Significance
Oncogene-induced senescence (OIS) is an initial barrier for cancer development. Reactive oxygen species (ROS) play critical roles in oncogenic Ras OIS. Senescent cells develop a senescence-associated secretory phenotype (SASP), which has important role in tumor suppression and tissue repair. However, the mechanisms underlying the SASP regulation are not clear. In this paper, we show that ROS-protein kinase Cδ (PKCδ)-protein kinase D1 (PKD1) axis is essential for SASP induction and maintenance via modulation of NF-κB activity. Considering the pivotal role of both SASP and ROS in systemic aging and age-related diseases, the link between ROS-PKCδ-PKD1 pathway and SASP regulation elucidated here may provide a new target to intervene in age-related inflammation and diseases.
Abstract
Oncogene-induced senescence (OIS) is an initial barrier to tumor development. Reactive oxygen species (ROS) is critical for oncogenic Ras OIS, but the downstream effectors to mediate ROS signaling are still relatively elusive. Senescent cells develop a senescence-associated secretory phenotype (SASP). However, the mechanisms underlying the regulation of the SASP are largely unknown. Here, we identify protein kinase D1 (PKD1) as a downstream effector of ROS signaling to mediate Ras OIS and SASP. PKD1 is activated by oncogenic Ras expression and PKD1 promotes Ras OIS by mediating inflammatory cytokines interleukin-6 (IL-6) and interleukin-8 (IL-8) via modulation of NF-κB activity. We demonstrate that ROS-protein kinase Cδ (PKCδ)-PKD1 axis is essential for the establishment and maintenance of IL-6/IL8 induction. In addition, ablation of PKD1 causes the bypass of Ras OIS, and promotes cell transformation and tumorigenesis. Together, these findings uncover a previously unidentified role of ROS-PKCδ-PKD1 pathway in Ras OIS and SASP regulation.
Cellular senescence is a permanent cell growth arrest state triggered either by telomere attrition (replicative senescence), or by many other stimuli, such as DNA damage, oxidative stress, and oncogenes activation, etc., without any detectable telomere shortening (premature senescence) (1). Oncogene-induced senescence (OIS) is of particular interest. OIS indeed occurs in premalignant human tumors and represents an initial barrier for cancer development in vivo (2). One well-characterized type of OIS is that induced by oncogenic Ras. Ras expression in normal primary cells induces premature senescence (3). ROS plays an important role in Ras OIS. ROS levels increase in Ras OIS, and ROS elimination prevent Ras OIS (4). Seladin-1 was identified as a downstream effector of ROS to mediate Ras OIS (5). Several positive ROS-generating feedback loops, such as ROS-protein kinase Cδ (PKCδ) loop (6), mitochondrial dysfunctional signaling (7), and DDB2-mediated pathway (8), were proposed to contribute to elevated ROS levels in Ras OIS. Despite steady progress in probing the roles of ROS in senescence, however, most of studies focus on the links between ROS and the p53, p16, and DNA damage response (DDR) pathways, little is known about other mechanisms that ROS might mediate Ras OIS.
Senescent cells develop a senescence-associated secretory phenotype (SASP) by secretion of numerous cytokines, chemokines, and other proteins (9, 10). SASP have multiple biological effects depending on the physiological context. Some SASP factors such as IL-6/IL-8 and others can suppress early tumor formation either by reinforcing senescence in an autocrine fashion (11, 12), or by stimulating the immune system to clear premalignant senescent cells in a paracrine manner (13). SASP also can facilitate tissue repair (14). On the other hand, SASP can promote tumor growth (15). Moreover, many proinflammatory factors in SASP might be an important source of the low-level chronic inflammation in aged mammalian tissues, hence may contribute to many age-related diseases in late life (15).
SASP is regulated mainly at mRNA level and depends on the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and C/EBPβ transcription factors (11, 12). Persistent DDR (16), p38 MAPK activity (17), and IL-1α (18) are essential for SASP induction. MicroRNAs miR-146a/b (19) and Klotho (20) are important negative regulators of SASP. It was known that ROS signaling plays a critical role in chronic inflammation (21) and in NLRP3 inflammasome-mediated IL-1β and IL-18 induction (22), and a previous study revealed that IL-8 receptor CXCR2 activation correlates with ROS production in Ras OIS (11). However, whether ROS signaling involved in SASP regulation is still unknown.
Protein kinase D (PKD) is a serine/threonine kinase family belongs to the Ca2+/Calmodulin-dependent kinase superfamily, and consists of PKD1, PKD2, and PKD3 isoforms. PKDs are mainly activated by PKC (23, 24). PKDs regulate many cellular functions, such as gene expression, cell adhesion and migration, and protein transport at the trans-Golgi network, etc. (23, 24). PKD1 is an important sensor of oxidative stress (25, 26), which is activated by ROS-PKCδ and subsequently induces NF-κB activity. However, whether PKD1 can play a role in Ras OIS and acts as a downstream effector of ROS-PKCδ loop in Ras OIS to regulate NF-κB–dependent SASP has not yet been elucidated so far. In this study, we report that PKD1 is a downstream effector of ROS-PKCδ cascade to mediate Ras OIS by regulating IL-6/IL-8 expression via modulation of NF-κB activity. Intervention of PKD1 leads to bypass Ras OIS and promotes cell transformation and tumorigenesis.
Results
PKD1 Is Activated During Ras-Induced Cell Senescence and Is Required for Ras OIS.
