Significance
Down-regulation of TGFβ signaling is a therapeutic strategy for several disease applications, particularly cancer and fibrosis. However, TGFβ blockade may have adverse effects because TGFβ signaling is necessary for formation and maintenance of normal blood vessels. Because side effects may vary between individuals due to innate differences in genetic makeup, it is important to find which interindividual genetic variants regulate responses to reduced TGFβ signaling and how these operate at the molecular level. Here we identified polymorphic variants of mouse a disintegrin and metalloprotease 17 that differentially regulate TGFβ signaling output and influence the severity of Tgfb1-dependent vascular pathology. This has relevance to risk assessment for clinical manifestations in TGFβ-driven diseases, as well as for prediction of desirable and undesirable responses to anti-TGFβ therapy.
Abstract
Outcome of TGFβ1 signaling is context dependent and differs between individuals due to germ-line genetic variation. To explore innate genetic variants that determine differential outcome of reduced TGFβ1 signaling, we dissected the modifier locus Tgfbm3, on mouse chromosome 12. On a NIH/OlaHsd genetic background, the Tgfbm3bC57 haplotype suppresses prenatal lethality of Tgfb1−/− embryos and enhances nuclear accumulation of mothers against decapentaplegic homolog 2 (Smad2) in embryonic cells. Amino acid polymorphisms within a disintegrin and metalloprotease 17 (Adam17) can account, at least in part, for this Tgfbm3b effect. ADAM17 is known to down-regulate Smad2 signaling by shedding the extracellular domain of TGFβRI, and we show that the C57 variant is hypomorphic for down-regulation of Smad2/3-driven transcription. Genetic variation at Tgfbm3 or pharmacological inhibition of ADAM17, modulates postnatal circulating endothelial progenitor cell (CEPC) numbers via effects on TGFβRI activity. Because CEPC numbers correlate with angiogenic potential, this suggests that variant Adam17 is an innate modifier of adult angiogenesis, acting through TGFβR1. To determine whether human ADAM17 is also polymorphic and interacts with TGFβ signaling in human vascular disease, we investigated hereditary hemorrhagic telangiectasia (HHT), which is caused by mutations in TGFβ/bone morphogenetic protein receptor genes, ENG, encoding endoglin (HHT1), or ACVRL1 encoding ALK1 (HHT2), and considered a disease of excessive abnormal angiogenesis. HHT manifests highly variable incidence and severity of clinical features, ranging from small mucocutaneous telangiectases to life-threatening visceral and cerebral arteriovenous malformations (AVMs). We show that ADAM17 SNPs associate with the presence of pulmonary AVM in HHT1 but not HHT2, indicating genetic variation in ADAM17 can potentiate a TGFβ-regulated vascular disease.
The TGFβ signaling pathway is a therapeutic target for numerous disease applications because elevated levels of ligands and signaling components have been shown to drive several pathological states, especially cancer and fibrosis (1). However, this signaling pathway is essential for formation and maintenance of the vascular system, as manifested by the vascular phenotypes of Tgfb1, Tgfbr1, Tgfbr2, Eng, and Acvrl1 gene knockout mice (2), and by congenital vasculopathies caused by mutations in orthologous human genes (3–5). Therefore, therapeutic downmodulation of TGFβ signaling has the potential to cause undesirable vascular outcomes (6), making investigation of the molecular mechanisms underlying such phenotypes clinically important.
Studies in mice have shown that, when the TGFβ1 pathway is disrupted, innate genetic differences between strains result in variable penetrance and severity of vascular phenotypes (7). Human Mendelian disorders of TGFβ and bone morphogenetic protein (BMP) signaling also show variable penetrance and severity that may be attributed, at least in part, to genetic variation within modifier loci (8, 9). One example is hereditary hemorrhagic telangiectasia (HHT), which is caused by loss-of-function mutations in ENG, ALK1, or SMAD4 that encode components of the TGFβ/BMP signaling pathways (4, 5, 10). HHT patients can suffer vascular malformations in multiple organ systems. They initially present with recurrent epistaxis but subsequently develop multiple cutaneous and mucosal telangiectases. Bleeding from gastrointestinal telangiectases can lead to chronic, debilitating, and treatment-refractory anemia. Some patients develop more severe arteriovenous malformations (AVMs) in the lung, brain, and/or liver, which can lead to severe complications. Lung AVMs occur in ∼30–50% of patients with HHT, causing right-to-left shunting due to a compromised pulmonary capillary filter, and potentially leading to life-threatening stroke (11).
Here, we characterized a TGFβ modifier locus on proximal mouse chromosome 12 (12) and found that, like mouse modifiers of common disease phenotypes (13, 14), Tgfbm3 is genetically complex with positive and negative elements that potentiate or suppress lethal prenatal Tgfb1−/− vascular dysgenesis. A C57 versus NIH haplotype over a subinterval of Tgfbm3, termed Tgfbm3b, functions to support Tgfb1-independent developmental angiogenesis and, in adults, potentiates circulating endothelial progenitor cell (CEPC) numbers, a surrogate marker for angiogenic capacity (15). We show that Tgfbm3b encodes a hypomorphic variant of a disintegrase and metalloprotease 17 (ADAM17) that, due to ineffectual down-regulation of TGFβRI signaling, potentiates TGFβ-mothers against decapentaplegic homologs 2 and 3 (Smad2/3) signaling downstream of the ligand. We also provide evidence that genetic variation within human ADAM17 associates with the presence of potentially life-threatening pulmonary AVMs in patients with HHT1 carrying ENG mutations, giving credence to the notion that genetic variation at ADAM17 may determine severity of TGFβ pathway-regulated disease in humans.
Results
Tgfbm3bC57 Is a Suppressor of Tgfb1−/− Prenatal Lethality.
