Significance
The mechanisms underlying Dollo’s Law, the assertion that the evolutionary loss of complex structures is irreversible, remain poorly characterized. In principle, such mechanisms could involve the improbability either of generating the mutations required for trait reappearance or of selecting for their fixation. Whereas most attention has focused on the former mechanism, we used experimental reversal of dentition reduction in cypriniform fishes to provide evidence for the operation of both within a single system.
Keywords: Astyanax mexicanus, transgenic
Abstract
The apparent irreversibility of the loss of complex traits in evolution (Dollo’s Law) has been explained either by constraints on generating the lost traits or the complexity of selection required for their return. Distinguishing between these explanations is challenging, however, and little is known about the specific nature of potential constraints. We investigated the mechanisms underlying the irreversibility of trait loss using reduction of dentition in cypriniform fishes, a lineage that includes the zebrafish (Danio rerio) as a model. Teeth were lost from the mouth and upper pharynx in this group at least 50 million y ago and retained only in the lower pharynx. We identified regional loss of expression of the Ectodysplasin (Eda) signaling ligand as a likely cause of dentition reduction. In addition, we found that overexpression of this gene in the zebrafish is sufficient to restore teeth to the upper pharynx but not to the mouth. Because both regions are competent to respond to Eda signaling with transcriptional output, the likely constraint on the reappearance of oral teeth is the alteration of multiple genetic pathways required for tooth development. The upper pharyngeal teeth are fully formed, but do not exhibit the ancestral relationship to other pharyngeal structures, suggesting that they would not be favored by selection. Our results illustrate an underlying commonality between constraint and selection as explanations for the irreversibility of trait loss; multiple genetic changes would be required to restore teeth themselves to the oral region and optimally functioning ones to the upper pharynx.
That complex traits do not reappear once lost is a macroevolutionary pattern of sufficient generality to have been proposed independently as “Dollo’s Law” (1, 2) and the “Law of Loss” (3). This pattern is frequently interpreted as evidence of a constraint on adaptive evolution resulting from the loss of genetic information required for trait development (2, 4, 5). Such information loss in the form of pseudogene formation has been documented (5), but most examples involve traits with relatively direct connections of genotype to phenotype, such as floral pigment (6) and hemoglobin (7). In contrast, the complex morphological features for which laws of irreversible evolution were formulated develop under the control of genes whose pleiotropy (8) is expected to preserve their function (4). Such pleiotropy is likely to be especially prominent in the case of irreversible loss of individual members of systems of repeated parts (9).
An alternative to constraint as an explanation for the irreversibility of the loss of complex structures is natural selection. For example, selection may act against the deleterious pleiotropic consequences of mutations in developmental regulatory genes, as proposed by Galis et al. (9). In addition, mutations whose reversal leads to reduced fitness may accumulate following structural loss, as has been shown in association with a shift in glucocorticoid receptor function (10). Finally, it is possible that some apparent instances of irreversible loss are simply the result of absence of selection for return of the structure (2).
An example of irreversible loss of morphological structures that allows inference of selection, as well as characterization of potential constraints, is reduction of dentition in the teleost fish order Cypriniformes, which includes minnows, suckers, loaches, and algae eaters. Teeth in this group were lost from the entire mouth cavity and upper pharynx at least 50 million y ago and remain only on the fifth ceratobranchial bones of the lower posterior pharynx (Fig. 1A), where they serve the function of mechanical processing of food, especially by chewing (11, 12). Such reduction of dentition is thought to have evolved as an adaptation to suction feeding in bottom deposits (11, 13, 14). At least two lines of evidence suggest that some cypriniforms have experienced selection for regaining of lost dentition. Several lineages have adopted piscivory, which in noncypriniform fishes is usually associated with oral teeth for gripping and upper pharyngeal teeth for transporting prey (11, 14, 15). These piscivorous cypriniforms have been suggested to be less-efficient predators than other taxa with more extensive dentition (11, 16, 17). In addition, the cypriniform species Danionella dracula has evolved dramatic fangs, not by regaining oral teeth but by sculpting the shape of bones of the jaw margin (18).
Fig. 1.
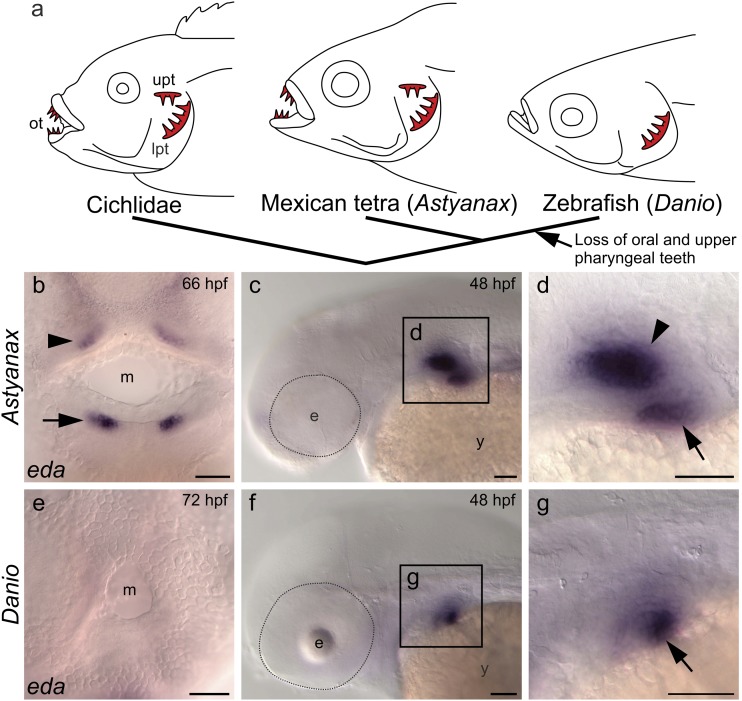
Regional loss of eda expression is associated with reduction of dentition in the zebrafish lineage. (A) Teeth were lost from the mouth and upper pharynx in the zebrafish (D. rerio) lineage after its divergence from that of the Mexican tetra (A. mexicanus). (B) eda expression in upper (arrowhead) and lower (arrow) jaws of Astyanax. (C and D) eda expression in upper (arrowhead) and lower (arrow) pharynx of Astyanax. (E) Absence of eda expression in the mouth of Danio. (F and G) eda expression in the lower (arrow) but not upper pharynx of Danio. All eda expression shown is in mesenchyme, as confirmed by sectioning (Fig. S1). Abbreviations: e, eye; hpf, hours postfertilization; lpt, lower pharyngeal teeth; m, mouth; ot, oral teeth; upt, upper pharyngeal teeth; y, yolk. (Scale bars, 50 μm.)