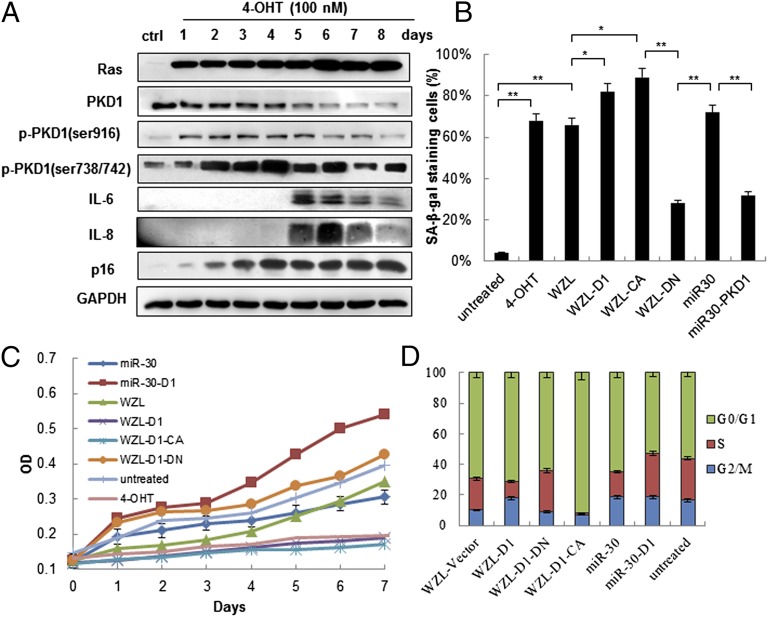
The existence of ROS-PKCδ loop in Ras OIS led us to explore whether PKD1 can be activated during Ras OIS and plays a role in Ras OIS. To investigate this possibility, we made use of the IMR90 cells stably expressing 4-hydroxytamoxifen (4-OHT)–inducible ER:H-RasV12 fusion protein (ER:Ras-IMR90 cells) (27). Consistent with previous findings with Ras induction, cells enter “senescence phase” around day 5–6, which characterized by p16 up-regulation and IL-6/IL-8 induction (Fig. 1A and Fig. S1 A and B). Then, the phosphorylated levels of PKD1 were analyzed by Western blot. Indeed, we detected increased phosphorylation of PKD1 on Ser738/742 and Ser916 sites (Fig. 1A), which indicated that PKD1 was activated during Ras OIS (28, 29). PKD1 phosphorylation increased slightly in the “mitotic phase” (day 1), rose substantially in the early “transition phase” (days 2–3), and reached to peak levels at late “transition phase” (day 4), thereafter slightly decreased when entering “senescence phase” (days 5–8) (Fig. 1A). Importantly, the timing of PKD1 activity reaching peak levels just preceded IL-6/IL-8 induction, implying that PKD1 might regulate SASP in Ras OIS.
Fig. 1.
PKD1 is activated during Ras-induced senescence and is required for Ras OIS. (A) PKD1 is activated by Ras induction. ER:Ras IMR90 cells were given 100 nM 4-OHT for the indicated days; fresh medium with 4-OHT was changed every other day. Cell lysates were collected every day for total eight days, and then analyzed for expression of the indicated proteins. (B–D) PKD1 overexpression enhances Ras OIS, whereas PKD1 silencing prevents Ras OIS. ER:Ras IMR90 cells expressing the indicated genes and shRNAs were induced to express Ras for 6 d. (B) Cells were stained for SA-β-gal. The percentage of cells positive for SA-β-gal in each sample was shown. At least 300 cells were counted for each sample. Error bars represent means + SD (n = 3) *P < 0.05, **P < 0.01. (C) Growth curves were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. (D) Cell cycle was measured. Values are mean ± SD of triplicate points from a representative experiment (n = 3), which was repeated three times with similar results.
To analyze the role of PKD1 in Ras OIS, we overexpressed PKD1 in pWZL-Hygro and pLPC-Puro retroviral vectors and silenced it, by using miR30 retroviral vector, in young ER:Ras IMR90 cells. After 6-d Ras induction, WZL-PKD1 induced more robust senescence phenotypes, which included elevated SA-β-gal activity (Fig. 1B and Fig. S1C), a biomarker for senescent cells, stronger cell growth retardation (Fig. 1C), reduced S and increased G1 compartments (Fig. 1D), and modest decreased numbers of colonies measured by colony-formation assay (Fig. S1D) compared with corresponding empty control vector-infected cells. In contrast, miR30-PKD1 resulted in much lower SA-β-gal activity (Fig. 1B and Fig. S1C), continuous cell growth (Fig. 1C) and cell cycle progression (Fig. 1D), and much more colony formation (Fig. S1D) compared with control miR30 cells. The similar results were achieved by using another independent construct miR30-PKD1#2 to knockdown PKD1 (Fig. S2 A–C). These results demonstrate that PKD1 is essential for Ras OIS and that PKD1 overexpression promotes Ras OIS, whereas PKD1 silencing prevents Ras OIS.
To determine the effect of PKD1 activity on Ras OIS, the constitutively active form of PKD1 (PKD1-CA) and kinase-dead PKD1 (PKD1-DN) mutants (30) were cloned in the WZL and LPC vectors and stably introduced into young ER:Ras IMR90 cells. PKD1-CA greatly enhanced Ras OIS, which was demonstrated by increased SA-β-gal activity (Fig. 1B and Fig. S1C), much stronger cell growth inhibition (Fig. 1C), severe G1 cell cycle arrest (Fig. 1D), and only few colonies (Fig. S1D), compared with control cells. By contrast, PKD1-DN expression reduced SA-β-gal staining (Fig. 1B and Fig. S1C), increased cell proliferation (Fig. 1C) and S compartment (Fig. 1D), and increased colony formation (Fig. S1D) compared with mock cells. The similar SA-β-gal results were achieved by using PKC/PKD inhibitor Gö6976 (31) or PKC/PKD activator PMA (Fig. S1E). Taken together, these results show that PKD1 activity is essential for Ras OIS. When the ability of Ras to activate PKD1 is blocked, Ras OIS cannot occur. Conversely, increasing PKD1 activity enhances Ras OIS.
PKD1 Mediates Ras OIS via Modulation of IL-6/IL-8 Expression and Secretion.
