Significance
Antibodies are important for recovery from viral infections and vaccine efficacy. To improve the ability of antibodies to bind and neutralize viral pathogens, antibody DNA sequences undergo a mutational process driven by the enzyme activation-induced deaminase (AID). However, high levels of antibody mutations are required to potently inhibit global strains of the retrovirus HIV-1. We provide evidence that a related enzyme, apolipoprotein B mRNA-editing enzyme catalytic polypeptide 3 (APOBEC3), can also mutate antibodies during retrovirus infection, but in a different DNA sequence context compared to AID. The findings demonstrate that APOBEC3 acts as a key player in generating virus-specific neutralizing antibodies and highlight a previously unidentified mechanism for antibody diversification that could be harnessed for vaccine development.
Keywords: humoral immunity, restriction factor, antibody repertoire profiling, affinity maturation, Friend retrovirus
Abstract
Somatic hypermutation (SHM) is an integral process in the development of high-affinity antibodies that are important for recovery from viral infections and vaccine-induced protection. Ig SHM occurs predominantly in germinal centers (GC) via the enzymatic activity of activation-induced deaminase (AID). In contrast, the evolutionarily related apolipoprotein B mRNA-editing enzyme, catalytic polypeptide 3 (APOBEC3) proteins are known to restrict retroviruses, including HIV-1. We previously reported that mouse APOBEC3 encodes Recovery from Friend virus 3 (Rfv3), a classical resistance gene in mice that promotes the neutralizing antibody response against retrovirus infection. We now show that APOBEC3/Rfv3 complements AID in driving Ig SHM during retrovirus infection. Analysis of antibody sequences from retrovirus-specific hybridomas and GC B cells from infected mice revealed Ig heavy-chain V genes with significantly increased C-to-T and G-to-A transitions in wild-type as compared with APOBEC3-defective mice. The context of the mutations was consistent with APOBEC3 but not AID mutational activity. These findings help explain the role of APOBEC3/Rfv3 in promoting the neutralizing antibody responses essential for recovery from retroviral infection and highlight APOBEC3-mediated deamination as a previously unidentified mechanism for antibody diversification in vivo.
Natural recovery from viral infections and vaccine-induced protection are typically associated with potent neutralizing antibodies (1), so it is of great importance to understand fully the mechanisms that generate high-affinity antibodies. Part of the process of affinity maturation to improve the recognition of foreign antigens involves somatic hypermutation (SHM), which occurs predominantly in germinal centers (GCs) via the enzymatic activity of activation-induced deaminase (AID) (2, 3). AID catalyzes the deamination of deoxycytidines to deoxyuridines in single-stranded Ig DNA. In vitro reconstitution and analysis of in vivo Ig mutations demonstrated that AID preferentially deaminates in the 5′-WRC-3′ context (W = A or T; R = purine = A or G; the underlined C corresponds to the deaminated deoxycytidine) (4–6). On the other hand, a deoxycytidine preceded by a pyrimidine, e.g., in the dinucleotide context YC (Y = C or T) is referred to as an AID “coldspot.” It is well established that AID is the critical enzyme mediating antibody class-switching and SHM. However, it remains unknown if other deaminases can complement AID in driving SHM.
The apolipoprotein B mRNA-editing enzyme, catalytic polypeptide 3 (APOBEC3) proteins are deoxycytidine deaminases expressed in lymphocytes, including T and B cells, that can restrict retroviruses, including HIV-1 (7). APOBEC3 becomes packaged into virions and inhibits reverse transcription in the next target cell. Retrovirus inhibition could occur through the enzymatic action of APOBEC3, which involves deaminating deoxycytidines in single-stranded reverse transcripts, resulting in G→A hypermutation in the retroviral plus strand (7). Notably, APOBEC3 prefers to deaminate in the YC dinucleotide context, with different APOBEC3 proteins exhibiting subtle variations in their preferred sequence contexts. Human APOBEC3G deaminates in the CCC context (8, 9), whereas mouse APOBEC3 (mA3) deaminates in the TYC context (10–15). Thus, the preferred deamination sites or sequence hotspots for AID and the APOBEC3 proteins are quite distinct; in fact, this property could be used to track their respective activities in vivo (16).