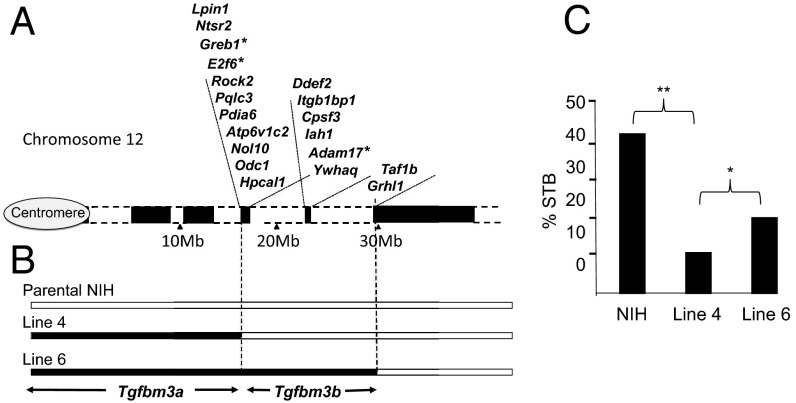
Tgfb1−/− mice are not viable. They either die prenatally from vascular dysgenesis 9.5–10.5 days postcoitum (16), or postnatally from lethal multifocal inflammation (17). Previously we mapped variant genomic loci that determine variable penetrance of Tgfb1−/− prenatal lethality (7, 9, 12, 18). We showed that a C57 Tgfbm3C57 haplotype on proximal chromosome 12 accentuates prenatal lethality of NIH.Tgfb1−/− embryos, albeit that the Tgfbm3NIH allele, acting alone, is insufficient to suppress C57.Tgfb1−/− embryo lethality (12). To further dissect Tgfbm3, we used marker-assisted backcross to generate Tgfb1+/− congenic sublines carrying different extents of C57 over the Tgfbm3 locus, which were defined by mapping using SSR and SNP markers (Fig. 1 A and B and Table S1). As seen within other genetic modifier loci (13, 14, 19), we found that Tgfbm3C57 is complex and possesses two modifying elements, a proximal enhancer (Tgfbm3aC57; centromere to Lpin1 gene) and a distal suppressor (Tgfbm3bC57; Ntsr2 to Grhl1) of Tgfb1−/− prenatal lethality (Fig. 1C).
Fig. 1.
Tgfbm3 is a complex locus harboring an enhancer (Tgfbm3a) and suppressor (Tgfbm3b) of Tgfb1−/− prenatal lethality. (A) Cartoon of Tgfbm3 on proximal mouse chromosome 12, indicating positions of genes within “islands” of gene-rich unique DNA (black blocks) interspersed by extensive regions (megabases) of repetitive DNA (white blocks). The human TGFBM3 syntenic region (2p23–25) lacks repetitive DNA and is thus far shorter (Fig. S3). Asterisks mark genes with amino acid polymorphisms between NIH and C57. (B) C57 regions within NIH.C57-Tgfbm3 congenic lines 4 and 6, aligned to A. Black blocks indicate C57; white blocks indicate NIH genomic DNA. The C57→NIH transition in line 4 occurs between rs49671069 and rs3724468, located just distal to Lpin1, and that of line 6 occurs between rs29180890 and rs31626421, located just distal to Taf1b. (C) Congenic test cross data generated by intraline NIH.Tgfb1+/− intercrosses of NIH, NIH.C57-Tgfbm3a (line 4), and NIH.C57-Tgfbm3a-Tgfbm3b (line 6). Survival to birth (STB) rates were estimated from the ratio of Tgfb1−/− neonates to Tgfb1+/+ neonates on the day of birth. *P = 0.07; **P < 0.01.
Genetic Variation at Tgfbm3b Modulates Nuclear Localization of SMAD2.
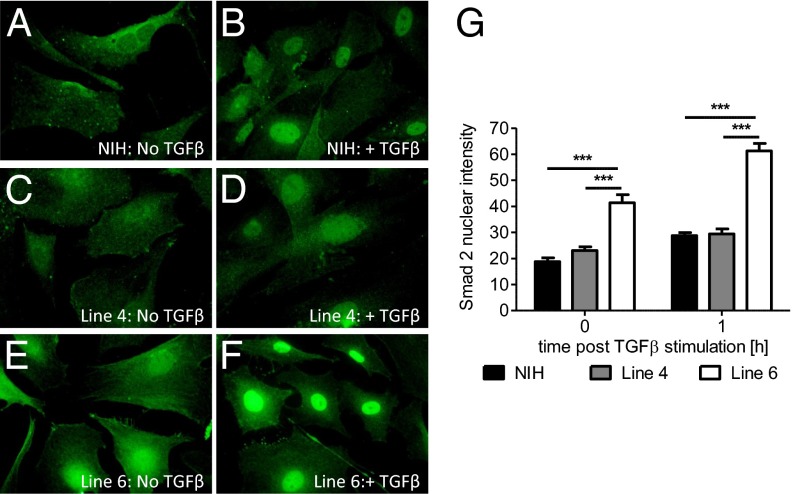
We focused on analysis of Tgfbm3b, because the ability to suppress Tgfb1−/− embryo-lethality suggests that this locus acts downstream of TGFβ1 to compensate for reduced ligand levels, whereas potentiation of a lethal phenotype might be achieved through a variety of mechanisms, not necessarily related to TGFβ signaling. Indeed, classical forward genetic screens for suppressors of gene knockout phenotypes have been used extensively in lower organisms to identify novel components of specific signaling pathways (20). To investigate the effect of variant Tgfbm3b on downstream canonical TGFβ signaling, we examined the nucleocytoplasmic distribution of endogenous Smad2 in wild-type primary mouse embryo fibroblasts (MEFs) derived from NIH.Tgfbm3C57 congenic lines 4 and 6, which differ over an ∼1.6-Mb interval of unique gene-rich DNA within Tgfbm3b (Fig. 1 A and B). Wild-type NIH and line 4 MEFs, homozygous for the Tgfbm3bNIH allele, showed predominantly cytoplasmic localization of Smad2, which relocated to the nucleus upon TGFβ treatment (Fig. 2 A–D, and G). In contrast, wild-type line 6 MEFs, which bear the C57 Tgfbm3bC57 allele, exhibited nuclear Smad2 staining before addition of TGFβ. The differential in nuclear Smad2 staining between line 4 and line 6 mice was further accentuated after TGFβ treatment (Fig. 2 E–G). The C57 allele of Tgfbm3b appeared to act in a recessive manner, because wild-type MEFs generated by intercrossing NIH.C57-Tgfbm3 line 4 with line 6 mice (NIH.Tgfbm3bC57/NIH), showed the same Smad2 distribution as those from line 4 MEFs (NIH.Tgfbm3bNIH/NIH) that bear only the NIH allele. Tgfbm3bC57 therefore appears to be a recessive Tgfb1 modifier that acts downstream of TGFβ1 to modulate Smad2 levels.
Fig. 2.