Next, we investigated the molecular mechanism through which PKD1 regulates Ras OIS. The sequential rise in PKD1 activity and IL-6/IL-8 levels as shown in Fig. 1A implies that PKD1 may mediate IL-6/IL-8 expression during Ras OIS. To test this hypothesis, IMR90 cells were treated with PKC/PKD inhibitor Gö6976 in the presence of Ras induction. Gö6976 treatment inhibited Ras-induced PKD1 activation and reduced p16 level compared with untreated cells. Importantly, Gö6976 treatment suppressed IL-6/IL-8 expression (Fig. S3A).
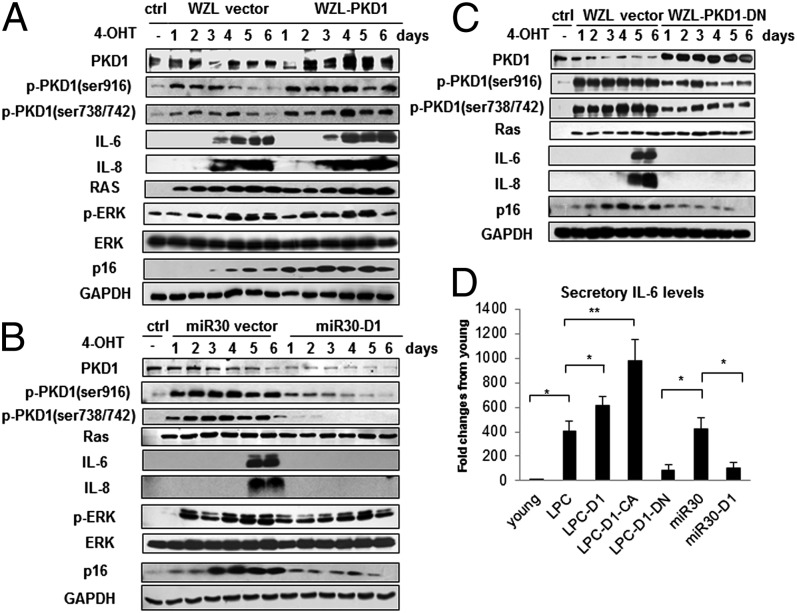
To further validate the role of PKD1 in this process, we made use of WZL-PKD1, WZL-PKD1-DN, and miR30-PKD1 plasmids. WZL-PKD1 enhanced PKD1 activity as measured by specific phospho-PKD1 antibodies (Fig. 2A). Strikingly, WZL-PKD1 led to much stronger induction of IL-6/IL-8 compared with control cells. p16 level was also higher than control cells. This result suggests that PKD1 might accelerate Ras OIS by promoting IL-6/IL-8 expression, which is consistent with previous report that IL-6/IL-8 can reinforce senescence (11, 12). Conversely, both miR30-PKD1 constructs diminished PKD1 activity and abolished or significantly lowered IL-6/IL-8 expression (Fig. 2B and Fig. S2D). Furthermore, PKD1-DN also blocked IL-6/IL-8 expression and significantly decreased p16 level (Fig. 2C), despite the endogenous PKD1 activity still remained. The secreted levels of IL-6 in supernatant detected by enzyme-linked immunoadsorbent assay (ELISA) obtained similar results (Fig. 2D and Fig. S2E). We also tested whether PKD1 could affect IL-6/IL-8 expression, p53/p21 and p16 pathways in the absence of Ras induction. Neither PKD1 overexpression nor PKD1 depletion stimulated IL-6/IL-8 expression and altered p53/p21 as well as p16 pathways without Ras expression (Fig. S3B). All together, these results reveal that PKD1 is an important mediator in Ras OIS by regulating IL-6/IL-8 induction.
Fig. 2.
PKD1 mediates Ras OIS via modulation of IL-6/IL-8 induction. (A–C) PKD1 overexpression promotes IL-6/IL-8 expression, and PKD1 silencing abolishes IL-6/IL-8 induction. ER:Ras IMR90 cells stably transduced with WZL-PKD1 (A), miR30-PKD1 (B), and WZL-PKD1-DN (C) were given 4-OHT for the indicated days. Cell lysates were then subjected to Western blot analysis for the indicated proteins. Data are representative of three independent experiments. (D) Supernatants collected from above cells at day 6 after Ras induction were assessed secretory levels of IL-6 measured by ELISA. Three independent experiments were analyzed. Error bars represent means + SD (n = 3) *P < 0.05, **P < 0.01.
Ras OIS depends on the sequential activation of Raf/MEK/ERK mitogenic signaling (32). PKD1 has been reported to facilitate ERK activation by phosphorylation of RIN1 (33). To investigate whether PKD1 participates in this pathway to mediate Ras OIS, we analyzed ERK activities as well as total ERK levels both in WZL-PKD1 and miR30-PKD1 cells. As shown in Fig. 2 A and B, neither WZL-PKD1 nor miR30-PKD1 altered ERK activities or endogenous ERK levels, which were measured by phospho-ERK and native ERK antibodies, respectively. This result ruled out the possibility that PKD1 involved in Ras OIS through regulation of upstream Raf/MEK/ERK signaling.
Senescent cells secret several matrix metalloproteinases (MMPs). PKD1 has been shown to negatively regulate many MMPs expression in breast cancer cell lines (34). We tested whether PKD1 also regulate MMPs expression in Ras OIS. PKD1 overexpression decreased MMP2 and MMP9 expression, whereas PKD1 inhibition increased MMP2 and MMP9 levels, compared with control cells (Fig. S3C). Thus, PKD1 negatively regulates MMPs expression in Ras OIS. To our knowledge, this is the first report that single gene PKD1 can both positively and negatively regulate different components of SASP.
PKD1 Regulates IL-6/IL-8 at mRNA Level and Depends on NF-κB Activity in Ras OIS.
IL-6/IL-8 are mainly up-regulated at mRNA level in Ras OIS (11, 12). To elucidate the mechanism by which PKD1 regulates IL-6/IL-8 expression, we used quantitative RT-PCR to determine mRNA levels of IL-6/IL-8. LPC-PKD1 significantly increased mRNA abundance of IL-6/IL-8 in Ras OIS, and PKD1-CA further elevated IL-6/IL-8 mRNA levels, compared with control cells. In contrast, PKD1-DN or PKD1 deletion markedly decreased IL-6/IL-8 mRNA (Fig. 3A and Figs. S2F and S4A).
