Significance
Autoimmune diseases are driven by immune cells that recognize self-tissues. A major goal for treatment strategies for autoimmune diseases is to turn off or tolerize self-reactive immune cells such as CD4 T cells that coordinate tissue damage in many autoimmune diseases. Autoimmune diseases are often diagnosed many years following their onset. The self-reactive CD4 T cells that must be tolerized, therefore, are previously activated or memory CD4 T cells. Little is known about whether tolerance can be induced in memory CD4 T cells. This paper demonstrates that memory CD4 T cells survive initial exposure to tolerance-inducing signals but that a second activation signal leads to cell death. This study has important implications for immunotherapeutic strategies for autoimmune diseases.
Abstract
A major goal for immunotherapy is to tolerize the immune cells that coordinate tissue damage in autoimmune and alloantigen responses. CD4 T cells play a central role in many of these conditions and improved antigen-specific regulation or removal of these cells could revolutionize current treatments. A confounding factor is that little is known about whether and how tolerance is induced in memory CD4 T cells. We used MHC class II tetramers to track and analyze a population of endogenous antigen-specific memory CD4 T cells exposed to soluble peptide in the absence of adjuvant. We found that such memory T cells proliferated and reentered the memory pool apparently unperturbed by the incomplete activation signals provided by the peptide. Upon further restimulation in vivo, CD4 memory T cells that had been previously exposed to peptide proliferated, provided help to primary responding B cells, and migrated to inflamed sites. However, these reactivated memory cells failed to survive. The reduction in T-cell number was marked by low expression of the antiapoptotic molecule B cell lymphoma 2 (Bcl2) and increased expression of activated caspase molecules. Consequently, these cells failed to sustain a delayed-type hypersensitivity response. Moreover, following two separate exposures to soluble antigen, no T-cell recall response and no helper activity for B cells could be detected. These results suggest that the induction of tolerance in memory CD4 T cells is possible but that deletion and permanent removal of the antigen-specific T cells requires reactivation following exposure to the tolerogenic antigen.
Immune memory is an important characteristic of the adaptive immune response with memory cells responding quickly and effectively upon reexposure to a pathogen (1–3). A number of differences between naive and memory cells contribute to this enhanced response. These include an increased sensitivity to the antigen, an enhanced effector response, and an altered location such that memory cells are positioned to act most effectively before reinfection (4, 5).
Memory cells can, however, also respond to nonpathogenic antigens such as autoantigens and alloantigens. In these instances, the superior responses of memory cells harm, rather than benefit, the host. A major goal in the treatment of autoimmune disease and in the prevention of transplant rejection is to remove or tolerize immune cells that specifically recognize auto- or alloreactive antigens, respectively (6, 7).
CD4 T cells are central coordinators of specific immune responses and as such play destructive roles in autoimmune diseases and in the rejection of allografts (8, 9). Whereas the signals involved in, and the molecular basis of, tolerance induction in naive T cells has been extensively characterized (10, 11), much less is known about how, or even whether, functional tolerance can be achieved in memory CD4 T cells. In comparison with naive T cells, memory T cells are much less dependent on costimulatory signals and are resistant to the induction of tolerance by molecules that block costimulatory pathways (12–14). However, memory T cells do not always act independently of costimulatory signals, suggesting that they may function abnormally if reactivated by antigen delivered in the absence of these signals (15, 16).
Soluble antigens are known to be good tolerogens for naive T cells (17). Moreover activation of effector or memory CD4 T cells with soluble antigen can induce reduced cytokine production, ex vivo proliferation, and the ability to cause inflammatory disease (18–20). However, there is no clear understanding of the fate of memory CD4 T cells activated with tolerizing signals and little evidence to explain why their effector responses may be reduced.
We therefore tested the effect of soluble antigen on memory T cells in vivo using fluorescent MHC class II tetramer reagents to track endogenous antigen-specific CD4 T cells and in vivo readouts of T-cell function. We found that CD4 memory T cells responded similarly to antigen delivered with or without adjuvant. However, the ability of memory CD4 T cells to survive further in vivo activation was severely limited following exposure to tolerizing antigen. Our data demonstrate that tolerance induction in memory CD4 T cells requires exposure to antigen at least twice, information that is highly relevant for clinical studies that aim to induce tolerance in memory CD4 T cells.
Results
Memory CD4 T Cells Proliferate and Up-Regulate Activation Markers in Response to Soluble Antigen and Then Reenter the Memory Pool.
To determine the consequences of activating memory CD4 T cells with antigen in vivo in the absence of overt costimulation, we first needed to generate a population of antigen-specific memory CD4 T cells that could be tracked. Memory cells were generated by immunizing C57BL/6 (B6) mice with the protein IAb restricted peptide, 3K, delivered with lipopolysaccharide (LPS). Mice immunized in this way are hereinafter referred to as memory mice. We chose this form of immunization as it allowed us to track an antigen-specific response using fluorescently labeled IAb/3K tetramers (Fig. 1). Our previous studies showed that such memory cells respond in a typical anamnestic fashion: in comparison with primary responding T cells, they reach their peak proliferative response faster, make more effector cytokines, and provide help to B cells more rapidly (21, 22).