Early passage NIH MEFs homozygous for Tgfbm3bC57 show higher basal and inducible nuclear Smad2 levels than those with Tgfbm3bNIH. (A–F) Smad2 staining of MEFs derived from wild-type mice of a parental NIH background (A and B), or on the line 4 (C and D) or line 6 (E and F) congenic backgrounds, before (A, C and E) and 1 h after (B, D, and F) stimulation with TGFβ 0.5 ng/mL. (G) Quantification of nuclear Smad2 intensity. *P ≤ 0.05. Each experiment was independently reproduced three times with three technical replicates within each experiment.
Tgfbm3b Is a Modifier of Circulating Endothelial Progenitor Cell Numbers.
Because Tgfb1 and Tgfbm3b interact to regulate vascular development in utero (Fig. 1C), we postulated that these loci may interact to regulate postnatal angiogenesis. In the adult, angiogenesis is uncommon except during wound healing and in disease states, such as cancer and retinopathy (21). Postnatal angiogenesis occurs predominantly by sprouting from existing blood vessels, but can be supported by endothelial progenitor cells (EPCs) that home to the angiogenic site after release from the bone marrow (22) or that appear by vasculogenesis local to the site of injury (23). Several studies have shown a strong association between CEPCs, active angiogenesis, and vascular outcomes in mice and humans (24–26). Importantly, angiogenic capacity and CEPC numbers correlate, and both are determined by innate genetic variation (15). However, as yet no variant genetic loci that modulate CEPC levels have been characterized.
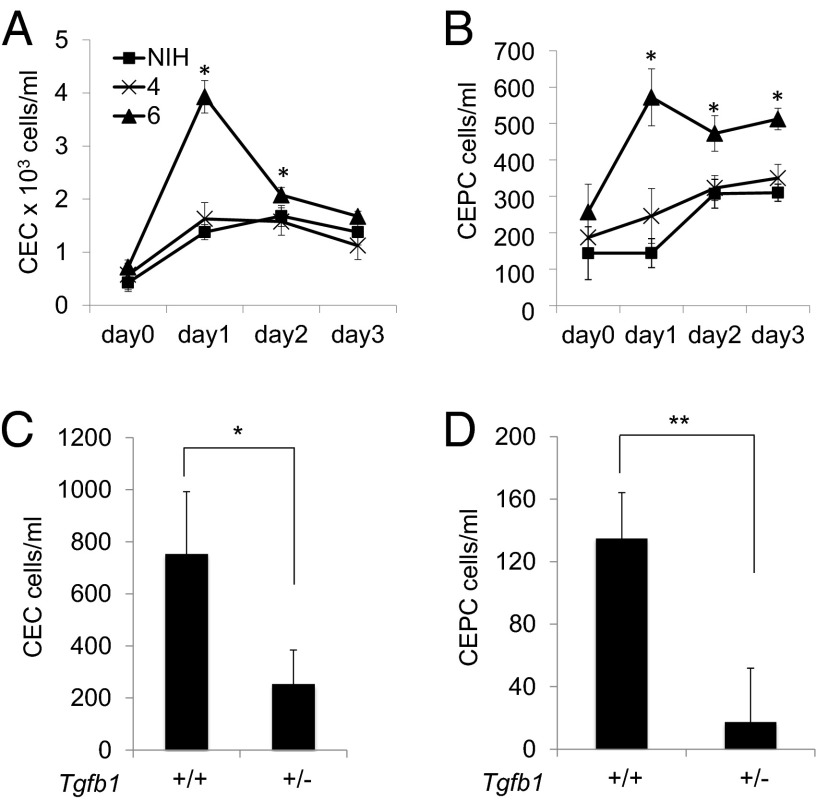
Because Tgfb1 has been shown to be necessary for a robust CEPC response to injury and associated reparative angiogenesis (26), we postulated that variants of Tgfbm3b may interact with Tgfb1 to regulate CEPC levels during postnatal angiogenesis. FACS analysis was used to monitor adult CEPC numbers in NIH and congenic NIH.C57-Tgfbm3 lines 4 and 6 following s.c. implantation of a highly angiogenic syngeneic carcinoma cell line. All three mouse lines showed elevation in CEPC (CD45−, CD117+, CD13+) and circulating endothelial cell (CEC) (CD45−, CD117−, CD13+) numbers, 1–2 d following administration of this angiogenic stimulus (Fig. 3 A and B). NIH and line 4 mice, both homozygous NIH for the major Tgfbm3bNIH allele, showed similar profiles of CEPC and CEC induction, with only a weak and transient increase in CEPC numbers. In contrast, line 6 mice, homozygous for the Tgfbm3bC57 allele, which suppresses Tgfb1−/− embryo lethality, elicited a much greater CEPC response than NIH or line 4 mice (Fig. 3 A and B). Significantly, differences in CEPC numbers between mice that harbor the two alternative Tgfbm3b genetic variants were not paralleled by differentials in total circulating CD45+ cells, or specifically in CD45+ CD11b+ F4.80+ Gr1− monocytes or CD45+ CD11b+ Gr1+ myeloid-derived suppressor cells (Fig. S1), implying that the effects observed were specific to EPCs. Importantly, loss of a single Tgfb1 allele severely reduced the accentuated CEPC and CEC responses to the angiogenic stimulus seen in line 6 mice (NIH.Tgfbm3abC57/C57) (Fig. 3 C and D), demonstrating that TGFβ1 is required for the activity of Tgfbm3bC57 in this assay and giving support to a model of genetic interaction between Tgfb1 and Tgfbm3b in regulating CEPC levels. We surmise that genetic variation within Tgfbm3b regulates CEPC numbers in response to an angiogenic stimulus. We believe that this is the first report of an endogenous polymorphic genetic modifier of CEPC number in vivo.
Fig. 3.
Tgfbm3b is a TGFβ1-dependent modifier of adult CEPC response to angiogenesis. Three mouse strains: (i) NIH, (ii) congenic NIH.Tgfbm3a line 4, and (iii) NIH.Tgfbm3ab line 6, were implanted with syngeneic CarB cells (2 × 105) expressing high VEGF and SDF1 levels, to provide an angiogenic stimulus. Blood was harvested at the indicated time points post-CarB implantation, and assayed by FACS analysis for (A) CD45−, CD13+, CD117− CECs, and (B) (CD45−, CD13+, and CD117+) CEPCs. Similar experiments were performed using NIH.Tgfbm3ab line 6 congenic mice that were either wild type or haploinsufficient for Tgfb1 (C and D). Each time point represents data from four mice per genotype, and each experiment was independently reproduced three times.