mA3 was first known as Recovery from Friend virus 3 (Rfv3), a dominant retroviral-resistance gene in C57BL/6 (B6) mice that promotes the neutralizing antibody response against Friend retrovirus (FV) infections (17–19). Polymorphisms in the Rfv3 gene of mice result in defective susceptible (s) alleles as in A.BY mice and functional resistant (r) alleles as in B6 mice (19–22). Previous evidence demonstrated that mA3/Rfv3 operates through an indirect mechanism to increase antigenic stimulation of immune cells via mA3-mediated release of noninfectious virus particles (23, 24). APOBEC3-deficient mice had no defects in antibody class-switching (23, 25). However, it remains possible that another mechanism of retrovirus restriction occurs through the deaminase activity of mA3. This previously proposed direct mechanism (18) stipulates that mA3 might directly mutate antibody genes, analogous to AID. Although hapten immunization studies in B6 WT versus mA3−/− mice failed to reveal an impact of mA3 on antibody affinity maturation (23, 25), hapten immunization does not recapitulate the immunological complexity of viral infections. We therefore evaluated whether mA3 was involved in SHM through sequence characterization of Ig mutations generated during FV infections. Our findings demonstrate that APOBEC3 can instigate Ig SHM during retrovirus infection in vivo.
Results
FV-Specific mAbs from mA3+/s Mice Exhibit Higher SHM and Stronger Binding to Native Virions.
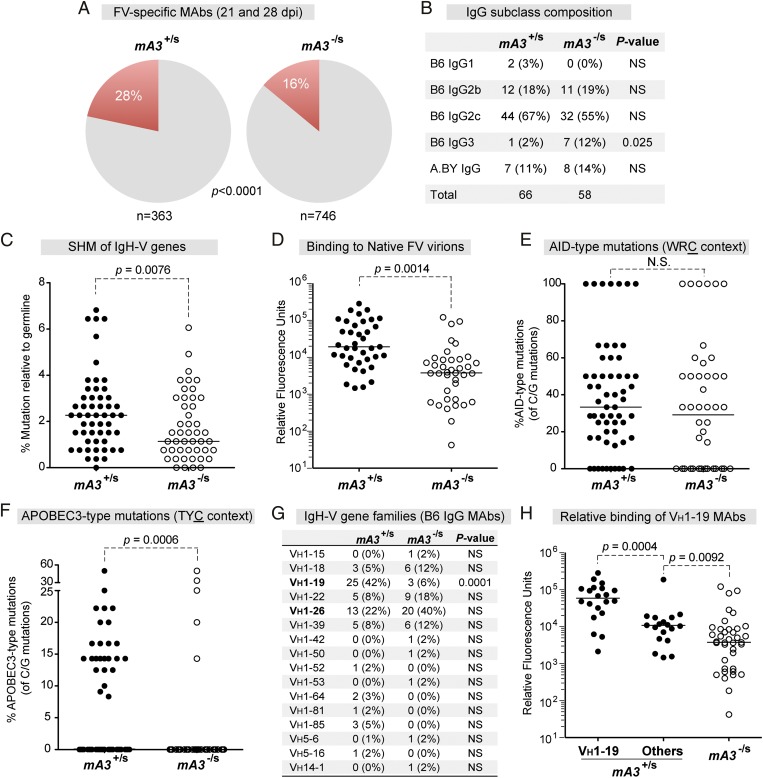
Investigation of mA3-mediated SHM was initiated by analyzing Ig heavy-chain (IgH) sequences from FV-specific monoclonal antibodies (mAbs) derived at 21 and 28 d postinfection (dpi). In total, 1,109 hybridomas were generated from Rfv3-resistant (B6 × A.BY)F1 and Rfv3-susceptible (B6 mA3−/− × A.BY)F1 mice (hereafter referred to as mA3+/s and mA3−/s, respectively) (Fig. S1 and Table S1). We found that a significantly higher fraction of antibodies from mA3+/s mice than from mA3-/s mice reacted to native virions (Fig. 1A). Because the mA3/Rfv3 phenotype affects the IgG response (18, 26), we focused the analyses on the IgG mAbs. IgG2c accounted for more than half of the mAbs from both cohorts of mice (Fig. 1B). We also found that a majority (88%) of the FV-specific mAbs were derived from B6 parental DNA (Fig. 1B). This result allowed us to compare most of our hybridoma sequences against B6 germline reference sequences in the IMGT database (27). IgG heavy-chain variable (VH) genes from mA3+/s mAbs exhibited significantly higher SHM levels than mA3−/s mAbs (Fig. 1C). Furthermore, the mA3+/s mAbs had significantly higher relative binding to native virions than mA3−/s mAbs (Fig. 1D). Thus, the presence of a resistant mA3 allele correlated with higher levels of antibody affinity maturation.
Fig. 1.