Identifying the genetic pathways altered in association with cypriniform dentition reduction is expected to provide insight into potential constraints leading to irreversibility, and is facilitated by the membership of the zebrafish (Danio rerio) model species in the group (12). One candidate gene for tooth loss encodes the TNF family ligand Ectodysplasin (Eda) (19), as zebrafish homozygotes for the nkt loss-of-function mutation in this gene completely lack adult dentition (20).
To investigate a potential role for altered Eda signaling in cypriniform dentition reduction, we first compared eda expression between the zebrafish and a relative with more extensive dentition, the characiform Mexican tetra, Astyanax mexicanus. We found that localized eda expression prefigured tooth-forming regions and that evolutionary loss of this expression is associated with cypriniform dentition reduction. Support for a causal relationship between eda expression loss and tooth loss was provided by our characterization of the dental phenotype of nkt mutants, which exhibited arrest of pharyngeal tooth developmental at a stage resembling that of the wild-type oral region. We next investigated the reversibility of cypriniform dentition reduction by producing a transgenic zebrafish line with continuous and ubiquitous expression of eda. Ectopic teeth were found in the upper pharynx of this line, but not in the oral cavity, suggesting different mechanisms underlying the irreversibility of tooth loss in these two regions. We propose that these mechanisms involve both natural selection and constraint caused by alteration of multiple developmental genetic pathways.
Results and Discussion
Regional Loss of Eda Expression Is Associated with Cypriniform Dentition Reduction.
The requirement for Eda function in the zebrafish pharyngeal dentition (20) suggests that if alteration of Eda signaling were involved in cypriniform dentition reduction, it likely involved changes in the location of signal transduction rather than loss of pathway component function. We therefore used in situ hybridization to compare eda expression in the zebrafish with that in a related species possessing oral as well as upper and lower pharyngeal teeth, the Mexican tetra, A. mexicanus (21) (Fig. 1A). Expression of eda in the zebrafish was detected in mesenchyme of the lower pharynx in the region from which teeth develop (Fig. 1 F and G and Fig. S1) but not in the mouth or upper pharynx (Fig. 1 E–G and Fig. S1). In contrast, eda expression is evident in tooth-forming mesenchyme of all three regions in A. mexicanus (Fig. 1 B–D and Fig. S1). The presence of eda expression in mesenchyme of the oral and pharyngeal dentition of outgroups in the family Cichlidae (22) indicates that its loss occurred in association with cypriniform tooth loss.
Loss of Eda Function in the Zebrafish Pharyngeal Dentition Phenocopies the Wild-Type Oral Region.
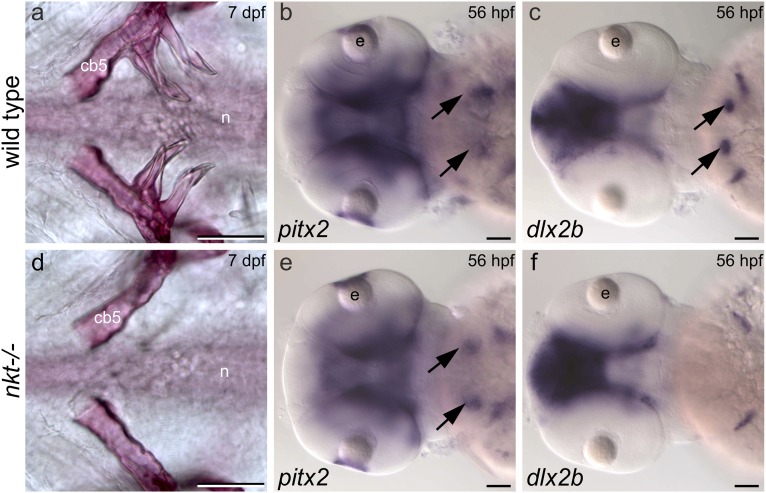
To investigate loss of eda expression as a potential cause of dentition reduction, we compared tooth initiation in the larval pharynx of wild-type and nkt mutant zebrafish. Mineralized teeth are visible in the wild-type pharynx at 3 d postfertilization (dpf) (12) but were completely lacking from the pharynx of 15 of 19 nkt homozygous larvae analyzed at 6–10 dpf (Fig. 2D). The remaining four nkt mutants examined possessed a single tooth on a single side of the pharynx. In contrast, all 16 homozygous wild-type siblings possessed at least three mineralized teeth on each side (Fig. 2A). These data are consistent with the necessity of eda expression for zebrafish tooth development.
Fig. 2.