Innate Genetic Variation of Adam17 Within Tgfbm3b Modifies TGFβ Signaling.
Whole genome SNP analysis showed that lines 4 and 6 were both ≥95% homozygous NIH, including the Tgfbm1 and Tgfbm2 loci (Table S1). It is therefore unlikely but not impossible that differences in TGFβ biology observed between congenic lines 4 and 6 were influenced by genetic variation at unlinked loci. Definitive proof that Tgfbm3b directly influences TGFβ signaling therefore required analysis of the variant genes within that locus. We sequenced the coding regions of candidates located within Tgfbm3b (Fig. 1A). Three genes, Greb1, E2f6, and Adam17, each showed amino acid polymorphisms between C57 and NIH mice (Table S2). E2f6 possesses a single p.Leu10Gln polymorphism, but comparative functional analysis of the two protein variants showed no significant influence on TGFβ signaling in vitro.
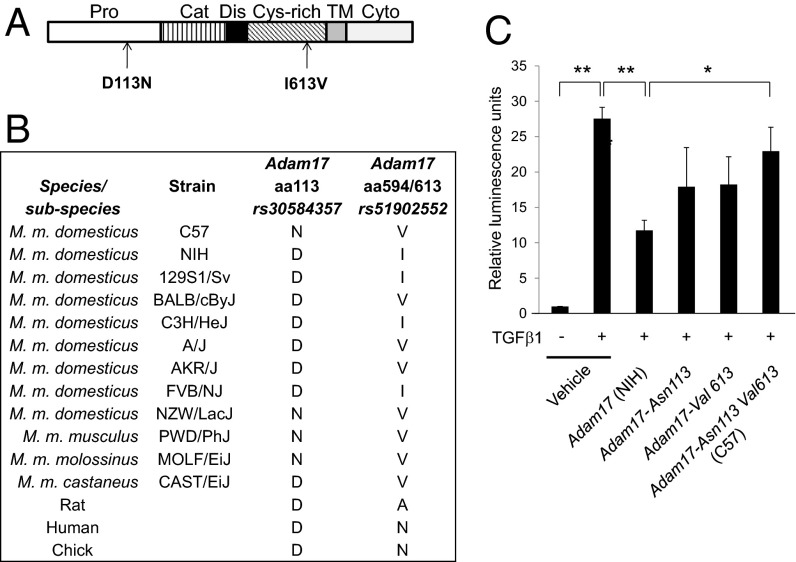
Adam17 possesses two nonsynonymous coding SNPs. This gene encodes a disintegrin and metalloprotease 17 (ADAM17), also termed tumor necrosis factor α converting enzyme (TACE), a transmembrane metalloprotease involved in cell surface shedding and activation of a number of growth factors, including TNFα, EGF-R ligands, Notch1, and Notch4 (27). A p.Asp113Asn variant in the C57 allele introduces a charge change at an evolutionarily conserved residue within the ADAM17 Pro domain. This domain is involved in ADAM17 polypeptide processing, subcellular trafficking, and activation (Fig. 4 A and B). The ADAM17 p.Asp113Asn variant has only been observed in one other Mus musculus domesticus strain, NZW/LacJ, and two wild-derived Mus subspecies, Mus musculus molossinus and Mus musculus musculus (Fig. 4B). The C57 ADAM17 p.Ileu613Val variant (Fig. 4A) is located in the cysteine-rich domain. This is a relatively common variant in laboratory mouse strains and is not evolutionarily conserved (Fig. 4B).
Fig. 4.
Adam17 polymorphic variants differentially regulate canonical TGFβ-SMAD signaling output. (A) Position of C57 amino acid substitutions within the ADAM17 protein. (B) Evolutionary conservation of ADAM17 amino acid residues 113 and 594/613 from chick to human. (C) TGFβ1/Smad2/3-mediated transcriptional responses of NIH 3T3 fibroblasts transfected with expression vectors encoding NIH.Adam17, Adam17Asp113Asn, Adam17Ileu613Val, or C57.Adam17 Asp113Asn,Ileu613Val. Cells were transfected with the indicated Adam17 expression constructs together with a Smad2/3-responsive pCAGA-luciferase construct. Note that exogenous ADAM17 expression was in vast excess to that of endogenous NIH 3T3 ADAM17 (Fig. S2). Activation of luciferase was assayed 24 h after addition, or not, of 1 ng/mL TGFβ. *P ≤ 0.05, **P ≤ 0.01. Each experiment was independently reproduced three times with three technical replicates within each experiment.
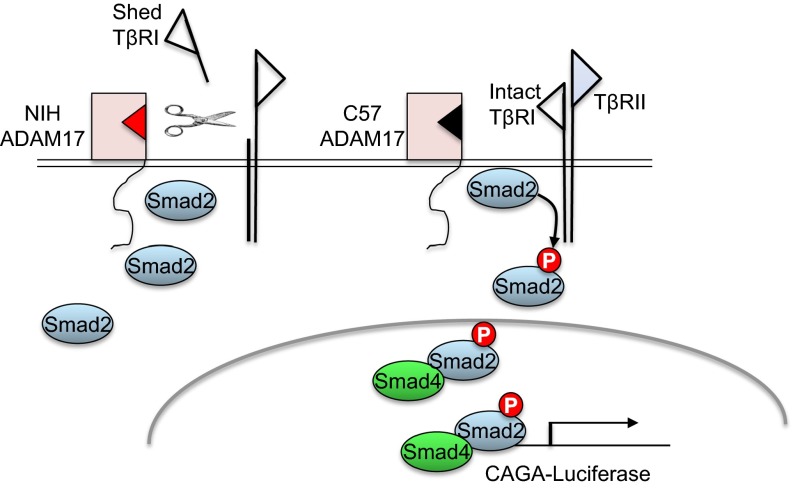
ADAM17 has been shown to cleave the TGFβ type I receptor, effectively reducing Smad2 signaling (28). We therefore tested the comparative activity of ectopically expressed ADAM17 variants to modulate TGFβ responsiveness in NIH 3T3 fibroblasts using a CAGA12-luciferase assay that reports on Smad2/3 transcriptional activity. As predicted, ectopic expression of the major (NIH) ADAM17 isoform reduced TGFβ-dependent Smad2/3 transcriptional output (Fig. 4C), presumably by shedding TβRI (28). Ectopic C57 ADAM17 protein levels were similar to those of the NIH variant (Fig. S2), but showed a 50% attenuation in the ability to reduce TGFβ-induced CAGA12-luciferase activity (Fig. 4C), suggesting that this isoform is hypomorphic with respect to TβRI sheddase activity. Interestingly, ADAM17 polypeptides harboring single C57 amino acid variants showed intermediate levels of CAGA12-luciferase activity between that of the C57 and NIH alleles (Fig. 4C), suggesting that both amino acid variants contribute to the overall decrease in TβRI sheddase activity. We conclude that the C57 genome encodes a recessive Adam17 variant that is hypomorphic for down-regulation of TβRI, resulting in a hyperactivated state of TβRI-Smad2/3 signaling that, in the presence of TGFβ2, TGFβ3, or maternal TGFβ1, may contribute to the rescue of line 6 Tgfb1−/− embryos from lethal vascular dysgenesis (Fig. 5).