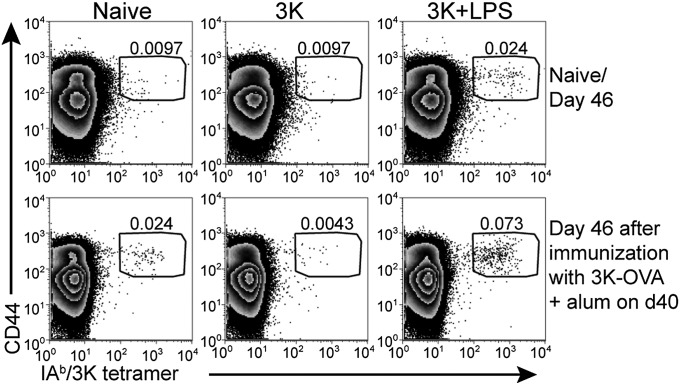
Fig. 1.
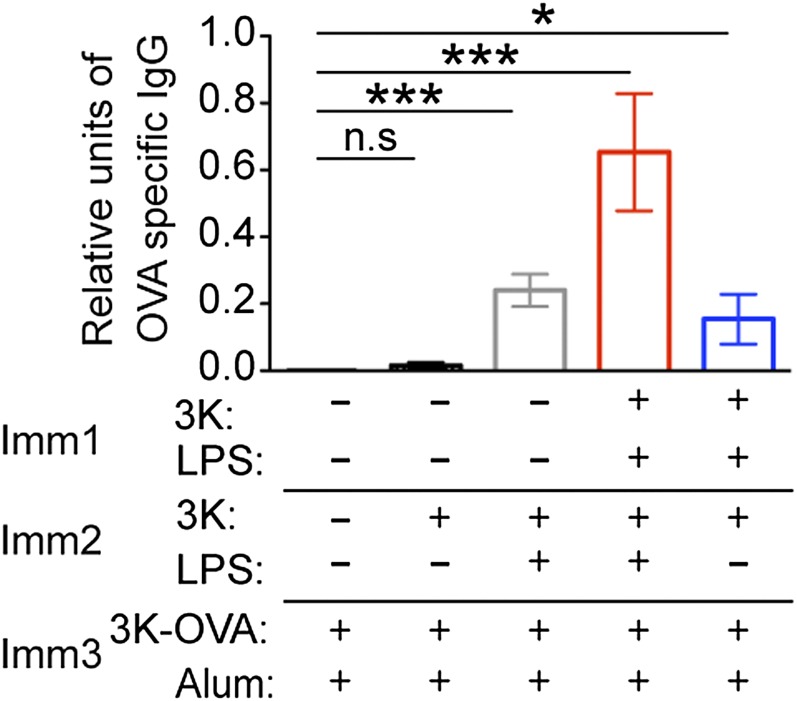
Immunization with 3K peptide and LPS generates memory CD4 T cells but injection of 3K into naive animals leads to antigen-specific T-cell tolerance. Naive B6 mice were injected with 3K peptide or 3K peptide and LPS i.v. and the percentages of live CD4+ dump negative cells that were IAb/3K tetramer+ CD44hi in the spleen examined 46 d later (Upper row). Alternatively, 40 d following immunization, these mice and naive controls were immunized with 3K-OVA delivered with alum i.p. and the percentages of IAb/3K tetramer+ CD44hi out of live CD4+ dump negative cells examined in the spleen after a further 6 d (Lower row). The data are representative plots from two experiments with four mice per group. The cells are gated on CD4+ live lymphocytes that do not express CD8, F4/80, MHC II, and B220. The numbers indicate the percentages of gated CD4 T cells that are CD44hi IAb/3K tetramer+.
To examine the response of memory CD4 T cells to antigen delivered in the absence of exogenous adjuvant, we injected 3K peptide i.v. alone into either control naive animals or memory mice. Injection of 3K into naive mice did not increase the numbers of IAb/3K-specific CD4 T cells measured 46 d later (Fig. 1). Thus, the soluble peptide could not induce a sustained immune response. Following immunization with 3K conjugated to ovalbumin protein (3K-OVA) and delivered with the adjuvant alum, the specific T cells in naive mice, or in mice previously immunized with 3K + LPS expanded but no 3K-specific CD4 T cells could be detected in mice previously given 3K peptide alone (Fig. 1 and Fig. S1). These data indicate that any 3K-specific T cells present in animals injected with peptide alone were either unable to respond to antigen or had been deleted. Therefore, the soluble peptide was tolerogenic.
In contrast to the response of naive CD4 T cells, we found that memory CD4 T cells increased in number following the injection of soluble peptide (Fig. 2A and Fig. S2A). Moreover, these reactivated memory cells reentered the memory pool and declined at a rate similar to that of memory CD4 T cells reactivated with 3K and LPS. However, reduced numbers of IAb/3K tetramer+ cells were found in memory mice injected with 3K peptide alone compared with those reactivated with antigen and adjuvant.
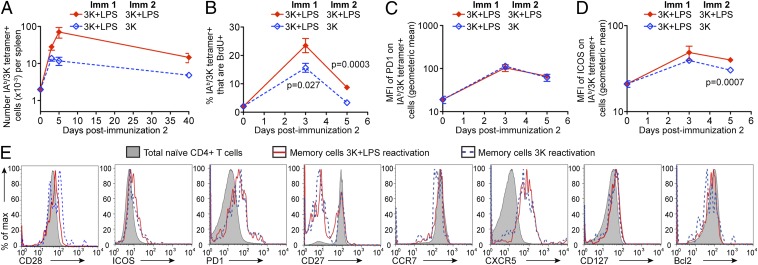
Fig. 2.