The nkt mutation in eda results in early arrest of pharyngeal tooth development. (A and D) Ventral views of alizarin stained 7-dpf larvae showing complete absence of teeth in an nkt mutant homozygote. A single tooth is sometimes formed on one side of these mutants (n = 4 of 19). (B and E) pitx2 is expressed in presumptive pharyngeal tooth epithelium (arrows) in the nkt mutant homozygote (n = 5 of 5), heterozygote (n = 11 of 11), and wild-type homozygote (n = 5 of 5). (C and F) dlx2b is expressed in pharyngeal tooth germs (arrows) of wild-type homozygotes (n = 3 of 3) and nkt heterozygotes (n = 12 of 12), but its expression is lacking in the pharynx of the nkt mutant homozygote (n = 5 of 5). Abbreviations: cb5, fifth ceratobranchial bone; e, eye; hpf, hours postfertilization; n, notochord. (Scale bars, 50 μm.)
The oral region of zebrafish larvae expresses the transcription factor pitx2, a marker of tooth-competent epithelium, but lacks the expression of markers of the dental placode, the earliest morphologically visible sign of tooth development (12, 21). We found that arrest of pharyngeal tooth development in nkt mutant homozygotes resembles that found in the wild-type oral region. Specifically, expression of pitx2 is present (Fig. 2 B and E), whereas that of the transcription factor dlx2b, a dental placode marker, is absent (Fig. 2 C and F). Taken together, our data are consistent with regional loss of eda expression as a cause of loss of teeth from the upper pharynx and mouth of a cypriniform ancestor.
Eda Overexpression Restores Teeth to the Upper Pharynx of the Zebrafish.
The hypothesis of constraint as the cause of the irreversibility of dentition reduction in cypriniforms predicts the difficulty of restoring lost teeth to the zebrafish through simple genetic changes. We therefore asked whether reversal of eda expression loss is sufficient to restore teeth by producing transgenic lines that express zebrafish eda under the control of the Xenopus laevis elongation factor ef1α promoter, which drives ubiquitous and continuous expression throughout development (23). Fish from these ef1α:eda lines exhibit supernumerary and bicuspid teeth associated with the fifth ceratobranchial bones (Fig. S2), phenotypes resembling those produced by activation of fibroblast growth factor (Fgf) or inhibition of bone morphogenetic protein (Bmp) signaling (24). Eda acts upstream of Fgf expression (25) and inhibits Bmp activity (26) during tooth development in the mouse; similar regulatory interactions may operate in the development of zebrafish teeth.
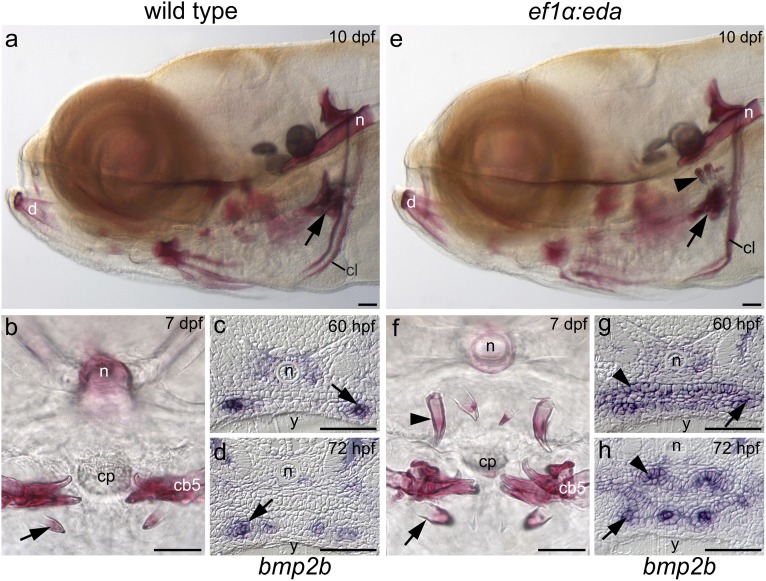
The most striking dental phenotype in the ef1α:eda transgenic line is the presence of ectopic teeth in the upper pharynx opposing those of the lower pharynx (Figs. 3 A, B, E, and F, 4 A, C, D, and F, and Movie S1). Both regions possessed teeth in cypriniform ancestors (12), suggesting that the ectopic teeth represent atavisms, reappearances of ancestral characteristics. It has been proposed that atavistic structures can only arise when a rudiment has been retained (27). In addition, the supernumerary teeth in Eda-overexpressing mice are thought to arise by rescue of a rudimentary tooth germ (28). We therefore examined whether the ability of Eda to restore lost upper pharyngeal teeth is dependent on the presence of rudiments in the upper pharynx. Expression of the tooth germ markers bone morphogenetic protein 2b (bmp2b) and ectodysplasin A receptor (edar) was lacking from the upper pharynx in wild-type zebrafish but present in the eda-overexpressing transgenics (Fig. 3 C, D, G, and H, and Fig. S3). In contrast, pitx2 was present in upper pharyngeal epithelium of wild type and eda-overexpressing zebrafish (Fig. S3). We conclude that although competence for tooth initiation exists in the upper pharynx of wild-type zebrafish, rudiments of teeth are absent. eda overexpression therefore appears to be inducing teeth de novo in the upper pharynx.
Fig. 3.
Ectopic Eda expression restores upper pharyngeal teeth to the zebrafish. Lower pharyngeal teeth (arrows) in lateral (A) and transverse (B) views of wild-type alizarin red-stained larvae. (C and D) bmp2b expression is limited to tooth germs (arrows) of the lower pharynx in wild-type. Upper pharyngeal teeth (arrowheads) in lateral (E) and transverse (F) views of ef1α:eda transgenic zebrafish. (G) bmp2b expression is induced in the upper pharyngeal epithelium (arrowhead) by ectopic eda expression and becomes limited to tooth germs (arrowhead) at later stages (H). Abbreviations: cb5, fifth ceratobranchial; cl, cleithrum; cp, chewing pad; d, dentary bone; n, notochord; y, yolk. (Scale bars, 50 μm.)