Fig. 5.
Model depicting effect of ADAM17 variant on the canonical TGFβ signaling pathway. TGFβ ligand induces heteroligomerization of TβRII and TβRI, activation of TβRI by TβRII, and phosphorylation of Smad2 by TβRI kinase. P-Smad2 oligomerizes with Smad4 to shuttle to the nucleus and instigate a Smad-mediated transcriptional response. The model proposes differential shedding and consequent inactivation of TβRI by the NIH versus C57 variants of ADAM17. The C57 ADAM17 isoform has relatively weak inhibitory activity against TβRI, consequently elevating nuclear P-Smad2/3 and Smad2/3-mediated transcription in cells harboring this variant.
ADAM17 Inhibition Potentiates CEPC Levels by Enhancing TGFβ Signaling.
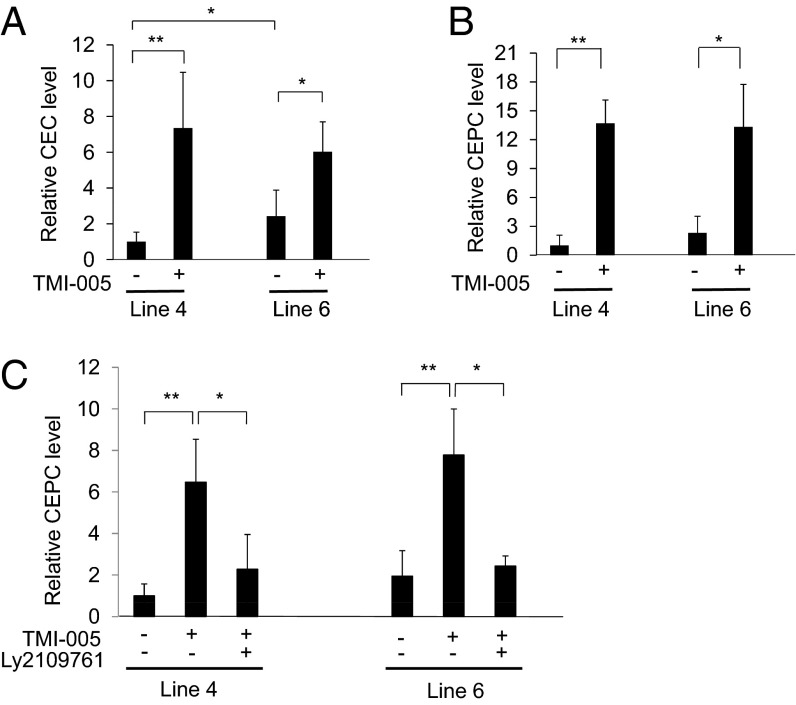
We postulated that genetic variation of Adam17 is also responsible for the modulatory effects of Tgfbm3b on CEPC levels. Based on the observation that Tgfbm3bC57, harboring the hypomorphic Adam17 allele, potentiated CEPC levels in response to the angiogenic stimulus, we hypothesized that pharmacological ADAM17 inhibition would accentuate release of CEPCs from the bone marrow. Indeed, systemic treatment of mice with an ADAM17-specific small molecule inhibitor, TMI-005 (29), enhanced CEPC and CEC numbers 16- to 30-fold (Fig. 6 A–C). This TMI-005–induced increase in CEPC response was observed even in mice carrying the hypomorphic Adam17C57 allele (line 6, Fig. 6 B and C), consistent with the fact that the hypomorphic C57 variant retains significant residual ADAM17 activity. Based on our genetic interaction analysis, we hypothesized that the effect of hypomorphic ADAM17 on CEPC and CEC responses in vivo was mediated by reduced shedding and consequent increased activation of the TGFβ type I receptor. In concordance with this hypothesis, the large induction in CEPC numbers observed in the presence of TMI-005 was completely ablated by Ly2109761 (Fig. 6C), a small molecule inhibitor of TβRI kinase (30). We therefore conclude that the stimulatory effect of ADAM17 inhibition on CEPC levels is mediated by enhanced TβRI signaling.
Fig. 6.
TβRI kinase activity mediates the effect of hypomorphic ADAM17 on CEPC numbers in vivo. Mice were treated, or not, with 50 mg/kg of a pharmacological ADAM17 inhibitor, TMI-005, and/or 100mg/kg of an orally available TβRI kinase inhibitor, Ly2109761, before and during induction of angiogenesis, as described in the legend to Fig. 3. Mice were dosed twice a day with drug or vehicle, from one day before CarB implantation. Blood samples were collected before tumor implantation (0 h) and 24 h post implantation. CEPC and CEC numbers were assayed as described in Fig. 3. (A) CEC and (B) CEPC numbers, in response to inhibition of ADAM17 by TMI-005. Elevated induction of (C) CEPCs in congenic mice was neutralized by treatment with the TβRI kinase inhibitor, Ly2109761. Each time point reports the mean of four mice per experiment, and each experiment was replicated three times. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Genetic Variation Within Human ADAM17 Associates with Pulmonary Vascular Lesions in HHT.