Memory cells are activated and reenter the memory pool following exposure to antigen in the absence of adjuvant. B6 mice immunized (Imm) with 3K peptide and LPS i.v 40 d previously (Imm1) were injected with 3K + LPS or 3K without adjuvant (Imm2). The numbers of IAb/3K tetramer+ CD44hi cells in the spleens of these mice were examined 3, 5, and 40 d after Imm2 (A). Some of these animals were injected with BrdU 15 h before the percentages of CD44hi IAb/3K tetramer+ BrdU+ were examined on days 3 and 5 after Imm2 (B). The expression of PD1 (C) or ICOS (D) on the CD44hi IAb/3K tetramer+ was examined before and 3 or 5 d after Imm2. The phenotype of the IAb/3K tetramer+ memory cells was examined at 40 d after Imm2 (E). In A–D, each point shows the mean of the group and error bars are SEM. The data are combined from two individual experiments with a total of six to eight mice per group per time point. In A, the x axis is set at the limit of detection as determined by staining spleen cells from naive animals. In E, the cells are gated on IAb/3K tetramer+ cells reactivated with 3K and LPS (red line) or 3K alone (blue dashed line) or total naive CD4 cells (filled histogram). The data are representative FACS plots from two experiments with three to four mice per group.
We examined the ability of the reactivated memory cells to enter the cell cycle to determine whether this reduction in cell number was due to decreased proliferation. The percentages of IAb/3K tetramer+ cells that were BrdU+ were examined before and 3 or 5 d following reimmunization with 3K peptide injected with or without LPS. Memory cells reactivated with peptide alone were less likely than those reactivated with peptide and LPS to be BrdU+ 3 and 5 d after the recall (Fig. 2B and Fig. S2B). This demonstrates that, as expected, LPS acts as an adjuvant to drive T-cell proliferation (23). Despite their reduced proliferative response, memory cells reactivated with peptide alone up-regulated markers of activation, including programmed cell death 1 (PD1) and inducible T-cell costimulator (ICOS) (Fig. 2 C and D). ICOS expression was slightly less well sustained on IAb/3K tetramer+ cells from mice injected with peptide alone.
To determine whether memory cell reactivation with or without adjuvant led to an alteration in the phenotype of the resultant memory cells, we examined the expression of a variety of cell-surface or intracellular molecules. We examined molecules important in cell survival [CD127, B cell lymphoma 2 (Bcl2)], costimulatory and inhibitory molecules (CD28, CD27, ICOS, and PD1) and chemokine receptors (CCR7 and CXCR5). Regardless of the presence of LPS during memory cell reactivation, the expression of these molecules was the same on the memory IAb/3K tetramer+ cells (Fig. 2E and Fig. S2C). These data suggest that despite the slightly reduced T-cell response in memory mice reactivated with peptide alone, cells of a similar phenotype repopulate the memory pool.
Memory Cells Exposed to Soluble Peptide Fail to Induce a Sustained Delayed-Type Hypersensitivity Response.
To find out whether or not the memory CD4 T cells that had been previously exposed to soluble antigen in the absence of adjuvant were functional, we tested their ability to drive a delayed type hypersensitivity (DTH) response, a characteristic of T helper 1 memory CD4 T cells (24). The 3K peptide conjugated to OVA protein was injected into the footpads of previously immunized animals. Mice that had been immunized once or twice with 3K peptide and LPS showed increased and prolonged footpad swelling (Fig. 3A). However, mice primed with 3K and LPS then injected with 3K peptide alone, had a significant increase in footpad swelling only on day 2 after the footpad injection. These data suggested that, whereas CD4 memory T cells exposed to soluble peptide may traffic to the DTH draining lymph node, they might fail to respond completely after they have reached the site.
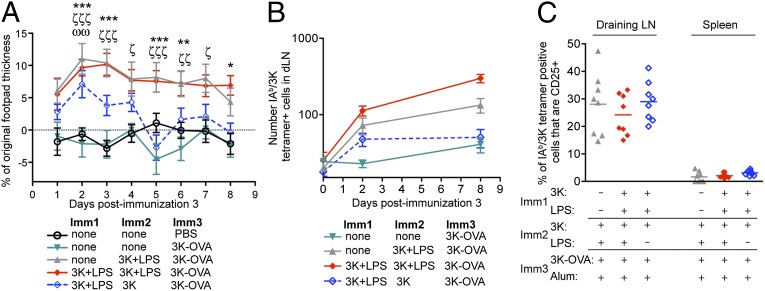
Fig. 3.