Fig. 4.
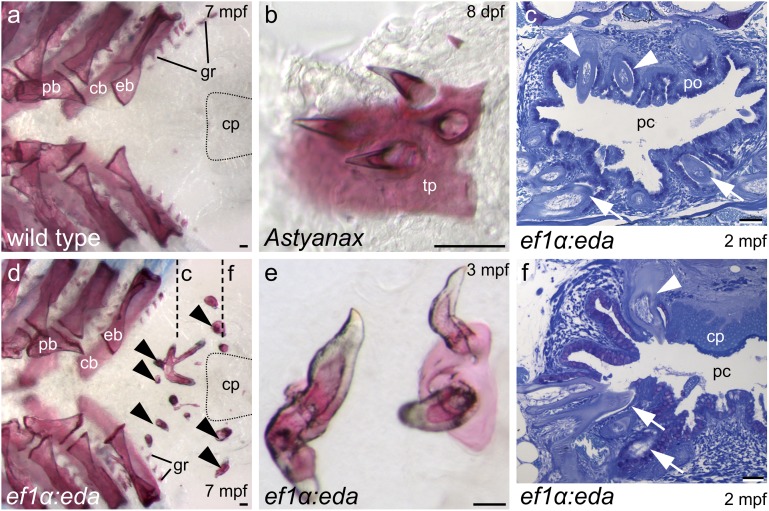
The upper of pharynx of adult zebrafish is only partially restored to the ancestral condition by ectopic eda expression. (A and D) Dorsal views of alizarin-stained pharyngeal arches with fifth ceratobranchials removed to show location of ectopic teeth (arrowheads in D) posterior to upper gill arch elements and dorsal to fifth ceratobranchials. Approximate position of the chewing pad is indicated by dotted lines. (E) Calcified tissue uniting ectopic teeth resembles upper pharyngeal toothplates of A. mexicanus (B). (C and F) Transverse sections (approximate plane of section indicated in D) reveal teeth in the vicinity of the palatal organ and projecting into the pharyngeal cavity (arrowheads in C) as well as lateral to the chewing pad (arrowhead in F). Lower pharyngeal teeth in (C and F) indicated by arrows. Abbreviations: cb, ceratobranchial; cp, chewing pad; eb, epibranchial; gr, gill raker; mpf, months postfertilization; pb, pharyngobranchial; pc, pharyngeal cavity; po, palatal organ; tp, toothplate. (Scale bars, 50 μm.)
The relative simplicity of the genetic change sufficient to restore upper pharyngeal teeth implicates selection rather than constraint as an explanation for the irreversibility of their loss in cypriniforms. One possibility is selection against deleterious pleiotropic effects of the mutations required to regain these teeth, as suggested by Seritrakul et al. (29). These authors found that retinoic acid treatment was sufficient to expand the zebrafish dentition but also resulted in early larval lethality. In contrast, ef1α:eda zebrafish survive to adulthood and are fertile. We suggest instead that restoration of the ancestral function of upper pharyngeal teeth requires additional modifications that may interfere with the current function of the posterior pharynx.
Pharyngeal teeth in cypriniform ancestors likely functioned in transport of captured prey to the esophagus (11, 14, 15). The upper teeth were attached to toothplates of dermal bone supported by endochondral bones or cartilages of the gill arches (30). Loss of these teeth was but one of numerous morphological changes occurring in association with acquisition of a food-processing function by the posterior pharynx (11, 14). Toothplates were lost, and although the gill arch elements that formerly supported them remain, these are located more anteriorly and underlie a muscular palatal organ that has assumed the transport function. In the zebrafish and other members of the family Cyprinidae, the lower pharyngeal teeth are opposed to a keratinized chewing pad attached to the base of the skull. Upper pharyngeal teeth in the ef1α:eda transgenic line frequently erupt into the pharyngeal cavity (Fig. 4 C and F) and, surprisingly, are in some cases supported by sheets of bone resembling toothplates (Fig. 4 B and E). The chewing pad remains in these fish, however, with the ectopic teeth located anterior or lateral to it (Fig. 4 C and F). Upper pharyngeal teeth were lost in the common ancestor of the order Cypriniformes (12), while the chewing pad has a more restricted distribution within the group (31). The combination of teeth and chewing pad in the transgenic line is therefore unlikely to represent an intermediate stage in evolution and could indeed be maladaptive. In addition, the ectopic teeth—even if attached to toothplates—are located posterior to the upper gill arch elements that supported them previously (Fig. 4 A and D). Upper pharyngeal teeth are unlikely to be selected for without reversals of these numerous other pharyngeal modifications, which—if occurring in isolation—would reduce the efficiency of the food-processing function.
Eda Overexpression Does Not Restore Teeth to the Mouth of the Zebrafish Despite Competence to Respond with Transcriptional Activation.