Because Adam17 interacts with Tgfb1 to modulate prenatal vascular development and adult CEPC levels in mice, we postulated that genetic variation in ADAM17 may modify TGFβ-regulated vascular disorders in humans. HHT is such a disorder, caused by haploinsufficiency of ENG, ACVRL1, or SMAD4, and considered to be a disease of excess angiogenesis and/or altered endothelial cell fate, resulting from perturbations in the TGFβ/BMP signaling pathways (31–33). Although HHT is rare, affecting 1 in 5–10,000 people worldwide, studies of this disease are valuable to our overall understanding of human vascular biology. We hypothesized that genetic variation within human ADAM17 may influence clinical severity of HHT, one feature of which is development of pulmonary AVMs. We therefore investigated genetic association between SNPs within ADAM17 and the presence of pulmonary AVM in HHT mutation carriers.
We genotyped six common independent ADAM17 tagSNPs (r2 < 0.7) in a cohort of 401 Dutch and Dutch Antillean HHT mutation carriers, together with 235 unaffected first degree relatives. Gamete competition analysis (34), a modified version of the transmission distortion test, revealed that three of the six ADAM17 SNPs showed significant association between the minor allele and presence of pulmonary AVM in HHT mutation carriers (P < 0.05, Table S3: rs10495565, rs12474540, and rs17524594). When Dutch patients were stratified by HHT subtype, significant association with pulmonary AVM was observed for HHT1 (mutant ENG), but not for HHT2 (mutant ACVRL1). Importantly, of 175 additional tagSNPs that spanned the human 2p25 genomic interval syntenic to Tgfbm3b (750 kb proximal and 1 Mb distal from ADAM17; Fig. S3) only one other SNP (rs2304401) showed significant genetic association to lung AVM in the Dutch cohort. In humans, a positive genetic association requires confirmation in an independent population. We therefore genotyped rs10495565 and rs12474540 (ADAM17), as well as rs2304401 in an additional French cohort of patients with HHT (n = 222). Both ADAM17 SNPs showed a strong trend toward genetic association between minor alleles with pulmonary AVM in HHT1 (n = 75, Table S4; P = 0.067) but not HHT2 mutation carriers (n = 147), with the statistical power being limited by the small size of this French HHT cohort. rs2304401 showed no genetic association. In conclusion, despite the statistical power constraints of working with small populations with a rare genetic disorder, the data strongly suggest that differential interactions between ADAM17 variants and the TGFβ signaling pathway can influence severity of vascular pathology in humans.
Discussion
In the current study, we report the presence of a polymorphic genetic locus in mice that regulates both Tgfb1-dependent prenatal and postnatal vascular biology. We show that the C57 allele of Tgfbm3b, a component of the larger Tgfbm3 locus on mouse chromosome 12, is a suppressor of Tgfb1−/− prenatal lethality and modulates CEPC levels in adults, a surrogate for angiogenic capacity. Although these genetic data might be confounded by the presence of a low level of genetic heterogeneity elsewhere in the genome (9), we provide molecular and pharmacological evidence that genetic variation of the Adam17 gene within Tgfbm3b is at least in part responsible for this modifier effect.
It is notable that the C57 mouse strain has a low angiogenesis potential and low CEPC levels (15), as well as manifesting complete penetrance of prenatal lethality of Tgfb1−/− embryos from vascular dysgenesis (7, 9). It is therefore counterintuitive that the C57 allele of Tgfbm3b would be proangiogenic, as manifested by enhanced adult CEPC numbers and suppression of Tgfb1−/− prenatal lethality in line 6 compared with line 4 mice. This may be explained if, in pure C57 mice, the proangiogenic activity of Tgfbm3bC57 is masked by stronger angiogenesis suppressor genes located elsewhere in the C57 genome, such as within Tgfbm3a. This paradox highlights the complexity of genetic interactions between endogenous variants observed in polygenic traits, such as angiogenesis, as has previously been documented in cancer susceptibility loci (35–37). In the current study, the confounding effects of unlinked modifier genes are largely, although not entirely, eliminated by utilization of the NIH congenic lines that differ only across Tgfbm3.
It seems counterintuitive that MEFs from pure NIH mice, which have a relatively high Tgfb1−/− embryo survival rate, have lower nuclear Smad2 levels compared with line 6 MEFs (NIH.Tgfbm3bC57). Moreover, adult NIH mice did not demonstrate an accentuated CEPC response to the angiogenic stimulus, despite a high Tgfb1−/− embryo survival rate. These facts might be reconciled if NIH alleles within the proximal Tgfbm3a haplotype support prenatal Tgfb1−/− development in a Smad2-independent fashion that does not influence CEPC levels in the adult. In the current study we restricted our analysis to Tgfbm3b because this locus not only modulated adult CEPC levels, but also suppressed Tgfb1−/− embryo lethality, suggesting a direct influence of Tgfbm3b on the TGFβ signaling pathway.
We present evidence that functional polymorphism within ADAM17 is a major contributor to the modifier effects of Tgfbm3b. ADAM17 is a sheddase that cleaves many substrates and, by so doing, is involved in processing and activating TNFα, Notch, EGFR, and amphiregulin, as well as suppressing TβRI signaling (27, 28). We found that the C57 Adam17 allele harbors two amino acid polymorphisms, p.Asp113→Asn and p.Ileu613→Val, which together result in a protein that is hypomorphic in down-modulating TGFβ signaling, culminating in higher levels of nuclear Smad2 signaling downstream of TβRI and enhanced Smad2/3-dependent transcriptional reporter activity. This effect may compensate somewhat for lack of TGFβ1 ligand in Tgfb1−/− embryos, allowing more of them to undergo normal embryonic development, possibly supported by TGFβ2, TGFβ3, or maternal TGFβ1.
Intriguingly, we found that inheritance of the hypomorphic C57 Adam17 variant or pharmacological inhibition of ADAM17 results in higher CEPC numbers in mice. Many studies have found that CEPC numbers correlate with productive angiogenesis and vascular stabilization. CEPC numbers have been positively associated with a reduced risk of vascular disease, as well as a better outcome after a cardiovascular incident (38). In stroke victims, CEPC numbers are lower compared with control subjects, whereas higher CEPC numbers correlate with a more favorable outcome (24, 25). An inverse correlation between CEPC number and aneurysm size has been reported in patients with idiopathic thoracic ascending aortic aneurysm (TAAA) (39), an intriguing observation because TAAD (TAAA with dissections) is a major health concern in patients who have mutations in TGFβ signaling pathway genes (40–42). The fact that genetic reduction of TGFβ1 or pharmacological inhibition of TβRI signaling neutralized the effects of ADAM17 hypoactivity on CEPC numbers, not only further illustrates the importance of interaction between ADAM17 activity and TGFβ signaling in vivo, but suggests that many of the effects of ADAM17 on angiogenesis are mediated via TGFβRI.