Memory CD4 T cells exposed to tolerance signals fail to sustain a DTH response. B6 mice immunized with 3K peptide with/without LPS i.v. as indicated were injected with 3K-OVA in the footpad 40 d after Imm2. The mice’s footpads were measured each day and the percentage change in footpad thickness was calculated (A). The numbers of IAb/3K tetramer+ cells in the popliteal lymph node draining the injected footpad were examined 0, 2, or 8 d after footpad injection (B). The percentages of IAb/3K tetramer+ cells in the spleen or draining lymph node that were CD25+ were examined 2 d following the footpad injection (C). Data are combined from two to three independent experiments with three to four mice per group. In A, the symbols above the graph show significant differences in footpad swelling of the different groups compared with that in naive animals injected with 3K-OVA on the indicated days. Mice immunized once with 3K + LPS: P < 0.05 = ζ; P < 0.01 = ζζ; P < 0.001 = ζζζ. Mice immunized once with 3K + LPS and once with 3K: P < 0.01 = ωω. Mice immunized twice with 3K + LPS: *P < 0.05; **P < 0.01; ***P < 0.001. In A and B, each symbol on the graph represents the mean of the group and error bars are SEM. In B, level of background staining is indicated by the day 0 time point of the primary immunized mice. In C, each symbol represents a mouse and the horizontal line shows the mean of the group.
To distinguish between these possibilities, we measured the numbers of IAb/3K-specific CD4 T cells in the draining popliteal lymph node. The numbers and percentages of IAb/3K tetramer+ cells were found to be above background in the draining lymph nodes of mice that had been immunized once or twice with 3K and LPS and also in memory mice that had been injected with 3K alone 2 d after footpad injection (Fig. 3B and Fig. S3A). There were more memory cells in the draining lymph nodes of mice immunized twice with 3K and LPS than in memory mice that had been injected with 3K alone; however, this probably reflected the difference in the total number of antigen-specific cells in these animals (Fig. S3B).
By day 8, the numbers of IAb/3K tetramer+ cells in the draining lymph node had increased in mice previously immunized once or twice with 3K and LPS. However, the numbers of specific cells remained the same in memory mice injected with 3K (Fig. 3B and Fig. S3A). This result, in combination with the failure of these cells to induce a sustained DTH response, suggested that once recruited to the DTH site, the memory cells were either unable to respond or to survive.
To distinguish between these two possibilities, we examined the surface expression of the high-affinity interleukin-2 receptor, CD25, on the activated T cells. Between 15% and 20% of IAb/3K tetramer+ cells in the draining lymph nodes of memory mice were CD25+ 2 d following footpad injection, regardless of their previous immunization history (Fig. 3C). In contrast, splenic IAb/3K tetramer+ cells from these animals, which had not been exposed to the antigen, did not up-regulate CD25. These data indicate that memory cells exposed to antigen in the absence of adjuvant are activated following migration to inflamed sites.
Memory Cells Exposed to Soluble Peptide Fail to Survive Following Reactivation in Vivo.
The apparent failure of memory cells exposed to soluble peptide to survive reactivation in the footpad may have been confounded by the lack of adjuvant in the final immunization. In addition, this local immunization did not reactivate all of the animal’s memory cells. To address these points, we systemically immunized memory mice that had or had not been exposed to tolerance signals with 3K-OVA delivered with the adjuvant alum. In some of these experiments, we included LPS in the final immunization; however, this did not alter the subsequent T-cell responses.
Memory cells generated by either one or two immunizations with 3K peptide and LPS increased in number following reactivation with 3K-OVA and alum and then entered a normal contraction phase. However, whereas memory CD4 T cells that had been reactivated with soluble peptide alone initially increased in number, the cells entered a rapid decline such that by day 9, their numbers were barely above the limit of detection (Fig. 4A).
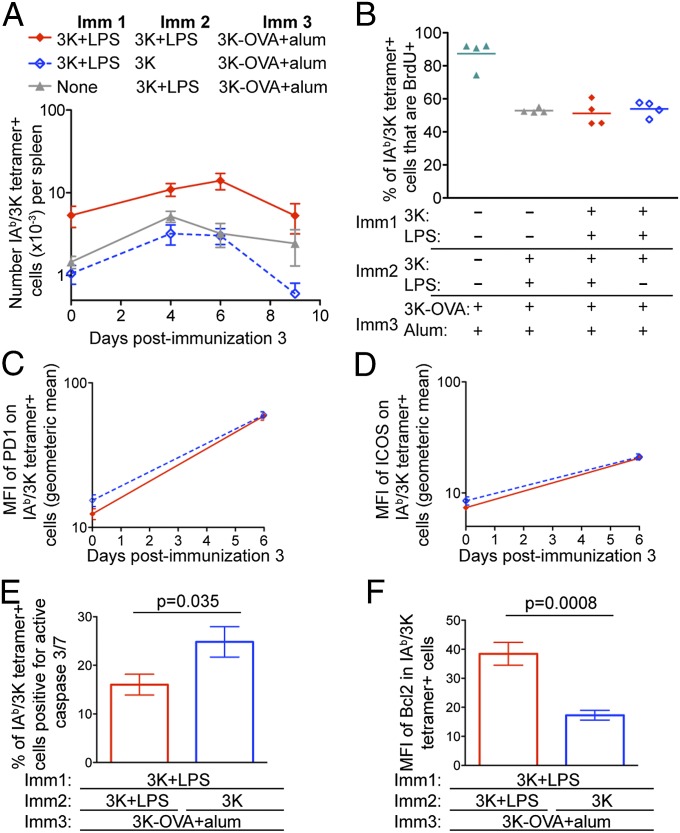
Fig. 4.