Characterization of hybridomas from Rfv3-resistant versus -susceptible mice. (A) FV-specific mAbs from mA3+/s and mA3−/s mice. Hybridomas were derived from splenocytes of FV-infected mice at 21 and 28 dpi. (B) IgG subclass composition of FV-reactive mAbs. (C) Mutation frequency relative to germline. IgG cDNA was sequenced and compared against B6 germline VH sequences. (D) Relative binding of mAbs to native virions at 21 dpi, as evaluated by time-resolved fluorescence ELISA. (E and F) AID (E) and mA3 (F) hotspot mutations. The percentages of WRC or TYC mutations relative to the number of C or G mutations per mAb were calculated, respectively. (G) VH gene distribution. The number of hybridomas belonging to each VH gene family is shown. (H) Data are identical to D, except that mA3+/s mAbs encoding VH1–19 were analyzed separately. In A, B, and G, the difference in percentages was evaluated using Fisher’s exact test. In C–F, median values are shown; differences between the two groups were evaluated using a two-tailed Mann–Whitney U test with P < 0.05 considered significant. NS, not significant.
APOBEC3 Deficiency Significantly Decreased mA3 Hotspot Mutations, but Not AID Hotspot Mutations, in FV-Specific Antibodies.
We investigated the possible direct role of mA3 in the SHM of the hybridomas by looking at the context of the mutations. AID preferentially mutates in the WRC trinucleotide context (4–6), whereas mA3 deaminates in the TYC trinucleotide context (10–15) (Fig. S2). Not unexpectedly, AID-type WRC mutations accounted for a significant fraction of C and G mutations in all the mAbs, regardless of origin (Fig. 1E). In contrast, mA3-type TYC mutations were found at significantly lower frequencies in mAbs from susceptible mA3−/s mice (Fig. 1F). Similar results were observed for the YC dinucleotide preference (P = 0.024 by Mann–Whitney U test) (Fig. S3A) and, in particular, for the TTC context (P < 0.0001) (Fig. S3B). Thus, the functional (resistant) mA3/Rfv3 phenotype was associated with mA3-type mutations in virus-specific Ig sequences.
The FV-Specific B-Cell Response Is Associated with Specific VH Genes.
Virus-specific antibodies may use immunodominant VH gene segments, as documented for VH1–46 in rotavirus infections (28) and for VH1–59 in HIV-1 CD4-induced antibodies (29) in humans. Therefore we examined whether certain VH genes predominated the FV-specific antibody response by analyzing the VH gene usage of the IgG mAbs (Fig. 1G and Table S1). We found that 16 of 109 possible VH genes were used, and that VH1–26 was found at high proportions in both mAb groups. The majority (60%) of the mA3-type mutations were detected in VH1–19, which was found at a significantly higher frequency in mA3+/s IgG mAbs than in mA3−/s IgG mAbs (Fig. 1G). Interestingly, the VH1–19 mAbs exhibited the highest binding to native virions (Fig. 1H). The majority of mA3-type TYC mutations in the VH1–19+ mAbs resulted in a methionine to isoleucine (M39I) substitution adjacent to the complementarity determining region 1 (CDR1) (Fig. S4), and M39I was detected preferentially in mA3+/s versus mA3−/s mAbs (72% versus 0%, respectively; Fisher's exact test, P < 0.05). Moreover, the mA3+/s mAbs encoding the TYC M39I mutation showed higher relative binding to native virions (Fig. S4). Thus, mA3/Rfv3 resistance correlated with VH1–19 IgG antibodies that harbored nonsynonymous TYC mutations. However, although a large number of hybridoma clones were analyzed, it was possible that some bias entered the analysis because of the expansion of select virus-specific B-cell clones by 21–28 dpi (Table S1). Investigating the impact of mA3 on mutational profiles in VH1–19 relative to other VH genes also would require a more extensive sequence dataset.
High-Throughput Evaluation of Ig SHM by Next-Generation Sequencing.