It is likely that these amino acid substitutions in C57 ADAM17 alter microdomains that differentially affect substrate specificities. In fact, the p.Ileu613Val variant is located within the cysteine-rich substrate recognition domain (43); thus, variation in Adam17 p.Asn113-Val613 may preferentially affect TβRI signaling over that of other substrates, such as TNFα. If the C57 ADAM17 variant reduced activation of EGFR, TGFα or TNFα processing, wild-type C57 mice might be expected to present with dramatic phenotypes, such as perinatal lethality (44) or inflammatory skin and bowel disease (45), observed in mice or humans lacking functional ADAM17, or with wavey fur and open eyelids, as observed in a more severe murine ADAM17 hypomorph (46), which is clearly not the case.
Notch is another ADAM17 substrate, and is a vascular quiescence factor with a known involvement in TGFβ and ALK1 signaling (32, 33). It is thus possible that reduction in Notch activation, due to reduced ADAM17 activity, may also contribute to hyperelevation of CEPC numbers and increased angiogenesis observed in the NIH.C57-Adam17 and TMI-005–treated mice (32). Nevertheless, the fact that specific pharmacological inhibition of TβRI completely suppressed the TMI-005–induced CEPC response, argues in favor of a major role of TGFβ signaling in mediating the effects of hypomorphic ADAM17 on CEPC number. We conclude that TGFβ1-TβRI-Smad2/3 signaling plays an important proangiogenic role not only during embryogenesis, but in release of adult bone-marrow–derived CEPCs to the blood, and that these processes are modulated by interaction between TβRI and genetic variants of Adam17.
Finally, in the current study, we provide evidence that genetic variation in human ADAM17 may influence clinical outcome of a TGFβ-regulated disease, HHT. We show genetic association between ADAM17 SNPs and the presence of pulmonary AVMs in patients with HHT, suggesting a contribution to the risk of lung AVM. The human study is limited by the small cohort size necessitated by working with a rare disease, and the SNPs that drive association between ADAM17 and PAVM in HHT remain to be identified. However, the fact that three of six independent SNPs (r2 < 0.7) showed significant genetic association in the Dutch population as well as marginal association in French HHT is compelling. Human variation in ADAM17 at the specific amino acid residues orthologous to those of the C57 variant (113 and 613) has not been reported to date. However, clusters of “potentially detrimental” amino acid variants lie close to the orthologous polypeptide positions of the C57 mouse variants, between residues 97–120 (Pro domain) and 505–610 (cysteine-rich domain) (Polyphen and SIFT analysis in dbSNP). These protein variants may contribute to differences in TGFβ signaling within the human population. Intriguingly, in both the Dutch and French populations, genetic association was only observed within HHT1 but not in HHT2. This may be of biological significance, because ACVRL1, mutated in HHT2, encodes a serine threonine kinase signaling receptor that is primarily activated by BMP9 rather than TGFβ, whereas ENG/endoglin, mutated in HHT1, is involved in presentation of various ligands to their respective type II receptors, including TGFβ1 and -3, activins, and BMPs.
In conclusion, here we show that genetic variants of mouse Adam17 can profoundly influence the outcome of vascular phenotypes in vivo, both pre- and postnatally. This explains one component of the genetic complexity that determines individualized responses to reduced TGFβ1 signaling. It is likely that human genetic variation in ADAM17, by differential effects on TβRI, will also determine risks and outcomes of more common human diseases, as well as clinical responses to TGFβ pathway-targeted therapies (1).
Materials and Methods
The parental mouse strains under study were NIH/OlaHsd and C57BL/6NTac, each carrying a Tgfb1Tm1n allele (17). NIH.C57-Tgfbm3 congenic mouse lines were generated by repeated backcross (more than seven generations) of NIH.C57.F1 mice to NIH/OlaHsd, with selection for the Tgfb1Tm1n allele on chromosome (Chr) 7 and for C57 SSR markers at Tgfbm3. Congenic lines were genotyped at Tgfbm1 (Chr 5) and Tgfbm2 (Chr 1) to confirm homozygosity for NIH and exclude the possibility of confounding effects due to genetic variation at these Tgfbm loci. Further methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Mamie Higgins and Minh Thu Luu for outstanding technical assistance and Jonathan Yingling for supplying Ly2109761. Human genotyping was performed by the Helen Diller Family Comprehensive Cancer Center Genome Core Facility. This work was funded by National Institutes of Health Grants HL078564, GM60514, CA116019, and an award from the HHT International Foundation (to R.J.A.). K.K. received a fellowship from the Uehara Memorial Foundation, Japan. F.F.C. received a fellowship from the Belgian American Educational Foundation; F.F.C. and M.B. were both recipients of American Heart Association fellowships; D.S.M. was the recipient of a Swiss National Foundation fellowship, and T.G.W.L. was the recipient of a fellowship from the Ter Meulen Fund, The Netherlands.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318761111/-/DCSupplemental.