Memory cells exposed to tolerance signals respond to further reactivation but fail to accumulate. B6 mice immunized with 3K with/without LPS i.v. as indicated were injected with 3K-OVA and alum i.p. 40 d after Imm2. After an additional 4, 6, or 9 d, the numbers of splenic IAb/3K tetramer+ cells were determined (A). Each point shows the mean of a total of 7–27 mice from seven separate experiments; error bars are SEM. The x axis is set at the limit of detection as determined by staining spleen cells from naive animals. On days 3–6 after Imm3, these mice were given drinking water containing BrdU and the percentages of CD44hi IAb/3K tetramer+ cells that were BrdU+ in each mouse were determined 6 d later (B). Each point represents one mouse and the line shows the mean of the group. Alternatively, the levels of expression of PD1 (C), ICOS (D), active caspase 3 and 7 (E), and Bcl2 (F) were determined in the antigen-specific CD4 T cells. In all experiments, data are representative of one to three independent experiments with three to four mice per group per time point.
The change in cell survival after day 6 was not due to a difference in cell proliferation as all memory cells incorporated similar levels of BrdU at this time point (Fig. 4B). In addition, the memory cells expressed similar levels of the activation molecules PD1 and ICOS at day 6 (Fig. 4 C and D). In contrast, memory cells exposed to antigen in the absence of adjuvant were more likely to contain active caspase 3 and 7 and expressed lower levels of the prosurvival molecule, Bcl2 (Fig. 4 E and F and Fig. S4). Therefore, whereas memory CD4 T cells exposed to antigen in the absence of adjuvant can certainly respond to reactivation, they fail to survive this process.
Memory CD4 T Cells Exposed to Soluble Antigen Provide Accelerated Help to B Cells.
We recently demonstrated that CD4 memory T cells provide accelerated help to primary responding B cells to produce an early class-switched antibody response (22). This ability was contained within a population of memory T cells that expressed CXCR5 at a high level. CXCR5 expression was similar on memory cells that had been reactivated in the absence or presence of adjuvant (Fig. 2E), suggesting that both populations may be able to provide this accelerated help. However, the poor survival of memory cells previously activated with antigen alone might suggest that they would be unable to interact successfully with naive B cells.
We tested whether this was the case by measuring the amount of anti-OVA IgG in the serum of memory mice reimmunized with 3K-OVA delivered with alum. At the early time point, day 5, primary responding animals made at most a small anti–OVA-specific response (Fig. 5). Mice that had been previously exposed once or twice to 3K and LPS produced plenty of anti-OVA, with mice primed twice producing more than those that had been primed only once, probably because they contained more 3K-specific T cells (day 0 in Fig. 4A). Therefore, the OVA-specific antibody at this time point is dependent on help provided by 3K-specific memory cells rather than help from either primary responding 3K- or OVA-specific CD4 T cells.
Fig. 5.
Memory CD4 T cells reactivated with antigen alone can provide help to B cells. B6 mice immunized with 3K with/without LPS i.v. as indicated were injected with 3K-OVA and alum i.p. 40 d after Imm2. After an additional 6 d, the amount of anti–OVA-specific IgG in the serum was examined. The data are combined from two independent experiments with four mice per group. Significant differences between the groups and primary responding mice are indicated by *P < 0.05, ***P < 0.001; ns, not significant, error bars are SEM.
Memory mice that had been immunized once with 3K and LPS contained similar numbers of IAb/3K-specific cells to memory mice reactivated with 3K peptide (Fig. 4A). The animals in these two groups made a similar anti-OVA IgG response. Therefore, despite their poor survival following reactivation, memory CD4 T cells that had been reactivated with antigen alone can still provide accelerated help to B cells.
Memory CD4 T Cells Are Deleted Following Exposure to Two Injections of Soluble Peptide.
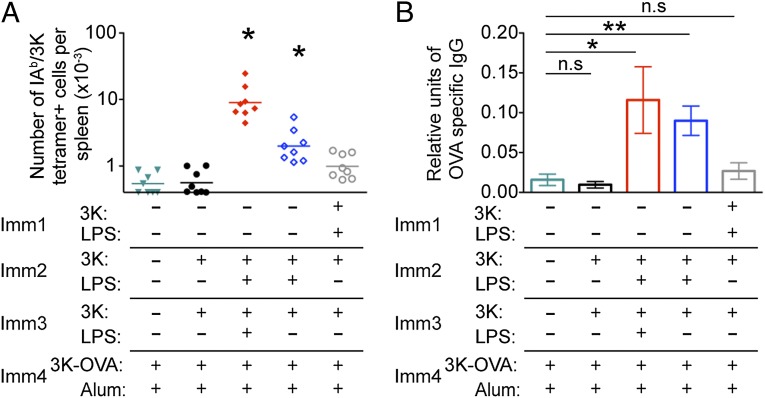
As memory CD4 T cells exposed to soluble antigen undergo apoptosis following further reactivation with antigen delivered with (Fig. 4) or without (Fig. 3) adjuvant, we reasoned that two injections of soluble antigen should lead to a loss of the antigen-specific T cells and the accelerated antibody responses. To test this, mice containing IAb/3K-specific cells were injected twice with 3K peptide i.v. 40 d apart. After an additional 40 d, these and control mice were immunized with 3K-OVA and alum. As expected (22), antigen-specific T cells could not be readily detected in primary immunized animals or in animals that had been injected twice with soluble peptide at this early time point. No IAb/3K tetramer+ cells above background could be detected in memory mice injected twice with 3K peptide 6 d after immunization (Fig. 6A and Fig. S5). Moreover, the anti-OVA response in these animals was the same as that in naive mice immunized with 3K-OVA and alum (Fig. 6B). Together these data demonstrate that deletional tolerance can be induced in memory CD4 T cells provided they are reactivated following exposure to the tolerizing antigen.