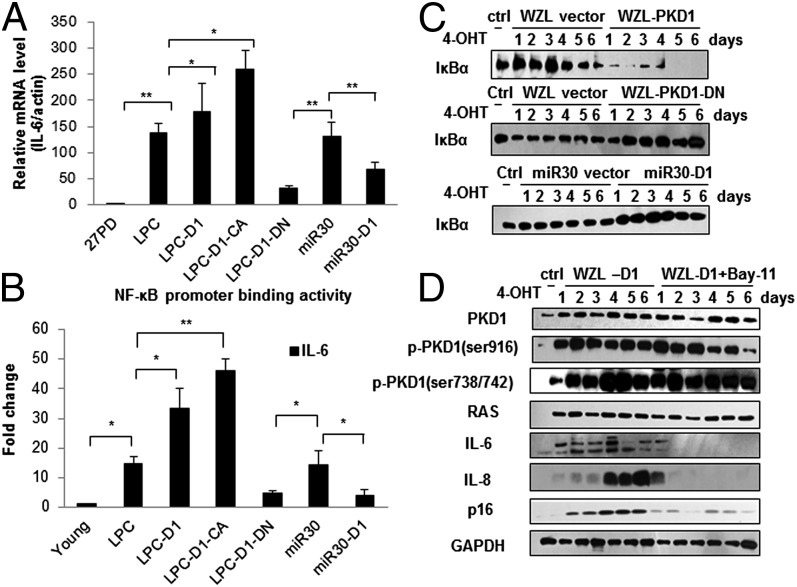
Fig. 3.
PKD1 regulates IL-6/IL-8 at mRNA level and depends on NF-κB activity in Ras OIS. (A) PKD1 regulates IL-6 at mRNA level. Total mRNA were extracted from ER:Ras IMR90 cells expressing indicated genes and shRNAs after 6 d Ras induction. Relative mRNA level of IL-6 was determined by quantitative PCR. mRNA levels in young IMR90 cells are considered as control. (B) PKD1 affects NF-κB binding activity to the promoter of IL-6 gene. ER:Ras IMR90 cells expressing indicated genes and shRNAs were given 4-OHT for 6 d, and then the lysates were analyzed by ChIP assay using an antibody against NF-κB. NF-κB binding to the IL-6 promoters in the indicated stable transfectants is represented relative to mouse IgG binding and is normalized to young IMR90 cells binding for each other. Error bars represent means + SD (n = 3) *P < 0.05, **P < 0.01 in A and B. (C) PKD1 affects IκBα degradation. ER:Ras IMR90 cells expressing WZL-PKD1, WZL-PKD1-DN, and miR30-PKD1, respectively, were given 4-OHT for the indicated times, and then subjected to Western blot to detect IκBα protein levels. (D) NF-κB inhibition abolishes PKD1-mediated IL-6/IL-8 expression. ER:Ras IMR90 cells expressing WZL-PKD1 were treated with solvent DMSO or IκB kinases inhibitor BAY 11-7082 (5 μM) in the presence of 4-OHT for the indicated days, then analyzed for expression of indicated proteins.
It is well known that IL-6/IL-8 induction mainly relies on the NF-κB activity in Ras OIS (11, 12). Because of precedent linking PKD1 to the activation of NF-κB under oxidative stress (25, 26), we asked whether PKD1 regulates IL-6/IL-8 expression via modulation of NF-κB activity in Ras OIS. Therefore, the binding activity of NF-κB to the promoter of IL-6 was detected by chromatin immunoprecipitation (ChIP). As shown in Fig. 3B, NF-κB promoter binding activity was dramatically increased in Ras OIS compared with young cells, which was in agreement with previous results (11, 12). The binding activity increased about twofold in PKD1 expressing cells compared with control cells. PKD1-CA further enhanced the binding activity. Conversely, PKD1-DN or PKD1 silencing markedly decreased the binding activity (Fig. 3B and Fig. S2G).
When activated under oxidative stress, PKD1 in turn phosphorylated a cytoplasmic IKKα-IKKβ-nemo complex, causing IκBα degradation and NF-κB nuclear translocation (25, 26). Indeed, we observed that IκBα gradually degradated to a very low level at day 5–6, indicating that NF-κB was increasingly activated (Fig. 3C). PKD1 overexpression greatly promoted IκBα degradation, whereas PKD1-DN and PKD1 depletion inhibited IκBα degradation. Thus, PKD1 regulates NF-κB activity by promoting IκBα degradation in Ras OIS. However, PKD1 does not directly phosphorylate the IκB kinase (IKK) complex due to the absence of direct interaction between PKD1 and IKK detected by coimmunoprecipitation assay (Fig. S4B).
Finally, we used a pharmacological method to confirm whether PKD1 depends on NF-κB activity to regulate IL-6/IL-8. BAY 11-7082, an inhibitor of IκB kinases, blunted IL-6/IL-8 induction in Ras OIS (Fig. S4C), which agreed with published data (11). In PKD1-expressed cells, treatment with BAY 11-7082 almost completely abrogated PKD1 ability to promote IL-6/IL-8 induction (Fig. 3D). Based on this evidence, we conclude that PKD1 relies on NF-κB activity to mediate IL-6/IL-8 expression in Ras OIS.
ROS-PKCδ Is Upstream Signaling to Activate PKD1 in Ras OIS.