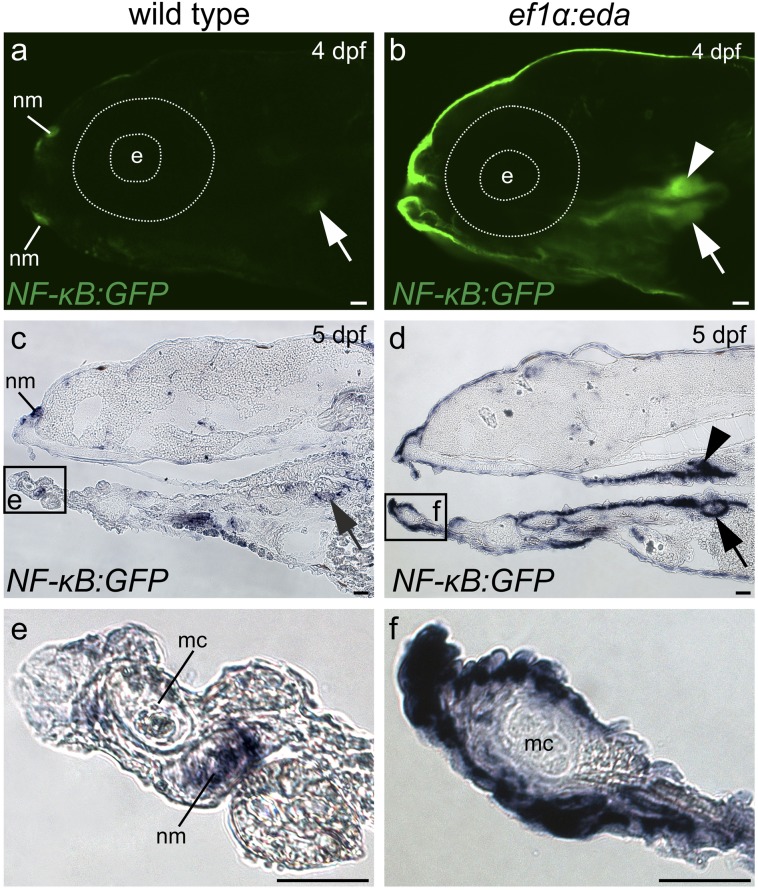
In contrast to the upper pharynx, we found no evidence for the induction of oral teeth in eda-overexpressing zebrafish using histology (Fig. 3 A and E) and a variety of tooth germ markers (Fig. S4) that have been shown to differ in their expression between wild-type zebrafish and A. mexicanus (21, 32). We therefore asked whether the oral epithelium is competent to respond to Eda. Transduction of this signal involves binding of the ligand-bound Edar receptor to an adaptor protein Edaradd and ultimately results in nuclear translocation of the transcription factor complex NF-κB (19). We found that both the receptor edar and the adaptor edaradd are expressed in the mouth of wild-type zebrafish in patterns resembling those of A. mexicanus (Fig. S5). In addition, eda overexpression is capable of inducing expression of GFP in the oral epithelium of a zebrafish reporter line (33) transgenic for NF-κB responsive elements (Fig. 5). Therefore, despite loss of the Eda inductive signal in the mouth, the downstream components of its signal transduction pathway have remained intact over a period likely to exceed 50 million y (12). Such maintenance of a genetic pathway in the absence of function contrasts with what has been reported for a pigment biosynthesis pathway in plants, in which loss of pathway function is associated with degenerative mutations in multiple components (6). It is therefore possible that the irreversibility of loss of morphological structures differs from that of other traits.
Fig. 5.
Competence to respond to Eda signaling is distributed throughout the oropharyngeal cavity. (A and B) Confocal imaging of GFP fluorescence and C-F) in situ hybridization analysis of gfp RNA expression in NF-κB reporter zebrafish. Oral expression is limited to neuromasts and pharyngeal expression to lower tooth germs (arrow) in fish lacking the ef1α:eda transgene (A, C, and E). Presence of this transgene (B, D, and F) induces reporter expression throughout the oropharyngeal cavity, including in lower (arrow) and upper (arrowhead) pharyngeal tooth germs. Abbreviations: dpf, days postfertilization; e, eye; mc, Meckel’s cartilage; nm, neuromast. (Scale bars, 50 μm.)
Because oral teeth were not induced by eda overexpression despite the retention of competence to respond with transcriptional activation, evolutionary changes in developmental genetic pathways in addition to Eda signaling were involved in cypriniform oral tooth loss or have accumulated subsequently. These genetic changes in multiple pathways likely comprise the constraint that has rendered loss of these teeth irreversible. Such a constraint would explain the appearance of bony projections from the jaw in D. dracula (18) and several lineages of fish-eating cypriniforms (12) in response to likely selection for the function provided by true oral teeth.
Selection, Constraint, and Irreversible Evolution.
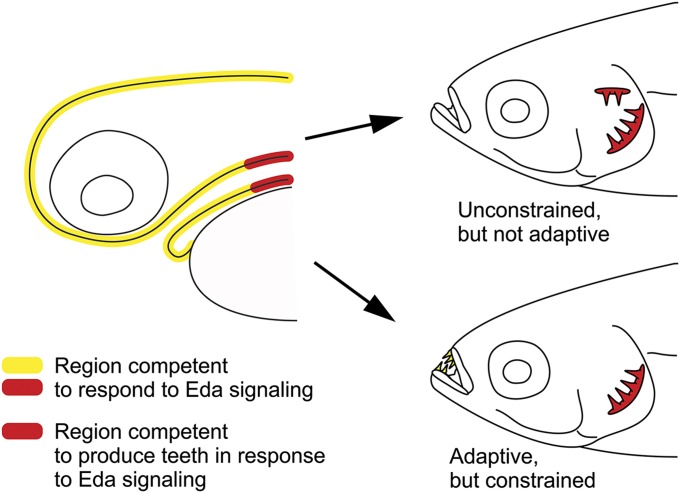
Our conclusions on the mechanisms underlying the irreversibility of dentition reduction in cypriniform fishes are summarized in Fig. 6. Although competence to respond to Eda signaling with transcription is present throughout the mouth and pharynx, only in the posterior pharynx does such a response lead to the induction of teeth. The ability to restore upper pharyngeal teeth with a single genetic change suggests that their evolutionary reappearance is relatively unconstrained in cypriniforms. The absence in nature of members of this group with upper pharyngeal teeth is therefore the result of such teeth not being adaptive in the current configuration of the pharynx. In contrast, reappearance of oral teeth would likely be adaptive in some cypriniform lineages, but is constrained by the absence in the oral region of gene products in addition to the Eda ligand that are necessary for tooth development.