Fig. 6.
Memory CD4 T cells reactivated twice with antigen alone do not survive and therefore cannot provide accelerated help for antibody production. B6 mice were immunized with/without 3K peptide and LPS i.v. as indicated with immunizations given 40 d apart. Six days following immunization with 3K-OVA and alum i.p., the numbers of splenic CD4 T cells that were IAb/3K tetramer+ CD44hi were examined (A) and the levels of anti-OVA IgG in the serum determined (B). In A, each point represents a mouse and the lines show the mean of the group, * indicates groups with means significantly greater than background, determined by staining naive splenoctyes with IAb/3K tetramer, the mean of which is indicated by the x axis. In B, error bars are SEM. In both, the data are combined from two experiments with four mice per group and in B, significant differences are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
Immunological memory is crucial as it protects the host from invaders to which it has previously been exposed. The superior responses of memory cells means that they are adept at responding to such pathogens, providing a larger and more rapid immune response. Protective immune memory is often considered a function of high-affinity antibody and killer CD8 T cells (1, 3). Memory CD4 T cells also play crucial roles in protecting the host from a diverse range of pathogens, including bacteria, viruses, and parasites (25–27).
The ability of memory CD4 T cells to respond in the absence of heightened costimulation presents a major hurdle for strategies that aim to induce specific T-cell tolerance in previously activated T cells (6, 7). In the experiments described here, we use a well-established method of tolerizing antigen-specific cells, the administration of antigen in the absence of components that stimulates innate immunity, an adjuvant. This method has been used for many years to tolerize, via deletion or the induction of anergy, naive lymphocytes (17, 28). However, its ability to tolerize memory CD4 T cells has not previously been examined in detail. Here we show that, unlike naive T cells, memory CD4 T cells respond very similarly to antigen given with or without adjuvant. The reactivated memory cells return to the memory and respond to further reactivation by up-regulating costimulatory molecules, migrating to inflamed lymph nodes, entering the cell cycle, and providing help to B cells. Together, these data strengthen the argument that memory CD4 T cells are intrinsically distinct from naive CD4 T cells (4).
Memory CD4 T cells were, however, permanently altered following activation with antigen alone. Despite normal expression of prosurvival molecules and long-term survival within the memory pool, further in vivo reactivation led to apoptotic cell death. The activation of either naive or memory T cells following immunization with antigen and adjuvant leads to a burst of cell proliferation followed by the death of the majority of the activated cells. Memory CD4 T cells previously exposed to antigen briefly expanded but entered a dramatic contraction phase that led to the deletion of the antigen-specific T cells. Taken together, these data indicate that these cells misinterpret activation signals, turning what should be a signal to increase in number into a signal to undergo cell death.
The molecular changes that underlie this altered survival remain unclear. Whereas costimulatory signals that promote T-cell activation and cell survival are closely linked (29), the intracellular molecules that transmit these signals can be distinct (30, 31). That the defect in cell survival occurs following further activation suggests that the stress induced by T-cell activation initiates apoptosis in the memory cells. T-cell activation leads to increased expression of proteins involved in autophagy allowing cells to maintain energy and nutrients during proliferation (32, 33). Autophagy and apoptosis are cellular pathways that can coregulate each other (34). We speculate that restimulated memory cells previously exposed to antigen alone fail to induce autophagy pathways that prevent immediate cell death. Intriguingly, inhibition of mammalian target of rapamycin, an inhibitor of autophagy, promotes the generation of memory CD8 T cells (35).
Injection of soluble antigen or altered peptide ligands into animals with ongoing autoimmune or allergic responses can lead to a reduction in disease (20, 36–40). The mechanisms or correlates of protection in these models vary from the induction of IL-10 producing cells (37, 39, 40), to reduced specific T-cell (20, 36) and antibody responses (38). Our data support the idea that the pathogenic T cells themselves are altered following administration of soluble antigen and that a double dose of soluble antigen is required to induce full tolerance. These different mechanisms of tolerance suggest that the initial generation of the memory cells may influence whether and how tolerance can be subsequently induced and that our results may not be applicable to all memory cells.
The distinctive functions of memory CD4 T cells have been recognized for many years (41). Here we demonstrate that the response of memory CD4 T cell to reactivation with soluble antigen is also markedly distinct from that of naive CD4 T cells. Whereas activation with soluble antigen alters the survival of memory CD4 T cells following reactivation, it does not, by itself, silence these cells. Our studies demonstrate that memory CD4 T cells must be activated at least twice, a first hit to damage the survival capacity of the cell, and a second hit to cause cell death. This finding has important implications for treatments for allergy, autoimmunity, and transplantation that aim to remove pathogenic CD4 T cells via peptide immunotherapy (42). Furthermore, understanding the molecular pathways that have gone awry in memory CD4 T cells exposed to tolerizing signals has the potential to highlight pathways to target or remove auto- or alloreactive T cells. Conversely, it may be possible to manipulate these pathways to increase the survival of memory CD4 T cells following infection or vaccination.