References
- 1.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-β family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20(9):556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Loeys BL, et al. Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med. 2006;355(8):788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 4.McAllister KA, et al. Six novel mutations in the endoglin gene in hereditary hemorrhagic telangiectasia type 1 suggest a dominant-negative effect of receptor function. Hum Mol Genet. 1995;4(10):1983–1985. doi: 10.1093/hmg/4.10.1983. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DW, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13(2):189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 6.Anderton MJ, et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol. 2011;39(6):916–924. doi: 10.1177/0192623311416259. [DOI] [PubMed] [Google Scholar]
- 7.Bonyadi M, et al. Mapping of a major genetic modifier of embryonic lethality in TGF β 1 knockout mice. Nat Genet. 1997;15(2):207–211. doi: 10.1038/ng0297-207. [DOI] [PubMed] [Google Scholar]
- 8.Kang HC, et al. Multiple self-healing squamous epithelioma (MSSE): Rare variants in an adjacent region of chromosome 9q22.3 to known TGFBR1 mutations suggest a digenic or multilocus etiology. J Invest Dermatol. 2013;133(7):1907–1910. doi: 10.1038/jid.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benzinou M, et al. Mouse and human strategies identify PTPN14 as a modifier of angiogenesis and hereditary haemorrhagic telangiectasia. Nat Commun. 2012;3:616. doi: 10.1038/ncomms1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallione CJ, et al. SMAD4 mutations found in unselected HHT patients. J Med Genet. 2006;43(10):793–797. doi: 10.1136/jmg.2006.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shovlin CL. Hereditary haemorrhagic telangiectasia: Pathophysiology, diagnosis and treatment. Blood Rev. 2010;24(6):203–219. doi: 10.1016/j.blre.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, et al. Epistatic interactions between modifier genes confer strain-specific redundancy for Tgfb1 in developmental angiogenesis. Genomics. 2005;85(1):60–70. doi: 10.1016/j.ygeno.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci USA. 2001;98(4):1787–1792. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuelson DJ, et al. Rat Mcs5a is a compound quantitative trait locus with orthologous human loci that associate with breast cancer risk. Proc Natl Acad Sci USA. 2007;104(15):6299–6304. doi: 10.1073/pnas.0701687104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaked Y, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis: Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7(1):101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Dickson MC, et al. Defective haematopoiesis and vasculogenesis in transforming growth factor-β 1 knock out mice. Development. 1995;121(6):1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni AB, et al. Transforming growth factor β 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Y, et al. Genetic modifiers interact with maternal determinants in vascular development of Tgfb1(-/-) mice. Hum Mol Genet. 2003;12(13):1579–1589. doi: 10.1093/hmg/ddg164. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian S, et al. Epistatic suppression of systemic lupus erythematosus: Fine mapping of Sle1 to less than 1 mb. J Immunol. 2005;175(2):1062–1072. doi: 10.4049/jimmunol.175.2.1062. [DOI] [PubMed] [Google Scholar]
- 20.St Johnston D. The art and design of genetic screens: Drosophila melanogaster. Nat Rev Genet. 2002;3(3):176–188. doi: 10.1038/nrg751. [DOI] [PubMed] [Google Scholar]
- 21.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 22.Gao D, et al. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim Biophys Acta. 2009;1796(1):33–40. doi: 10.1016/j.bbcan.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tongers J, Roncalli JG, Losordo DW. Role of endothelial progenitor cells during ischemia-induced vasculogenesis and collateral formation. Microvasc Res. 2010;79(3):200–206. doi: 10.1016/j.mvr.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobrino T, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38(10):2759–2764. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 25.Yip HK, et al. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39(1):69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 26.Wara AK, et al. TGF-β1 signaling and Krüppel-like factor 10 regulate bone marrow-derived proangiogenic cell differentiation, function, and neovascularization. Blood. 2011;118(24):6450–6460. doi: 10.1182/blood-2011-06-363713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: A molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32(8):380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Xu P, Lamouille S, Xu J, Derynck R. TACE-mediated ectodomain shedding of the type I TGF-β receptor downregulates TGF-β signaling. Mol Cell. 2009;35(1):26–36. doi: 10.1016/j.molcel.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thabet MM, Huizinga TW. Drug evaluation: Apratastat, a novel TACE/MMP inhibitor for rheumatoid arthritis. Curr Opin Investig Drugs. 2006;7(11):1014–1019. [PubMed] [Google Scholar]
- 30.Li HY, et al. Optimization of a dihydropyrrolopyrazole series of transforming growth factor-β type I receptor kinase domain inhibitors: Discovery of an orally bioavailable transforming growth factor-β receptor type I inhibitor as antitumor agent. J Med Chem. 2008;51(7):2302–2306. doi: 10.1021/jm701199p. [DOI] [PubMed] [Google Scholar]
- 31.Dupuis-Girod S, et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA. 2012;307(9):948–955. doi: 10.1001/jama.2012.250. [DOI] [PubMed] [Google Scholar]
- 32.Larrivée B, et al. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell. 2012;22(3):489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JH, Peacock MR, George SC, Hughes CC. BMP9 induces EphrinB2 expression in endothelial cells through an Alk1-BMPRII/ActRII-ID1/ID3-dependent pathway: Implications for hereditary hemorrhagic telangiectasia type II. Angiogenesis. 2012;15(3):497–509. doi: 10.1007/s10456-012-9277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange K, Sinsheimer JS, Sobel E. Association testing with Mendel. Genet Epidemiol. 2005;29(1):36–50. doi: 10.1002/gepi.20073. [DOI] [PubMed] [Google Scholar]
- 35.Moore JH, Williams SM. Epistasis and its implications for personal genetics. Am J Hum Genet. 2009;85(3):309–320. doi: 10.1016/j.ajhg.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuelson DJ, Aperavich BA, Haag JD, Gould MN. Fine mapping reveals multiple loci and a possible epistatic interaction within the mammary carcinoma susceptibility quantitative trait locus, Mcs5. Cancer Res. 2005;65(21):9637–9642. doi: 10.1158/0008-5472.CAN-05-1498. [DOI] [PubMed] [Google Scholar]
- 37.Nagase H, Mao JH, de Koning JP, Minami T, Balmain A. Epistatic interactions between skin tumor modifier loci in interspecific (spretus/musculus) backcross mice. Cancer Res. 2001;61(4):1305–1308. [PubMed] [Google Scholar]
- 38.Werner N, et al. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102(6):565–571. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- 39.Parietti E, et al. Presence of circulating endothelial progenitor cells and levels of stromal-derived factor-1α are associated with ascending aorta aneurysm size. Eur J Cardiothorac Surg. 2011;40(1):e6–e12. doi: 10.1016/j.ejcts.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 40.Lindsay ME, et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet. 2012;44(8):922–927. doi: 10.1038/ng.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doyle AJ, et al. Mutations in the TGF-β repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat Genet. 2012;44(11):1249–1254. doi: 10.1038/ng.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loeys BL, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37(3):275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 43.Takeda S, Igarashi T, Mori H, Araki S. Crystal structures of VAP1 reveal ADAMs’ MDC domain architecture and its unique C-shaped scaffold. EMBO J. 2006;25(11):2388–2396. doi: 10.1038/sj.emboj.7601131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peschon JJ, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282(5392):1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 45.Blaydon DC, et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N Engl J Med. 2011;365(16):1502–1508. doi: 10.1056/NEJMoa1100721. [DOI] [PubMed] [Google Scholar]
- 46.Hassemer EL, et al. The waved with open eyelids (woe) locus is a hypomorphic mouse mutation in Adam17. Genetics. 2010;185(1):245–255. doi: 10.1534/genetics.109.113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.