Fig. 6.
The irreversibility of cypriniform dentition reduction results from the action of both selection and constraint. Left side indicates locations of competence to respond to Eda signaling and to do so with tooth production in an extant larval cypriniform. Right side indicates “forbidden” morphologies that represent reversal of cypriniform dentition reduction. The Upper morphology does not exist in nature because of the complexity of selection required to restore ancestral pharyngeal function. The Lower morphology does not exist because of constraint on the ability to produce oral teeth.
Previous studies have identified likely constraints rendering structural loss irreversible, as in the mutational degradation of dental proteins in association with tooth loss in birds (34). Selection as an explanation for Dollo’s Law has proven more elusive, but has been suggested to be responsible for the difficulty of regaining lost digits in lizards (9). Our results suggest that both selection and constraint have acted to render loss in a single organ system in a single lineage irreversible. Interestingly, these causes may share an underlying similarity. Whereas constraints have been considered alternatives to selection as explanations for macroevolutionary patterns (35), others have argued that many forms of constraint are simply the result of selection itself (36). Our results provide evidence for such a relationship between selection and constraint. We suggest that the irreversibility of tooth loss in both the oral and upper pharyngeal regions of cypriniforms is the result of a requirement for multiple genetic changes to reacquire them. In the former case, teeth themselves cannot be reacquired by single mutations; in the latter, teeth may arise in variant individuals, but multiple mutations—many of which may not be advantageous individually—are required to restore their ancestral function. The requirement for multiple mutations has also been proposed to act as a constraint on increasing dental complexity in mammals (37) and resembles the original explanation for Dollo’s Law (1): the statistical improbability of retracing a long sequence of evolutionary steps (38).
Materials and Methods
Fish Strains.
Wild-type zebrafish of the inbred Tü line were obtained from the Zebrafish International Resource Center. Green fluorescent protein reporter lines Tg(dlx2b:gfp)cs1 and Tg(NFκB:EGFP)nc1 were used to visualize tooth germs (39) and sites of NF-κB activation (33), respectively, in the zebrafish. Loss of Eda function in the zebrafish was achieved with the nkt allele (20). A. mexicanus individuals used for cloning genes originated from the population in La Cueva de El Pachón, whereas those used for in situ hybridization were from a commercial population believed to originate from La Cueva Chica (21). Pigmentation in zebrafish larvae was inhibited with 0.003% 1-phenyl-2-thiourea.
Construction of Transgenic Zebrafish Lines Overexpressing Eda.
A zebrafish eda cDNA produced by reverse-transcriptase–mediated (RT)-PCR was ligated into plasmid pTAL200R150G (40) to replace the EGFP coding region. The resulting plasmid, pEF1α:EDA was coinjected with mRNA encoding tol2 transposase into the blastomeres of one-celled zebrafish embryos. Initial analyses were performed directly on injected fish, with injection of the unaltered pTAL200R150G serving as a negative control. Several transgenic lines were produced by injecting this construct into the Tg(dlx2b:gfp)cs1 reporter line; the analyses reported here were conducted on a single line, Tg(ef1α:eda)cs3, containing a single insertion of the transgene.
Cloning and Sequence Analysis.
RT-PCR was used to amplify fragments of eda, edar, and edaradd from the zebrafish and A. mexicanus, and fibroblast growth factor 4 (fgf4) from A. mexicanus. PCR products were cloned into plasmid pCR4-TOPO (Life Technologies) and sequenced. The sequences were translated and their orthology determined by BLAST searches of GenBank.
In Situ Hybridization.
Whole-mount in situ hybridization was carried out with digoxigenin-labeled riboprobes and the specimens were either cleared in glycerol for observation or sectioned at 4 μm following embedding in glycol methacrylate, as previously described (21). Additional specimens were sectioned at 10 μm after embedding in paraffin and were subjected to in situ hybridization following a modified version of the protocol of O’Neill et al. (41).
Antisense riboprobes used included zebrafish bmp2a (32), bmp2b (32), dlx2b (42), eda (nucleotide positions 115–1194 in GenBank NM_001115065), edar (positions 204–994 in NM_001115064), edaradd (the region matching human EDARADD positions 141–521 in GenBank NM_145861), fgf4 (42), pitx2 (42), and sonic hedgehog (shh) (21). Probes for A. mexicanus bmp2a, bmp2b, dlx2b, pitx2, and shh were as previously described (21, 32). A. mexicanus edar and edaradd probes correspond to the positions listed for their zebrafish orthologs, and the eda and fgf4 probes for this species comprised the regions corresponding to zebrafish positions 769–1151 (NM_001115064) and 538–887 (NM_131635.2), respectively. The sequences of these A. mexicanus cDNAs have been deposited in GenBank (www.ncbi.nlm.nih.gov/genbank) under accession nos. KJ767495–KJ767498.
Histology.
Clearing and skeletal staining with alizarin red followed Hanken and Wassersug (43) for adults and Wise and Stock (44) for larvae. Adult specimens for sectioning were fixed in formaldehyde, decalcified in Poly-NoCal (Polysciences), dehydrated through an ethanol series, and embedded in glycol methacrylate. The 5-μm sections were cut with glass knives and stained with toluidine blue.