Materials and Methods
Animals and Immunizations.
Animals were housed and handled either at National Jewish Health or The University of Glasgow in accordance with local Animal Care and Use Committee or UK Home Office regulations, respectively. Female C57BL/6 mice (The Jackson Laboratory or Harlan) were first immunized at 6–8 wk of age. To generate memory cells, mice were immunized i.v. with 10 µg of 3K peptide (FEAQKAKANKAVD) (JPT) and 10 µg of LPS (Sigma). To induce tolerance, age-matched naive mice and mice that had been immunized with 3K peptide and LPS 32–40 d earlier (memory mice) were injected with 10 µg of 3K peptide i.v. The 3K was conjugated to maleimide-activated OVA as described by the manufacturer (Pierce). A total of 5 µg of 3K-OVA in 25 µL of PBS was injected into the footpads of restrained mice. Footpad measurements were taken prior and each day following the injection using a Series 500 Caliper (Mitutoyo). The 3K-OVA peptide/protein conjugates were tumbled with alum (Alydrogel; Brenntag) for 2 h at room temperature and each mouse received 1 μg of 3K/OVA with 0.1 mg of alum intraperitoneally (i.p). BrdU was delivered either as an i.p. injection of 1 mg in PBS or continuously for 3 d in drinking water at 0.8 mg/mL. The BrdU drinking water was changed daily and protected from light at all times.
Flow Cytometry.
Phycoerythrin-labeled IAb/3K was produced as described (43). Single-cell suspensions from the spleens of immunized mice were stained with tetramer at 37 °C for 2 h. Antibodies to surface markers were added and the cells incubated for an additional 20 min. Tetramer-positive cells were defined by gating on live CD4 + CD44hi cells that were CD8, B220, F4/80, MHC class II negative (the dump gate). Antibodies were either from BD Biosciences, eBioscience, or BioLegend. For analysis of intracellular caspase activation, MHC class II tetramer-stained cells were incubated with a fluorescent inhibitor of caspase (FLICA) containing a DEVD sequence that binds to active caspase 3 and 7 as described by the manufacturer (Molecular Probes, Life Technologies). These cells were then fixed using cytofix/cytoperm (BD Biosciences) and stained with anti-Bcl2 (BioLegend). A total of 2–5 million events were collected on a CyAn ADP (Beckman Coulter), and data analyzed using FlowJo version 9.6 (TreeStar).
ELISA.
The 96-well immulon plates were coated with ovalbumin protein at 100 μg/mL in PBS overnight at 4 °C. Following washing, the plates were blocked with 10% (vol/vol) FCS/PBS before serum samples were added and titrated down the plate. To determine relative units, we used a positive control serum sample from B6 mice that contained OVA-specific antibody on each plate. We also included titrated serum from naive mice to set the level of background OD. The samples were incubated overnight at 4 °C. Plates were washed and alkaline phosphatase-conjugated anti-IgG1 (BD Biosciences) detection antibodies were added. Plates were washed again and p-nitrophenyl phosphate substrate diluted in glycine buffer was added to each well. Plates were allowed to develop and 405 nm absorbance values were collected on Elx808 microplate reader.
Statistics.
Data are presented as indicated in the figure legends. Statistical significance was determined using either Student’s two-tailed t test (when two groups are compared) or ANOVA (when more than two groups are compared) followed by Bonferroni posttest analysis (for normally distributed data) or Dunn’s multiple comparison test (for nonparametric data) to determine differences between the groups. All statistical analysis was performed using GraphPad Prism software (version 5).
Supplementary Material
Acknowledgments
The work was supported by a fellowship from Arthritis Research UK (19905) (to M.M.), National Institutes of Health Grants AI-18785 and AI-22295, and the US Department of Defense US Army Medical Research and Materiel Command W81XWH-07-1-0550 (to P.M. and J.W.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406218111/-/DCSupplemental.