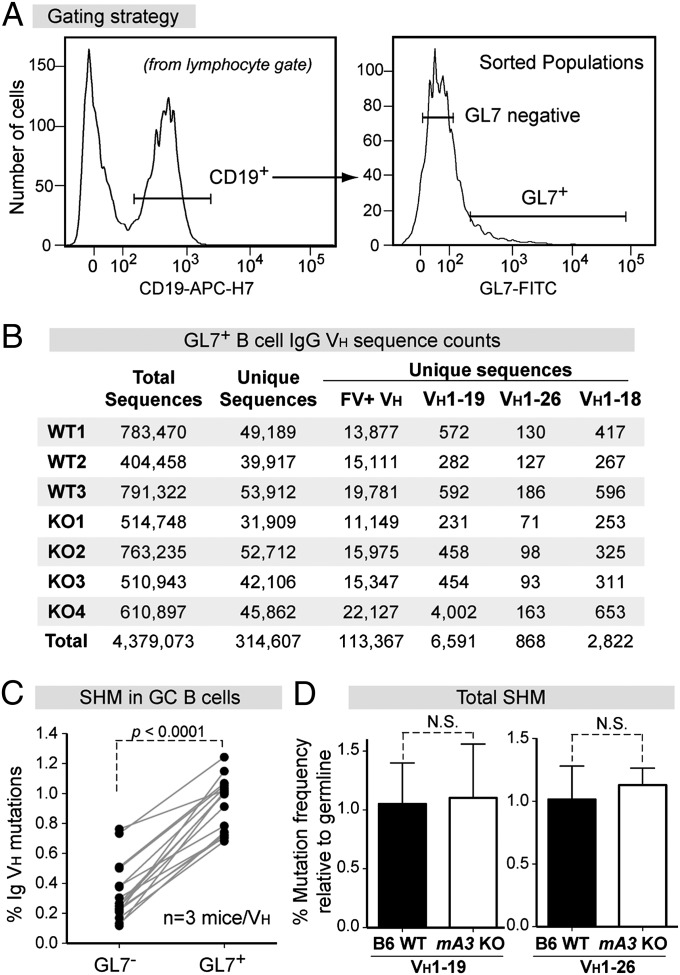
As a more robust method to obtain large numbers of diverse VH sequences for detection of mA3-type mutations, we used next-generation sequencing (NGS) to quantify the frequency of IgG VH mutations in B6 WT (n = 3) and mA3−/− (KO) mice (n = 4). The analyses focused on GC B cells because these cells are enriched for antigen-specific antibodies and are the sites of SHM (2). Mice were infected with FV, and splenocytes were harvested for cell sorting of GC B cells at 7 dpi (Fig. 2A) to increase the likelihood of capturing early SHM events before clonal expansion. Barcoded primers were used to amplify IgG cDNA sequences using high-fidelity polymerase, and the amplicons were pooled and run on a 2 × 250 bp paired-end Illumina Mi-Seq. Overall, we obtained >4 million VH sequences (Fig. 2B). To minimize bias caused by clonal expansion, identical sequences were collapsed into 314,607 unique VH sequences (Fig. 2B and Table S2). Of these unique sequence reads, 36%, including the two major VH genes, VH1–19 and VH1–26, grouped with the 16 VH genes in the FV-specific mAb panel (Fig. 2B). We focused the analysis of NGS data on these 16 FV-mAb VH genes because these were documented to generate FV-specific IgG antibodies (Fig. 1G). Validating the experimental strategy, SHM levels were 3.5-fold (range, 1.7- to 8.7-fold) higher in sorted GL7+ GC B cells than in GL7− (non-GC) B cells (Fig. 2C). FV-mAb VH sequences from GC B cells were compared with germline sequences to calculate SHM frequencies. No significant defects in total SHM was observed in mA3−/− mice as compared with B6 WT mice (Fig. 2D and Fig. S5A).
Fig. 2.
NGS of GC B-cell VH genes from B6 WT versus mA3 KO mice. (A) Sorting strategy. RNA extracted from CD19+GL7+ cells were used for cDNA synthesis followed by IgG VH PCR with Illumina primers. (B) Sequence read counts from individual WT and KO mice. To minimize selection bias, identical sequences were collapsed into a single unique sequence. FV+ VH corresponds to the 16 VH genes in the FV-specific mAbs in Fig. 1G. (C) Validation of sorted GC B cells. The average mutation frequencies of paired GL7+ and GL7− populations from the three WT mice were computed for each of the 16 VH genes. Differences were evaluated using a two-tailed paired Student t test. (D) Total mutation frequency in WT (n = 3) and KO (n = 4) mice for VH1–19 and VH1–26 sequences. Differences were evaluated using a two-tailed unpaired Student t test with P < 0.05 considered as significant.