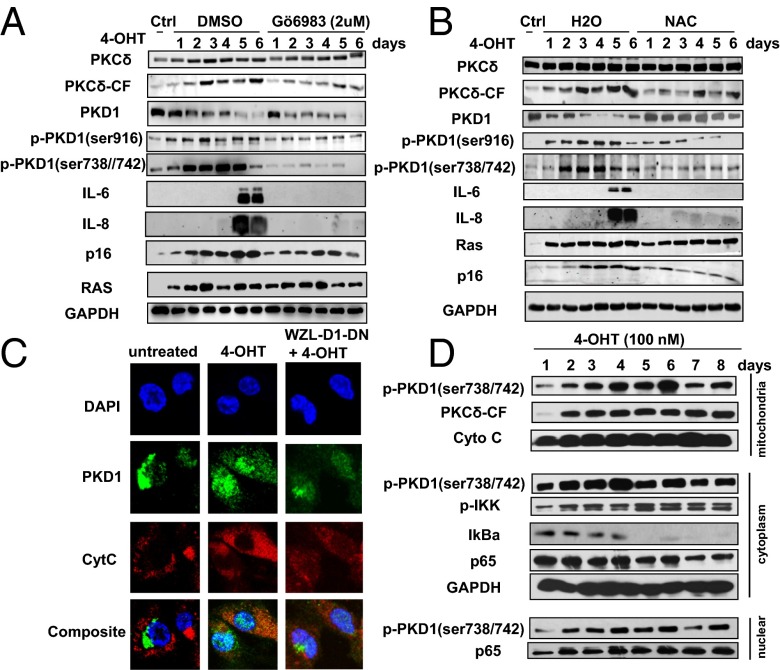
To test whether PKD1 is activated by ROS-PKCδ loop in Ras OIS (6), two PKCδ inhibitors, Gö6983 and Ro 31-8425, were used to treat IMR90 cells. Both treatments significantly reduced the levels of catalytically active fragment of PKCδ (PKCδ-CF), which was shown to be increased during Ras OIS in a previous report (6) and in our results (Fig. 4A and Fig. S5A). PKCδ inhibition greatly abolished PKD1 activity and PKD1-mediated IL-6/IL-8 expression. Next, we used ROS scavenger N-acetyl-cysteine (NAC) to inhibit ROS production. ROS elimination significantly reduced PKCδ-CF levels and markedly inhibited PKD1 activity and IL-6/IL-8 induction (Fig. 4B). The effects of ROS-PKCδ intervention on IL-6/IL-8 induction were further confirmed by ELISA results (Fig. S5B), mRNA levels of IL-6 (Fig. S5C), and NF-κB binding activity to IL-6 promoter (Fig. S5D). As a positive control, p38 MAPK inhibitor SB203580 reduced secreted level and mRNA level of IL-6, as well as NF-κB promoter binding activity, which was consistent with published data (17). Similar results were obtained by using another ROS scavenger, EU.K.-134 (Fig. S6 A–E). Altogether, these results indicate that PKD1 is activated by upstream ROS-PKCδ signaling and acts as a downstream effector of ROS-PKCδ to regulate SASP in Ras OIS.
Fig. 4.
ROS-PKCδ is upstream signaling to activate PKD1 in Ras OIS. (A) PKCδ inhibition blocks PKD1 activation and IL-6/IL-8 induction. ER:Ras IMR90 cells were given 4-OHT in the presence of solvent DMSO or Gö6983 (2 μM) for the indicated times; fresh medium with 4-OHT and Gö6983 was changed every other day. Cell lysates were then subjected to Western blot analysis for the indicated proteins. (B) Removal of ROS inhibits PKCδ-PKD1 activation and blocks IL-6/IL-8 induction. ER:Ras IMR90 cells treated with solvent or NAC (10 mM) for indicated times were subjected to Western blot analysis. (C) PKD1 translocates to the mitochondria. ER:Ras IMR90 cells and PKD1-DN–infected cells were seeded on glass coverslips and cultured with or without 4-OHT for 4 d, and then stained with phospho-PKD1 (ser738/742), green; anti-cytochrome c, red; and nuclei, DAPI, blue. (D) The coincidence of the kinetics of PKD1 activation, IKK complex phosphorylation, IκBα degradation, and p65 translocation to nucleus after Ras induction. ER:Ras IMR90 cells were cultured with 4-OHT and collected in the mitochondrial isolation buffer at indicated time; then, mitochondrial, cytoplasmic, and nuclear fractions were isolated and subjected to Western blot for the indicated proteins.
Mitochondria are an important source of ROS induced by Ras (4) and PKD1 has been shown to translocate to mitochondria under oxidative stress (26). These data led us to evaluate whether the induction of ROS by Ras promotes the translocation of PKD1 to the mitochondria. Immunofluorescence results revealed that PKD1 was primarily localized in a perinuclear region in young growing ER:Ras IMR90 cells (Fig. 4C), as in a previous report (26). However, in Ras-induction cells, PKD1 partially colocalized with the mitochondria, as judged by the overlap in staining with the mitochondrial marker cytochrome c. In contrast, no colocalization between mitochondria and PKD1-DN was observed under Ras induction due to lack of response to upstream ROS-PKCδ signal (Fig. 4C).
We further determined the kinetics of PKD1 activation, IKK phosphorylation, IκBα degradation, as well as p65 translocation upon Ras induction by isolation of mitochondria, cytoplasmic, and nuclear organelles. In the time-course assay, we found phosphorylated PKD1 gradually enriched to the mitochondria, peaking at day 4 to day 6 (Fig. 4D). PKCδ-CF was also recruited to mitochondria. Cytochrome c signal proved the purity of the mitochondrial preparations. Phospho-PKD1 in the cytoplasmic fraction also reached to peak level at day 4, which was concomitant with the increasingly elevated IKK phosphorylation, peaking at day 5. Accordingly, IκBα gradually degraded to very low level at day 5. p65 RelA also gradually decreased in cytoplasmic fraction (Fig. 4D). Meanwhile, we observed that p65 RelA gradually translocated to the nuclear fraction. We also detected the localization of p-PKD1 to nuclear fraction, which was similar to imaging results (Fig. 4D). p65 RelA translocated to the nucleus after Ras induction was confirmed by immunofluorescence imaging (Fig. S5E). These results suggest that the activation and translocation of PKD1 to the mitochondria relay the ROS-PKCδ signal to phosphorylate cytoplasmic IKK, cause IκBα degradation and subsequent NF-κB translocation to the nucleus, and ultimately result in IL-6/IL-8 induction.