Genotyping.
Zebrafish possessing the ef1α:eda transgene were distinguished from wild-type siblings by the presence of upper pharyngeal tooth germs, which behaved as a simple Mendelian trait when visualized by reporter expression from dlx2b:gfp or NFκB:GFP transgenes in living larvae. Clipped sections of adult caudal fins and whole larvae subjected to in situ hybridization or alizarin red staining were used for detecting the nkt allele. DNA was extracted by digestion with proteinase K and subjected to PCR with primers GTCGCTACAGTCACAACAGATG and ATAAGCAGTAGAGTCCAGGGAC. Restriction enzyme digestion was used to detect an XbaI recognition site absent in the wild type allele and present in the nkt alelle.
Imaging.
Conventional light microscopy used a Zeiss Axiovert 135 inverted compound microscope, a Zeiss SV11 stereomicroscope, or a Leica MZ FLIII fluorescent microscope, the former two of which were mounted with Zeiss Axiocam digital cameras. Confocal microscopy was carried out with a Nikon A1R Resonant Scanning Confocal and TIRF system.
X-ray synchrotron microtomography was performed at SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (proposal nos. 2008A1754 and 2009B1911). The micro computed tomography (microCT) was taken at beamline BL20B2 with 900 projections at an X-ray energy of 15 keV at 7.97 μm/pixel. The exposure time for one projection was 15 ms. The data were processed with AVIZO software (Maxnet).
Supplementary Material
Acknowledgments
Aaron Garnett and Daniel Meulemans Medeiros provided advice and discussion; Gilson Sanchez and Pamela Diggle assisted with imaging; and Matthew Harris and John Rawls provided zebrafish lines prior to publication. This study was supported by Grants IOS-0446720 and IOS-1121855 from the US National Science Foundation (to D.W.S.) and National Institutes of Health Grant R03 DE016328-01 (to D.W.S.), and included a portion of the MA thesis of S.R.A.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Database deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KJ767495–KJ767498).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321171111/-/DCSupplemental.
References
- 1.Dollo L. Les lois de l’évolution. Bull Soc Belge Géol Pal Hydr. 1893;7:164–166. [Google Scholar]
- 2.Collin R, Miglietta MP. Reversing opinions on Dollo’s Law. Trends Ecol Evol. 2008;23(11):602–609. doi: 10.1016/j.tree.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Arber A. The law of loss in evolution. Proc Linn Soc Lond. 1919;131(1):70–78. [Google Scholar]
- 4.Marshall CR, Raff EC, Raff RA. Dollo’s law and the death and resurrection of genes. Proc Natl Acad Sci USA. 1994;91(25):12283–12287. doi: 10.1073/pnas.91.25.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Futuyma DJ. Evolutionary constraint and ecological consequences. Evolution. 2010;64(7):1865–1884. doi: 10.1111/j.1558-5646.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- 6.Zufall RA, Rausher MD. Genetic changes associated with floral adaptation restrict future evolutionary potential. Nature. 2004;428(6985):847–850. doi: 10.1038/nature02489. [DOI] [PubMed] [Google Scholar]
- 7.Near TJ, Parker SK, Detrich HW., 3rd A genomic fossil reveals key steps in hemoglobin loss by the antarctic icefishes. Mol Biol Evol. 2006;23(11):2008–2016. doi: 10.1093/molbev/msl071. [DOI] [PubMed] [Google Scholar]
- 8.Carroll SB. Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell. 2008;134(1):25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Galis F, Arntzen JW, Lande R. Dollo’s law and the irreversibility of digit loss in Bachia. Evolution. 2010;64(8):2466–2476, discussion 2477–2485. doi: 10.1111/j.1558-5646.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 10.Bridgham JT, Ortlund EA, Thornton JW. An epistatic ratchet constrains the direction of glucocorticoid receptor evolution. Nature. 2009;461(7263):515–519. doi: 10.1038/nature08249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sibbing FA. In: Cyprinid Fishes: Systematics, Biology and Exploitation. Winfield IJ, Nelson JS, editors. New York: Chapman & Hall; 1991. pp. 377–412. [Google Scholar]
- 12.Stock DW. Zebrafish dentition in comparative context. J Exp Zoolog B Mol Dev Evol. 2007;308(5):523–549. doi: 10.1002/jez.b.21187. [DOI] [PubMed] [Google Scholar]
- 13.Gosline WA. Considerations regarding the phylogeny of cypriniform fishes with special reference to structures associated with feeding. Copeia. 1973;1973:761–776. [Google Scholar]
- 14.Sibbing FA, Osse JWM, Terlouw A. Food handling in the carp (Cyprinus carpio): Its movement patterns, mechanisms and limitations. J Zool. 1986;210:161–203. [Google Scholar]
- 15.Hyatt KD. In: Fish Physiology. Hoar WS, Randall DJ, Brett JR, editors. Vol VIII. San Francisco: Academic; 1979. pp. 71–119. [Google Scholar]
- 16.Portz DE, Tyus HM. Fish humps in the two Colorado River fishes: A morphological response to cyprinid predation? Environ Biol Fishes. 2004;71(3):233–245. [Google Scholar]
- 17.de Graaf M, Dejen E, Osse JWM, Sibbing FA. Adaptive radiation of Lake Tana’s (Ethiopia) Labeobarbus species flock (Pisces, Cyprinidae) Mar Freshw Res. 2008;59(5):391–407. [Google Scholar]