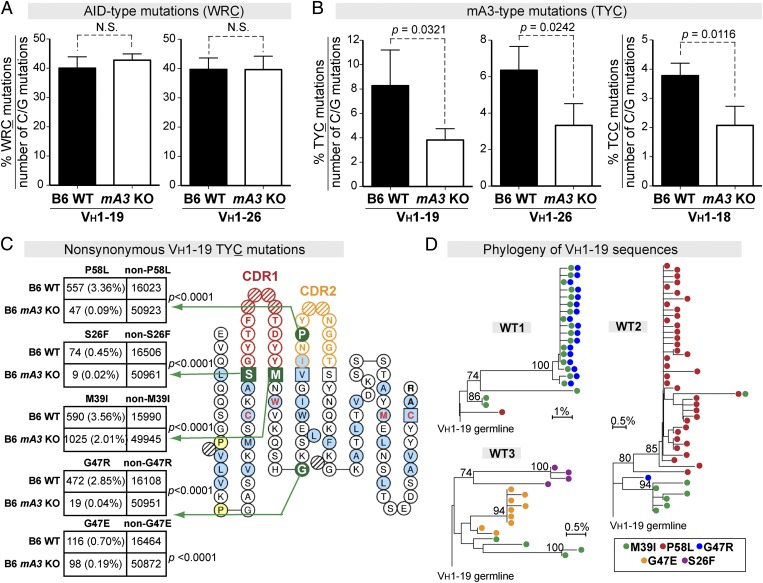
GC B Cells from FV-Infected APOBEC3 KO Mice Had Normal WRC Mutational Frequencies but Significantly Reduced TYC Mutations in Specific VH Genes.
We next counted AID-type (WRC) mutations in each of the 16 different FV-mAb VH genes and found that the frequency of AID-type mutations was not significantly different in WT and mA3−/− mice (Fig. 3A and Fig. S5B). Thus, mA3 deficiency did not result in any detectable change in AID-mediated SHM at 7 dpi. No significant differences in mA3-type mutations were detected between WT and mA3−/− mice for 13 of the 16 FV-mAb VH genes (Fig. S5C) or for 89 other VH genes not found in the FV-mAb panel (Table S3). However, mA3−/− mice showed significantly fewer TYC mutations in VH1–19 and VH1–26 (Fig. 3B). In addition, significantly fewer TCC mutations were observed for VH1–18 (Fig. 3B). The TYC mutations in VH1–19 resulted in five amino acid substitutions that were more prevalent in WT mice (P < 0.0001 by Fisher’s exact test) (Fig. 3C). These substitutions included the VH1–19 M39I mutation, which also was detected in the FV-specific IgG mAb panel (Fig. S4). P58L was more prevalent in WT than in mA3−/− VH1–26 sequences, whereas M39I was significantly more prevalent in WT VH1–18 sequences (P < 0.0001 by Fisher’s exact test in both cases) (Fig. S6). The nonsynonymous VH1–19 TYC mutations that correlated with mA3 function emerged in different mA3+/+ mice and belonged to separate lineages, indicating that these TYC mutations arose independently (Fig. 3D). Overall, the NGS data provide in vivo evidence that mA3 generated a significant proportion of the TYC mutations in VH1–19, VH1–26, and VH1–18 genes that were associated with the FV-specific IgG response.
Fig. 3.
mA3 promotes TYC antibody mutations in specific VH genes. (A and B) The percentages of (A) AID hotspot and (B) mA3 hotspot mutations relative to the number of C or G mutations were evaluated in GC B-cell VH genes from WT (n = 3) and mA3 KO (n = 4) mice. Data are shown for VH1–19 and VH1–26, which are the major VH genes in the FV-specific mAb panel in Fig. 1G. The percentage of TCC mutations in VH1–18 is shown in B. Differences were evaluated using a two-tailed unpaired Student t test with P < 0.05 considered as significant. (C) Prevalence of nonsynonymous TYC mutations in WT versus mA3 KO IgH sequences. TYC mutations from B6 WT (n = 16,680 noncollapsed sequence reads from three mice) and B6 mA3 KO (n = 51,096 noncollapsed sequence reads from four mice) sequences were combined, and those sequence reads that resulted in amino acid changes were evaluated in 2 × 2 contingency analyses. (Left) Five nonsynonymous VH1–19 TYC substitutions found in >0.4% of sequence reads in WT mice were compared with sequence reads in KO mice by Fisher’s exact test. Percentages from the entire VH1–19 sequence dataset are shown in parentheses. (Right) IMGT collier-de-perles plot (27) showing the relative positions of the predicted amino acid mutations. (D) Ontogeny of nonsynonymous VH1–19 TYC mutations in individual B6 WT mice. Phylogenetic trees were constructed using the neighbor-joining method, and clades supported with >70% bootstrap are indicated. Each dot within each line corresponds to a unique sequence. Note that M39I was detected in all three mice and that lineages with two TYC mutations could emerge.