ROS-PKCδ-PKD1 Signaling Is Required for Initiation and Maintenance of IL-6/IL-8 Expression and Secretion.
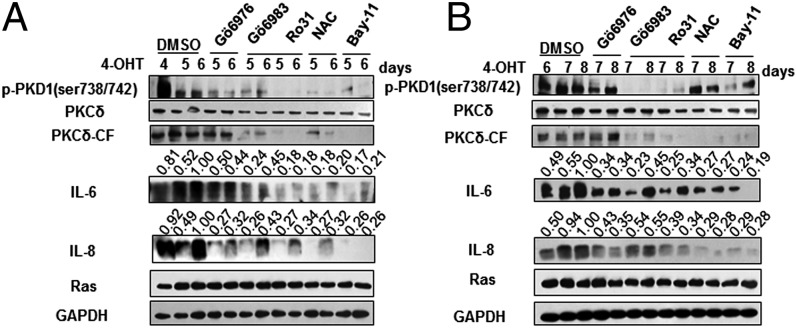
To further analyze the role of ROS-PKCδ-PKD1 axis in initiation and maintenance of IL-6/IL-8 expression in Ras OIS, we sought to inhibit this pathway at different time points after Ras induction. First, we investigated whether interruption of this pathway at day 4 could suppress the initial production of IL-6/IL-8. After 2 d of treatment, perturbation of ROS and PKCδ suppressed not only PKCδ-CF but also PKD1 activity (Fig. 5A). However, Gö6976 only blocked PKD1 activation and had no effect on PKCδ-CF. Importantly, all interventions greatly reduced IL-6/IL-8 induction compared with untreated cells (Fig. 5A). As a positive control, NF-κB inhibitor BAY 11-7082 suppressed IL-6/IL-8 expression. The same results were achieved by using EU.K.-134 (Fig. S6F). This result suggests that ROS-PKCδ-PKD1 signaling is at least partially required for initial production of IL-6/IL-8 in Ras OIS.
Fig. 5.
ROS-PKCδ-PKD1 signaling is required for the initiation and maintenance of IL-6/IL-8 induction. (A) Early interruption of this axis suppresses the initial induction of IL-6/IL-8. ER:Ras IMR90 cells were induced to express Ras for 4 d, then indicated inhibitors were added for another 1 or 2 d in the presence of 4-OHT. The indicated proteins were analyzed by Western blot. (B) Late intervention of this pathway inhibits the sustained production of IL-6/IL-8. ER:Ras IMR90 cells were cultured with 4-OHT for 6 d, then indicated inhibitors were added for another 1 or 2 d in the presence of 4-OHT. The indicated proteins were subjected to Western blot analysis. BAY 11-7082–treated cells serve as positive control. The densitometry data were analyzed by ImageJ software and normalized to the highest signal in the corresponding row.
Next, we examined whether intervention of ROS-PKCδ-PKD1 pathway at day 6 could block sustained production of IL-6/IL-8. Indeed, inhibition of this cascade greatly abolished IL-6/IL-8 expression compared with untreated cells (Fig. 5B and Fig. S6F). Taken together, these results support that ROS-PKCδ-PKD1 signaling is required for initiation and maintenance of IL-6/IL-8 induction in Ras OIS.
We further extended this study to replicative senescent 2BS cells to verify whether the ROS-PKCδ-PKD1 axis also plays an important role in IL-6/IL-8 induction in replicative senescence. The inhibition of this signaling not only prohibited PKCδ and PKD1 activation, but also decreased IL-6/IL-8 secretion compared with untreated senescent cells (Fig. S7A). PKD1 and PKD1-CA dramatically increased IL-6/IL-8 secretion in senescent 2BS cells, whereas PKD1 deletion decreased IL-6/IL-8 secretion (Fig. S7B). Therefore, these results indicate that the ROS-PKCδ-PKD1 pathway also exists in replicative senescence and is required for IL-6/IL-8 expression.
PKD1 Deficiency Promotes Cell Transformation and Tumorigenesis.
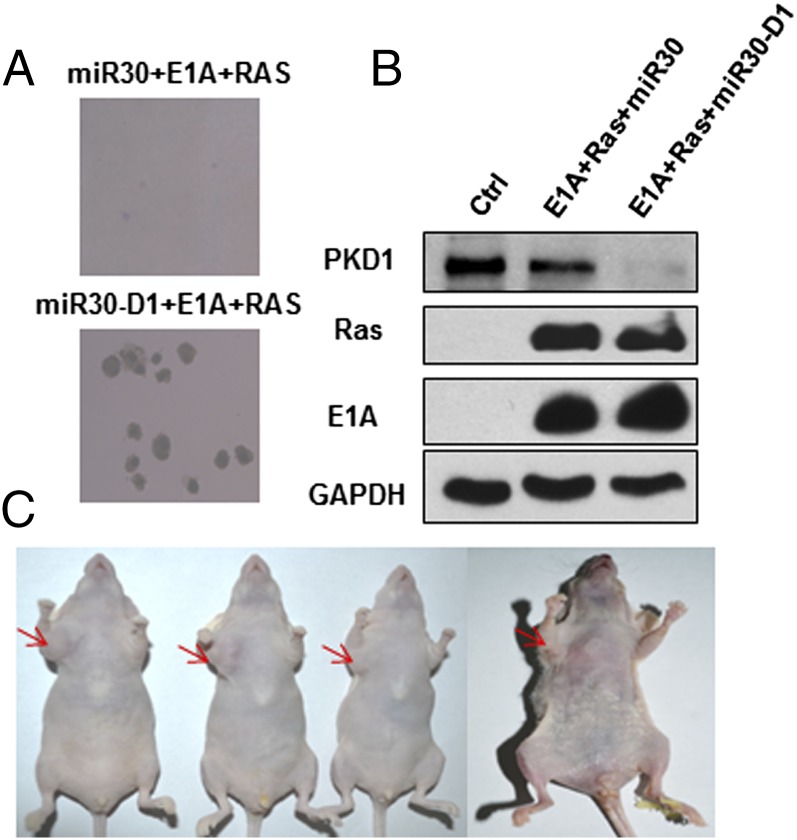
We further tested whether bypass of Ras OIS caused by PKD1 deficiency would permit cell transformation in IMR90 cells. Primary human fibroblasts can be efficiently transformed by a combination of Ras, E1A, and MDM2 (35). We tested whether PKD1 depletion could functionally replace MDM2 expression. IMR90 cells infected with Ras, E1A, and miR30-PKD1 or miR30-PKD1#2 formed robust anchorage-independent growth colonies in soft agar, whereas Ras, E1A, and miR30 empty vector-infected cells only formed rare and small colonies in soft agar, which agreed with previous data (35) (Fig. 6A and Fig. S2H). The coexpression of Ras and E1A, as well as PKD1 knockdown, was confirmed by Western blot (Fig. 6B). When injected s.c. in immunodeficient nude mice, coexpression of Ras, E1A, and miR30-PKD1 IMR90 cells grew large tumors within 5 wk (three of four mice grew large tumors, and one mouse grew relatively small tumor), whereas no tumor was observed (0 out of 4) in the opposite flank in the same mice injected with Ras, E1A, and miR30 empty vector coexpressing cells (Fig. 6C), which was in agreement with previous finding that Ras and E1A coexpressing human fibroblasts had no ability to develop tumors in nude mice (35). Additional injected animals and the representative kinetics of tumor growth are shown in Fig. S8. Therefore, PKD1 knockdown functionally replaces MDM2 and cooperates with Ras and E1A to transform normal human fibroblasts.
