson, M. & Carling, D. (1994) J. Biol. Chem. 269, 19509-19515. [PubMed] [Google Scholar]
- 38.Hardie, D. G. (1989) Prog. Lipid Res. 28, 117-146. [DOI] [PubMed] [Google Scholar]
- 39.Gaussin, V., Hue, L., Stalmans, W. & Bollen, M. (1996) Biochem. J. 316, 217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlson, C. L. & Winder, W. W. (1999) J. Appl. Physiol. 86, 669-674. [DOI] [PubMed] [Google Scholar]
- 41.Kim, K. H. (1997) Annu. Rev. Nutr. 17, 77-99. [DOI] [PubMed] [Google Scholar]
- 42.Ruderman, N. B., Saha, A. K., Vavvas, D. & Witters, L. A. (1999) Am. J. Physiol. 276, E1-E18. [DOI] [PubMed] [Google Scholar]
- 43.Rahman, S. M., Dobrzyn, A., Dobrzyn, P., Lee, S.-H., Miyazaki, M. & Ntambi, J. M. (2003) Proc. Natl. Acad. Sci. USA 100, 11110-11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orci, L., Cook, W. S., Ravazzola, M., Wang, M., Park, B.-H., Montesano, R. & Unger, R. H. (2004) Proc. Natl. Acad. Sci. USA 101, 2058-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atkinson, L. L., Fischer, M. A. & Lopaschuk, G. D. (2002) J. Biol. Chem. 277, 29424-29430. [DOI] [PubMed] [Google Scholar]
- 46.Dobrzyn, A. & Ntambi, J. M. (2004) Trends Cardiovasc. Med. 14, 77-81. [DOI] [PubMed] [Google Scholar]