Discussion
The genetic link between mA3 and retrovirus-neutralizing antibody responses and the evolutionary relationship of mA3 to AID raised the possibility that mA3 has a direct effect on Ig SHM. However, testing this hypothesis required controlling for potential indirect effects of mA3-mediated FV restriction on antibody responses (23–25). Because AID and mA3 preferentially mutate at different sequence contexts, we quantified AID-hotspot WRC mutations to evaluate if antibody SHM was impaired. Moreover, in the NGS studies, we minimized the indirect effects of mA3 by analyzing mice in the B6 background [which do not develop splenomegalic disease (30)] at an early time point (7 dpi), when mA3 had no significant impact on the viral antigenic load (24, 31). TYC mutations in FV-specific antibodies and associated GC B-cell VH genes decreased significantly in Rfv3-susceptible mice, but this decrease could not have been an indirect effect of mA3 on SHM, because mA3 deficiency did not alter the frequency of WRC mutations. Because TYC is the mA3 deamination hotspot (10–15), we concluded that mA3 directly generated a significant proportion of antibody TYC mutations during retrovirus infection in vivo.
AID evolved primarily to regulate antibody diversity, whereas the APOBEC3 proteins evolved to restrict retroviruses. Interestingly, phylogenetic analyses suggested that AID and APOBEC3 diverged from a primordial deaminase during vertebrate evolution (32), coinciding with the development of the adaptive immune system (33). Because AID still retains some antiretroviral activity (34), the functional divergence between AID and APOBEC3 may be incomplete. Our findings suggest that mA3 has not completely diverged from AID with respect to antibody SHM. However, it remains to be determined whether mA3-mediated antibody SHM is just an accident of evolution. It may be beneficial for the host to use a deaminase with a distinct deamination preference relative to AID to ensure a wider range of possible antibody mutations during infection. However, it is notable that, in contrast to AID (3), the impact of mA3 on antibody SHM was not global: it was restricted to VH genes linked to the FV-specific antibody response, notably VH1–19. FV-specific antibodies encoding VH1–19 were preferentially selected in mA3+/s mice and exhibited higher binding to native virions. Thus, VH1–19+ B cells likely underwent extensive proliferation in GCs, because these cells were selected for binding to viral antigens. Notably, mA3 was expressed primarily in the cytoplasm (35) and may have limited access to Ig DNA in the nucleus. We hypothesize that extensive nuclear membrane breakdown during cell division may allow mA3 to access Ig DNA of rapidly expanding GC B cells. Further work will be required to test this proliferative model of mA3-mediated antibody SHM.
Evidence from several studies suggest that our findings in mice likely extend to the primate APOBEC3s. Mice encode only a single mA3 gene, but humans and rhesus macaques have seven APOBEC3 members that preferentially mutate in the YC context (8–15). APOBEC3A and APOBEC3B have already been linked to mutating genomic DNA (36, 37). Moreover, coincubation of a partially single-stranded IgG DNA substrate with human tonsil nuclear B-cell lysates in vitro resulted in mutations in the CCC context, suggesting a potential role for APOBEC3G in mutating antibodies (38). However, without knocking down the primate APOBEC3 genes in vivo, it remains possible that coldspot AID activity was responsible for the antibody YC mutations in primates. This uncertainty highlights the importance of the current studies that used mA3-defective mice to attribute antibody TYC mutations directly to mA3. Together, the in vivo data on mA3-defective mice and aforementioned studies on primate APOBEC3 activity suggest that the ability of APOBEC3 to mutate antibodies is evolutionarily conserved.
A role for mA3 in promoting Ig SHM during retrovirus infection could have significant implications for human B-cell immunity. In particular, one of the most challenging goals of HIV-1 vaccine development is to elicit neutralizing antibodies that can potently inhibit global strains. These broadly neutralizing antibodies (bNAbs) are characterized by extensive levels of SHM, mutating at an average of 26% relative to germline (39). Most of these bNAbs harbored mutations relative to germline that fit the APOBEC3 deamination hotspot. The data based on mA3-deficient mice provide a strong rationale for testing the impact of human APOBEC3 proteins in antibody SHM and for identifying strategies such as IFN-α regulation (31) to modulate APOBEC3 activity in vivo. These efforts may be key to generating highly mutated antibodies with broad specificity against HIV-1 and other human pathogens for universal vaccine applications.
Materials and Methods
Mouse Strains.
B6, A.BY, and BALB/c mice were obtained through The Jackson Laboratory. B6 mA3−/− mice (18) were backcrossed for nine generations into B6 mice. All mice were handled in accordance with the recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (40) and were approved by the University of Colorado Institutional Animal Care and Use Committee [permit no. B-89709(10)1E]. Mice were infected with FV, and spleens were harvested at distinct time points postinfection. See SI Materials and Methods for further details.
Hybridoma Production and Characterization.
mA3+/s and mA3-/s splenocytes were fused with SP2/0 myeloma cells for hybridoma production. ELISAs were used to type and quantify the mAbs and evaluate their relative binding to native Friend murine leukemia virus virions. Hybridoma RNA was extracted and used to amplify IgH genes by RT-PCR for Sanger sequencing. See SI Materials and Methods for further details.