Fig. 6.
PKD1 deficiency promotes cell transformation and tumorigenesis. (A) Normal IMR90 cells were transduced with Ras, E1A, and miR30 vector or miR30-D1. The two stable cell lines grew in soft agar as described. (B) Levels of PKD1, Ras, and E1A in the above two cell lines were determined by Western blot. (C) The transformation cells (miR30-D1+Ras+E1A) can form tumors in nude mice. About 5 × 106 cells were collected, and resuspended in 0.1 mL of PBS, and then injected into mice. Control and transformed cells were injected to the left and right forelimb armpit, respectively. Tumor development was photographed at 5 wk after injection.
Discussion
ROS play critial role in Ras OIS (4). Several ROS-generating positive feedback loops were proposed to contribute to elevated ROS and mediate Ras OIS (6–8). The relationships between ROS and p53, p16, and DDR pathways were the main focus in these studies. Senescent cells also develop SASP. However, the potential link between ROS and SASP has not yet been elucidated. In this study, we identified PKD1 as a downstream effector of ROS signaling to regulate IL-6/IL-8 induction via modulation of NF-κB activity. Thus, we deciphered a previously unidentified connection between ROS and SASP, to our knowledge for the first time.
ROS levels increase gradually and reach to peak at senescence phase and remain at high level for a long interval (4, 6–8). The kinetics of PKD1 activation after Ras induction is slow and chronic (Fig. 1A), coincident with the rise of ROS levels. The SASP also takes several days to develop. We demonstrate that the kinetics of PKD1 activation coincide with its translocation to mitochondria, IKK phosphorylation, and subsequent IκBα degradation as well as p65 translocation to nucleus, and ultimately IL-6/IL-8 induction (Fig. 4D). However, PKD1 does not directly phosphorylate the IKK complex (Fig. S4B). The mechanisms through which PKD1 induces IKK complex phosphorylation remain to be defined in Ras OIS.
OIS is proved to be a powerful antitumor mechanism (2). ROS signaling plays pivotal role in both OIS (4–8) and other stress-induced premature senescence such as irradiation (36). Elimination of ROS results in cells escaping cell senescence or reentering cell cycle. Therefore, in contrast to the general view of ROS causing cancer (37), the indispensable role of ROS in establishing and maintaining cell senescence program suggests that ROS may have tumor suppressive function in a certain physiological context. IL-6/IL-8 are critical for the establishment of cell senescence, the disruption of which leads to the escape of Ras OIS (11, 12). SASP factors also can stimulate immune system to clear premalignant senescent cells through so called “senescence surveillance” mechanism (13). We show here that ROS-PKCδ-PKD1 signaling is required for the induction of IL-6/IL-8 in Ras OIS. Early intervention of this pathway abolished IL-6/IL-8 induction and led to bypass Ras OIS (Figs. 2 B–D and 4 A and B and Figs. S2, S3A, and S6). Most importantly, we displayed that PKD1 depletion promoted cell transformation and tumorigenesis (Fig. 6 A and C and Fig. S2H). Thus, our data confirm the undisputed importance of ROS signaling in antitumor function. We speculate that elevated ROS in senescent cells may use multiple mechanisms to exert its anti-cancer effect, including cell cycle arrest, cytokinesis blockage, SASP, and senescence surveillance, etc.
PKD1 has controversial roles in cancer development. It has been reported that PKD1 could promote pancreatic cancer development (38). In contrast, some studies suggested PKD1 might have anticancer function in androgen-independent prostate cancer (39), breast cancer (34), and gastric cancer (40). Moreover, the epigenetic silencing of PKD1 promoter region was found in both gastric cancer (41) and breast cancer (34). These findings suggested that PKD1 could potentially act as a tumor suppressor at early stage of tumor development in certain types of cancers. In this study, we show that PKD1 can enhance Ras OIS by promoting IL-6/IL-8 induction, thus preventing cancer occurrence through induction of cell senescence program. Accordingly, PKD1 silencing leads to bypass Ras OIS, and promotes cell transformation and tumorigenesis (Fig. 6 A and C and Fig. S2H). Meanwhile, we also reveal that PKD1 can negatively regulate MMPs in Ras OIS (Fig. S3C), which may limit the ability of SASP to alter tissue microenvironment to promote cancer metastasis in late life. Thus, our data provide evidence to support that PKD1 could potentially act as a tumor suppressor to prevent cancer development at an early stage in the context of oncogenic Ras activation.