- 18.Britz R, Conway KW, Rüber L. Spectacular morphological novelty in a miniature cyprinid fish, Danionella dracula n. sp. Proc Biol Sci. 2009;276(1665):2179–2186. doi: 10.1098/rspb.2009.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikkola ML. TNF superfamily in skin appendage development. Cytokine Growth Factor Rev. 2008;19(3–4):219–230. doi: 10.1016/j.cytogfr.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Harris MP, et al. Zebrafish eda and edar mutants reveal conserved and ancestral roles of ectodysplasin signaling in vertebrates. PLoS Genet. 2008;4(10):e1000206. doi: 10.1371/journal.pgen.1000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stock DW, Jackman WR, Trapani J. Developmental genetic mechanisms of evolutionary tooth loss in cypriniform fishes. Development. 2006;133(16):3127–3137. doi: 10.1242/dev.02459. [DOI] [PubMed] [Google Scholar]
- 22.Fraser GJ, et al. An ancient gene network is co-opted for teeth on old and new jaws. PLoS Biol. 2009;7(2):e31. doi: 10.1371/journal.pbio.1000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson AD, Krieg PA. pXeX, a vector for efficient expression of cloned sequences in Xenopus embryos. Gene. 1994;147(2):223–226. doi: 10.1016/0378-1119(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 24.Jackman WR, et al. Manipulation of Fgf and Bmp signaling in teleost fishes suggests potential pathways for the evolutionary origin of multicuspid teeth. Evol Dev. 2013;15(2):107–118. doi: 10.1111/ede.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Häärä O, et al. Ectodysplasin regulates activator-inhibitor balance in murine tooth development through Fgf20 signaling. Development. 2012;139(17):3189–3199. doi: 10.1242/dev.079558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pummila M, et al. Ectodysplasin has a dual role in ectodermal organogenesis: Inhibition of Bmp activity and induction of Shh expression. Development. 2007;134(1):117–125. doi: 10.1242/dev.02708. [DOI] [PubMed] [Google Scholar]
- 27.Hall BK. Atavisms and atavistic mutations. Nat Genet. 1995;10(2):126–127. doi: 10.1038/ng0695-126. [DOI] [PubMed] [Google Scholar]
- 28.Peterkova R, Lesot H, Peterka M. Phylogenetic memory of developing mammalian dentition. J Exp Zoolog B Mol Dev Evol. 2006;306(3):234–250. doi: 10.1002/jez.b.21093. [DOI] [PubMed] [Google Scholar]
- 29.Seritrakul P, et al. Retinoic acid expands the evolutionarily reduced dentition of zebrafish. FASEB J. 2012;26(12):5014–5024. doi: 10.1096/fj.12-209304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson GJ. Gill arches and the phylogeny of fishes, with notes on the classification of vertebrates. Bull Am Mus Nat Hist. 1969;141(4):475–552. [Google Scholar]
- 31.Doosey MH, Bart HL., Jr Morphological variation of the palatal organ and chewing pad of catostomidae (teleostei: cypriniformes) J Morphol. 2011;272(9):1092–1108. doi: 10.1002/jmor.10966. [DOI] [PubMed] [Google Scholar]
- 32.Wise SB, Stock DW. Conservation and divergence of Bmp2a, Bmp2b, and Bmp4 expression patterns within and between dentitions of teleost fishes. Evol Dev. 2006;8(6):511–523. doi: 10.1111/j.1525-142X.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 33.Kanther M, et al. Microbial colonization induces dynamic temporal and spatial patterns of NF-κB activation in the zebrafish digestive tract. Gastroenterology. 2011;141(1):197–207. doi: 10.1053/j.gastro.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sire J-Y, Delgado SC, Girondot M. Hen’s teeth with enamel cap: From dream to impossibility. BMC Evol Biol. 2008;8:246. doi: 10.1186/1471-2148-8-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: A critique of the adaptationist programme. Proc R Soc Lond B Biol Sci. 1979;205(1161):581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- 36.Shanahan T. Why don’t zebras have machine guns? Adaptation, selection, and constraints in evolutionary theory. Stud Hist Philos Biol Biomed Sci. 2008;39(1):135–146. doi: 10.1016/j.shpsc.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Harjunmaa E, et al. On the difficulty of increasing dental complexity. Nature. 2012;483(7389):324–327. doi: 10.1038/nature10876. [DOI] [PubMed] [Google Scholar]
- 38.Gould SJ. Dollo on Dollo’s law: Irreversibility and the status of evolutionary laws. J Hist Biol. 1970;3:189–212. doi: 10.1007/BF00137351. [DOI] [PubMed] [Google Scholar]
- 39.Jackman WR, Stock DW. Transgenic analysis of Dlx regulation in fish tooth development reveals evolutionary retention of enhancer function despite organ loss. Proc Natl Acad Sci USA. 2006;103(51):19390–19395. doi: 10.1073/pnas.0609575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006;174(2):639–649. doi: 10.1534/genetics.106.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Neill P, McCole RB, Baker CVH. A molecular analysis of neurogenic placode and cranial sensory ganglion development in the shark, Scyliorhinus canicula. Dev Biol. 2007;304(1):156–181. doi: 10.1016/j.ydbio.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackman WR, Draper BW, Stock DW. Fgf signaling is required for zebrafish tooth development. Dev Biol. 2004;274(1):139–157. doi: 10.1016/j.ydbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Hanken J, Wassersug R. The visible skeleton. Funct Photog. 1981;16(4):22–26, 44. [Google Scholar]
- 44.Wise SB, Stock DW. bmp2b and bmp4 are dispensable for zebrafish tooth development. Dev Dyn. 2010;239(10):2534–2546. doi: 10.1002/dvdy.22411. [DOI] [PubMed] [Google Scholar]