References
- 1.Huster KM, Stemberger C, Busch DH. Protective immunity towards intracellular pathogens. Curr Opin Immunol. 2006;18(4):458–464. doi: 10.1016/j.coi.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.McKinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology. 2010;130(1):1–9. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plotkin SA. Vaccines: Correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47(3):401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 4.MacLeod MK, Clambey ET, Kappler JW, Marrack P. CD4 memory T cells: What are they and what can they do? Semin Immunol. 2009;21(2):53–61. doi: 10.1016/j.smim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2012;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 6.Nepom GT, St Clair EW, Turka LA. Challenges in the pursuit of immune tolerance. Immunol Rev. 2011;241(1):49–62. doi: 10.1111/j.1600-065X.2011.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Getts DR, et al. Current landscape for T-cell targeting in autoimmunity and transplantation. Immunotherapy. 2011;3(7):853–870. doi: 10.2217/imt.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Fan H, Jiang S. CD4(+) T-cell subsets in transplantation. Immunol Rev. 2013;252(1):183–191. doi: 10.1111/imr.12038. [DOI] [PubMed] [Google Scholar]
- 9.Raychaudhuri S, et al. BIRAC Consortium YEAR Consortium Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat Genet. 2009;41(12):1313–1318. doi: 10.1038/ng.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bour-Jordan H, et al. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011;241(1):180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappert P, Schwartz RH. Induction of T cell anergy: Integration of environmental cues and infectious tolerance. Curr Opin Immunol. 2010;22(5):552–559. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164(1):265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 13.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhai Y, et al. The CD154-CD40 T cell costimulation pathway is required for host sensitization of CD8(+) T cells by skin grafts via direct antigen presentation. J Immunol. 2002;169(3):1270–1276. doi: 10.4049/jimmunol.169.3.1270. [DOI] [PubMed] [Google Scholar]
- 15.MacLeod M, et al. CD4 memory T cells survive and proliferate but fail to differentiate in the absence of CD40. J Exp Med. 2006;203(4):897–906. doi: 10.1084/jem.20050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ndejembi MP, et al. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177(11):7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 17.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1(4):327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 18.Verbeek R, van der Mark K, Wawrousek EF, Plomp AC, van Noort JM. Tolerization of an established alphaB-crystallin-reactive T-cell response by intravenous antigen. Immunology. 2007;121(3):416–426. doi: 10.1111/j.1365-2567.2007.02592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cells are tolerized upon exposure to parenchymal self-antigen. J Immunol. 2002;169(7):3622–3629. doi: 10.4049/jimmunol.169.7.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leech MD, Chung CY, Culshaw A, Anderton SM. Peptide-based immunotherapy of experimental autoimmune encephalomyelitis without anaphylaxis. Eur J Immunol. 2007;37(12):3576–3581. doi: 10.1002/eji.200737148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLeod MK, et al. CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. Proc Natl Acad Sci USA. 2008;105(38):14521–14526. doi: 10.1073/pnas.0807449105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLeod MK, et al. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J Immunol. 2011;186(5):2889–2896. doi: 10.4049/jimmunol.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAleer JP, Zammit DJ, Lefrançois L, Rossi RJ, Vella AT. The lipopolysaccharide adjuvant effect on T cells relies on nonoverlapping contributions from the MyD88 pathway and CD11c+ cells. J Immunol. 2007;179(10):6524–6535. doi: 10.4049/jimmunol.179.10.6524. [DOI] [PubMed] [Google Scholar]
- 24.Black CA. Delayed type hypersensitivity: Current theories with an historic perspective. Dermatol Online J. 1999;5(1):7. [PubMed] [Google Scholar]
- 25.Wilkinson TM, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18(2):274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 26.Lumsden JM, et al. Protective immunity induced with the RTS,S/AS vaccine is associated with IL-2 and TNF-α producing effector and central memory CD4 T cells. PLoS ONE. 2011;6(7):e20775. doi: 10.1371/journal.pone.0020775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boom WH, et al. Human immunity to M. tuberculosis: T cell subsets and antigen processing. Tuberculosis (Edinb) 2003;83(1–3):98–106. doi: 10.1016/s1472-9792(02)00054-9. [DOI] [PubMed] [Google Scholar]
- 28.Endres RO, Grey HM. Antigen recognition by T cells. II. Intravenous administration of native or denatured ovalbumin results in tolerance to both forms of the antigen. J Immunol. 1980;125(4):1521–1525. [PubMed] [Google Scholar]
- 29.Song J, Lei FT, Xiong X, Haque R. Intracellular signals of T cell costimulation. Cell Mol Immunol. 2008;5(4):239–247. doi: 10.1038/cmi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guy CS, et al. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat Immunol. 2013;14(3):262–270. doi: 10.1038/ni.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okkenhaug K, et al. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;2(4):325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- 32.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204(1):25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh CM, Bell BD. T cell intrinsic roles of autophagy in promoting adaptive immunity. Curr Opin Immunol. 2010;22(3):321–325. doi: 10.1016/j.coi.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferraro E, Cecconi F. Autophagic and apoptotic response to stress signals in mammalian cells. Arch Biochem Biophys. 2007;462(2):210–219. doi: 10.1016/j.abb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauer L, et al. Modulation of the allergic immune response in BALB/c mice by subcutaneous injection of high doses of the dominant T cell epitope from the major birch pollen allergen Bet v 1. Clin Exp Immunol. 1997;107(3):536–541. doi: 10.1046/j.1365-2249.1997.d01-953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell JD, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009;206(7):1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasselberg A, Schön K, Tarkowski A, Lycke N. Role of CTA1R7K-COL-DD as a novel therapeutic mucosal tolerance-inducing vector for treatment of collagen-induced arthritis. Arthritis Rheum. 2009;60(6):1672–1682. doi: 10.1002/art.24566. [DOI] [PubMed] [Google Scholar]
- 39.O’Neill EJ, Day MJ, Wraith DC. IL-10 is essential for disease protection following intranasal peptide administration in the C57BL/6 model of EAE. J Neuroimmunol. 2006;178(1-2):1–8. doi: 10.1016/j.jneuroim.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakken BJ, et al. Inhibition of adjuvant-induced arthritis by interleukin-10-driven regulatory cells induced via nasal administration of a peptide analog of an arthritis-related heat-shock protein 60 T cell epitope. Arthritis Rheum. 2002;46(7):1937–1946. doi: 10.1002/art.10366. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed R, Gray D. Immunological memory and protective immunity: Understanding their relation. Science. 1996;272(5258):54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 42.Sabatos-Peyton CA, Verhagen J, Wraith DC. Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr Opin Immunol. 2010;22(5):609–615. doi: 10.1016/j.coi.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8(6):675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.