Sorting for GC B Cells and Illumina Sequencing.
Spleens were harvested from B6 WT and B6 mA3−/− mice at 7 dpi and then were disassociated and stained with anti-CD19-APC-H7 and GL7-FITC (BD Biosciences). CD19+GL7+ and CD19+GL7− populations were sorted using a FACSAria cell sorter (BD Biosciences). RNA extracted from sorted B cells was used to amplify IgH with barcoded Illumina primers. Amplicons were pooled and run on a 2 × 250 bp Illumina Mi-Seq. Custom Perl scripts were used to match and analyze against reference VH sequences in the IMGT database. See SI Materials and Methods for further details.
Supplementary Material
Acknowledgments
We thank W. Greene, L. Wysocki, R. Pelanda, R. Torres, L. Pujunauski, J. Hagman, C. Dege, L. van Dyk, E. Janoff, D. Frank, and B. Palmer for reagents, technical advice and/or assistance. This work was supported by National Institutes of Health (NIH) Grant R01 AI090795 (to M.L.S.), the University of Colorado Department of Medicine Early Career Scholar Program (to M.L.S.), and by the NIH Division of Intramural Research (to K. J. Hasenkrug). K.H. was the recipient of a Robert D. Watkins Pre-doctoral Fellowship from the American Society for Microbiology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the National Center for Biotechnology Information GenBank database (accession nos. KJ147322–KJ47430) and Sequence Read Archive (accession no. SRP040999).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403361111/-/DCSupplemental.
References
- 1.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: What we know, what we don’t know, what we should know. Nat Med. 2004;10(8):806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 2.Peled JU, et al. The biochemistry of somatic hypermutation. Annu Rev Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102(5):553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Pham P, Bransteitter R, Petruska J, Goodman MF. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 2003;424(6944):103–107. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- 5.Larijani M, Frieder D, Basit W, Martin A. The mutation spectrum of purified AID is similar to the mutability index in Ramos cells and in ung(-/-)msh2(-/-) mice. Immunogenetics. 2005;56(11):840–845. doi: 10.1007/s00251-004-0748-0. [DOI] [PubMed] [Google Scholar]
- 6.Zheng NY, Wilson K, Jared M, Wilson PC. Intricate targeting of immunoglobulin somatic hypermutation maximizes the efficiency of affinity maturation. J Exp Med. 2005;201(9):1467–1478. doi: 10.1084/jem.20042483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2009;364(1517):675–687. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beale RC, et al. Comparison of the differential context-dependence of DNA deamination by APOBEC enzymes: Correlation with mutation spectra in vivo. J Mol Biol. 2004;337(3):585–596. doi: 10.1016/j.jmb.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 9.Refsland EW, Hultquist JF, Harris RS. Endogenous origins of HIV-1 G-to-A hypermutation and restriction in the nonpermissive T cell line CEM2n. PLoS Pathog. 2012;8(7):e1002800. doi: 10.1371/journal.ppat.1002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop KN, et al. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004;14(15):1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 11.Yu Q, et al. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat Struct Mol Biol. 2004;11(5):435–442. doi: 10.1038/nsmb758. [DOI] [PubMed] [Google Scholar]
- 12.Esnault C, et al. APOBEC3G cytidine deaminase inhibits retrotransposition of endogenous retroviruses. Nature. 2005;433(7024):430–433. doi: 10.1038/nature03238. [DOI] [PubMed] [Google Scholar]
- 13.Jern P, Stoye JP, Coffin JM. Role of APOBEC3 in genetic diversity among endogenous murine leukemia viruses. PLoS Genet. 2007;3(10):2014–2022. doi: 10.1371/journal.pgen.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langlois MA, Kemmerich K, Rada C, Neuberger MS. The AKV murine leukemia virus is restricted and hypermutated by mouse APOBEC3. J Virol. 2009;83(22):11550–11559. doi: 10.1128/JVI.01430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petit V, et al. Murine APOBEC1 is a powerful mutator of retroviral and cellular RNA in vitro and in vivo. J Mol Biol. 2009;385(1):65–78. doi: 10.1016/j.jmb.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 16.Vartanian JP, et al. Massive APOBEC3 editing of hepatitis B viral DNA in cirrhosis. PLoS Pathog. 2010;6(5):e1000928. doi: 10.1371/journal.ppat.1000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chesebro B, Wehrly K. Identification of a non-H-2 gene (Rfv-3) influencing recovery from viremia and leukemia induced by Friend virus complex. Proc Natl Acad Sci USA. 1979;76(1):425–429. doi: 10.1073/pnas.76.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santiago ML, et al. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science. 