Chronic inflammation is a hallmark of aging and is implicated in many age-related diseases (9). Senescent cells accumulate with age in many tissues and are present at sites of age-related pathology (42). SASP is proposed to have important roles in senescence-associated inflammation, systemic aging, and age-related diseases (15). Thus, cellular senescence and the associated SASP as the well established tumor suppressive mechanism in early life might promote aging and age-related diseases in late-life. ROS levels increase with aging in various mammals, including mice and humans (43). Similar to SASP, elevated ROS signaling, which represents as an anticancer mechanism by establishing and maintaining cell senescence in early life, may drive aging and age-related diseases in late life, which is the so called “free radical theory of aging.” In this study, we elucidate the link between ROS-PKCδ-PKD1 signaling and senescence-associated inflammation (Figs. 4 and 5 and Figs. S6 and S7). Our findings suggest the possibility to mitigate the deleterious effects of SASP by specifically inhibiting this pathway in late life. It is also worthwhile to investigate whether intervention of this cascade could delay and reduce the onset of age-related diseases and extend lifespan in the future.
In summary, we report here that PKD1 promotes Ras OIS by up-regulation of NF-κB activity and subsequent IL-6/IL-8 induction. ROS-PKCδ-PKD1 axis is essential for initiation and maintenance of IL-6/IL-8. Ablation of PKD1 leads to bypass Ras OIS and promotes cell transformation and tumorigenesis.
Materials and Methods
Antibodies, reagents, and cell lines used in this study are described in SI Materials and Methods. All experiments were processed according to the standard protocols. Plasmids, viral infection, real-time PCR, ChIP, immunoblot analysis, mitochondrial isolation, SA-β-gal staining, cell cycle analysis, ELISA, ROS measurement, soft agar assay, tumorigenic assay, etc., are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Yun Wang for plasmids and Dr. Masashi Narita for plasmids and cell lines. This work was supported by National Key Basic Research Program of China Grants 2013CB530801 and 2014CB910503 and National Natural Science Foundation of China Grant 81370455.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310972111/-/DCSupplemental.
References
- 1.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collado M, Serrano M. Senescence in tumours: Evidence from mice and humans. Nat Rev Cancer. 2010;10(1):51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 4.Lee AC, et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem. 1999;274(12):7936–7940. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 5.Wu C, Miloslavskaya I, Demontis S, Maestro R, Galaktionov K. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature. 2004;432(7017):640–645. doi: 10.1038/nature03173. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi A, et al. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol. 2006;8(11):1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 7.Moiseeva O, Bourdeau V, Roux A, Deschênes-Simard X, Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol Cell Biol. 2009;29(16):4495–4507. doi: 10.1128/MCB.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy N, et al. DDB2, an essential mediator of premature senescence. Mol Cell Biol. 2010;30(11):2681–2692. doi: 10.1128/MCB.01480-09. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol Med. 2010;16(5):238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133(6):1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479(7374):547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 14.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30(8):1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009;106(40):17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhaumik D, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany, NY Online) 2009;1(4):402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011;13(3):254–262. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 21.Franceschi C, et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 23.Fu Y, Rubin CS. Protein kinase D: Coupling extracellular stimuli to the regulation of cell physiology. EMBO Rep. 2011;12(8):785–796. doi: 10.1038/embor.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozengurt E. Protein kinase D signaling: Multiple biological functions in health and disease. Physiology (Bethesda) 2011;26(1):23–33. doi: 10.1152/physiol.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003;22(1):109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storz P, Döppler H, Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol. 2005;25(19):8520–8530. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young AR, et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23(7):798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J. 1996;15(22):6220–6230. [PMC free article] [PubMed] [Google Scholar]
- 29.Waldron RT, Rozengurt E. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J Biol Chem. 2003;278(1):154–163. doi: 10.1074/jbc.M208075200. [DOI] [PubMed] [Google Scholar]
- 30.Storz P, Döppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol. 2004;24(7):2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gschwendt M, et al. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392(2):77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- 32.Lin AW, et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12(19):3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, et al. The RAS effector RIN1 directly competes with RAF and is regulated by 14-3-3 proteins. Mol Cell Biol. 2002;22(3):916–926. doi: 10.1128/MCB.22.3.916-926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eiseler T, Döppler H, Yan IK, Goodison S, Storz P. Protein kinase D1 regulates matrix metalloproteinase expression and inhibits breast cancer cell invasion. Breast Cancer Res. 2009;11(1):R13. doi: 10.1186/bcr2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seger YR, et al. Transformation of normal human cells in the absence of telomerase activation. Cancer Cell. 2002;2(5):401–413. doi: 10.1016/s1535-6108(02)00183-6. [DOI] [PubMed] [Google Scholar]
- 36.Passos JF, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15(2):247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 38.Guha S, Tanasanvimon S, Sinnett-Smith J, Rozengurt E. Role of protein kinase D signaling in pancreatic cancer. Biochem Pharmacol. 2010;80(12):1946–1954. doi: 10.1016/j.bcp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaValle CR, et al. Protein kinase D as a potential new target for cancer therapy. Biochim Biophys Acta. 2010;1806(2):183–192. doi: 10.1016/j.bbcan.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shabelnik MY, et al. Differential expression of PKD1 and PKD2 in gastric cancer and analysis of PKD1 and PKD2 function in the model system. Exp Oncol. 2011;33(4):206–211. [PubMed] [Google Scholar]
- 41.Kim M, et al. Epigenetic inactivation of protein kinase D1 in gastric cancer and its role in gastric cancer cell migration and invasion. Carcinogenesis. 2008;29(3):629–637. doi: 10.1093/carcin/bgm291. [DOI] [PubMed] [Google Scholar]
- 42.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of ageing—where do we stand? Front Biosci. 2008;13:6554–6579. doi: 10.2741/3174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.