2008;321(5894):1343–1346. doi: 10.1126/science.1161121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda E, et al. Mouse APOBEC3 restricts friend leukemia virus infection and pathogenesis in vivo. J Virol. 2008;82(22):10998–11008. doi: 10.1128/JVI.01311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okeoma CM, Petersen J, Ross SR. Expression of murine APOBEC3 alleles in different mouse strains and their effect on mouse mammary tumor virus infection. J Virol. 2009;83(7):3029–3038. doi: 10.1128/JVI.02536-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santiago ML, et al. Persistent Friend virus replication and disease in Apobec3-deficient mice expressing functional B-cell-activating factor receptor. J Virol. 2011;85(1):189–199. doi: 10.1128/JVI.01838-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanville B, et al. Adaptive evolution of Mus Apobec3 includes retroviral insertion and positive selection at two clusters of residues flanking the substrate groove. PLoS Pathog. 2010;6:e1000974. doi: 10.1371/journal.ppat.1000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santiago ML, Benitez RL, Montano M, Hasenkrug KJ, Greene WC. Innate retroviral restriction by Apobec3 promotes antibody affinity maturation in vivo. J Immunol. 2010;185(2):1114–1123. doi: 10.4049/jimmunol.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DS, et al. Noninfectious retrovirus particles drive the APOBEC3/Rfv3 dependent neutralizing antibody response. PLoS Pathog. 2011;7(10):e1002284. doi: 10.1371/journal.ppat.1002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuji-Kawahara S, et al. Persistence of viremia and production of neutralizing antibodies differentially regulated by polymorphic APOBEC3 and BAFF-R loci in friend virus-infected mice. J Virol. 2010;84(12):6082–6095. doi: 10.1128/JVI.02516-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britt WJ, Chesebro B. Use of monoclonal anti-gp70 antibodies to mimic the effects of the Rfv-3 gene in mice with Friend virus-induced leukemia. J Immunol. 1983;130(5):2363–2367. [PubMed] [Google Scholar]
- 27.Lefranc MP, et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37(Database issue):D1006–D1012. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weitkamp JH, et al. VH1-46 is the dominant immunoglobulin heavy chain gene segment in rotavirus-specific memory B cells expressing the intestinal homing receptor alpha4beta7. J Immunol. 2005;174(6):3454–3460. doi: 10.4049/jimmunol.174.6.3454. [DOI] [PubMed] [Google Scholar]
- 29.Huang CC, et al. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci USA. 2004;101(9):2706–2711. doi: 10.1073/pnas.0308527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persons DA, et al. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet. 1999;23(2):159–165. doi: 10.1038/13787. [DOI] [PubMed] [Google Scholar]
- 31.Harper MS, et al. IFN-α treatment inhibits acute Friend retrovirus replication primarily through the antiviral effector molecule Apobec3. J Immunol. 2013;190(4):1583–1590. doi: 10.4049/jimmunol.1202920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conticello SG, Thomas CJ, Petersen-Mahrt SK, Neuberger MS. Evolution of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases. Mol Biol Evol. 2005;22(2):367–377. doi: 10.1093/molbev/msi026. [DOI] [PubMed] [Google Scholar]
- 33.Hirano M, Das S, Guo P, Cooper MD. The evolution of adaptive immunity in vertebrates. Adv Immunol. 2011;109:125–157. doi: 10.1016/B978-0-12-387664-5.00004-2. [DOI] [PubMed] [Google Scholar]
- 34.Metzner M, Jäck HM, Wabl M. LINE-1 retroelements complexed and inhibited by activation induced cytidine deaminase. PLoS ONE. 2012;7(11):e49358. doi: 10.1371/journal.pone.0049358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doehle BP, et al. The betaretrovirus Mason-Pfizer monkey virus selectively excludes simian APOBEC3G from virion particles. J Virol. 2006;80(24):12102–12108. doi: 10.1128/JVI.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burns MB, Temiz NA, Harris RS. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat Genet. 2013;45(9):977–983. doi: 10.1038/ng.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landry S, Narvaiza I, Linfesty DC, Weitzman MD. APOBEC3A can activate the DNA damage response and cause cell-cycle arrest. EMBO Rep. 2011;12(5):444–450. doi: 10.1038/embor.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pham P, Zhang K, Goodman MF. Hypermutation at A/T sites during G.U mismatch repair in vitro by human B-cell lysates. J Biol Chem. 2008;283(46):31754–31762. doi: 10.1074/jbc.M805524200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein F, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153(1):126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Committee on Care and Use of Laboratory Animals (1985) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.