Significance
The work identifies dATP8B as a critical factor for proper odorant and 11-cis-vaccenyl acetate pheromone sensitivity. These defects are attributed to a failure to translocate lipids, because mutants defective for dATP8B enzymatic activity do not rescue, but a functional vertebrate homolog fully rescues the pheromone detection defects. We identified abnormal localization of Or67d receptors as a likely cause for the phenotypes, and overexpression of Or67d can rescue 11-cis-vaccenyl acetate sensitivity and loss of spontaneous spiking in the lipid flippase mutant. We suggest dATP8B is important for lipid translocation in olfactory neurons, which in turn is important for proper subcellular trafficking of receptor subunits.
Keywords: olfaction, aminophospholipid translocase
Abstract
In Drosophila melanogaster, the male-specific pheromone cVA (11-cis-vaccenyl acetate) functions as a sex-specific social cue. However, our understanding of the molecular mechanisms underlying cVA pheromone transduction and its regulation are incomplete. Using a genetic screen combined with an electrophysiological assay to monitor pheromone-evoked activity in the cVA-sensing Or67d neurons, we identified an olfactory sensitivity factor encoded by the dATP8B gene, the Drosophila homolog of mammalian ATP8B. dATP8B is expressed in all olfactory neurons that express Orco, the odorant receptor coreceptor, and the odorant responses in most Orco-expressing neurons are reduced. Or67d neurons are severely affected, with strongly impaired cVA-induced responses and lacking spontaneous spiking in the mutants. The dATP8B locus encodes a member of the P4-type ATPase family thought to flip aminophospholipids such as phosphatidylserine and phosphatidylethanolamine from one membrane leaflet to the other. dATP8B protein is concentrated in the cilia of olfactory neuron dendrites, the site of odorant transduction. Focusing on Or67d neuron function, we show that Or67d receptors are mislocalized in dATP8B mutants and that cVA responses can be restored to dATP8B mutants by misexpressing a wild-type dATP8B rescuing transgene, by expressing a vertebrate P4-type ATPase member in the pheromone-sensing neurons or by overexpressing Or67d receptor subunits. These findings reveal an unexpected role for lipid translocation in olfactory receptor expression and sensitivity to volatile odorants.
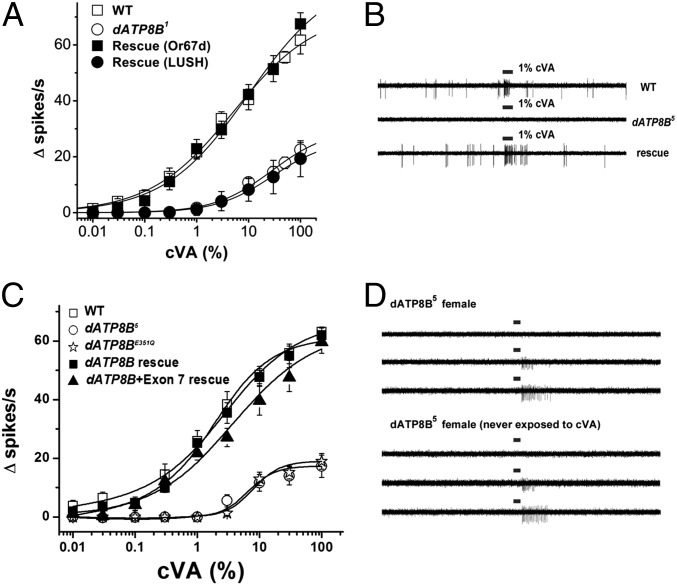
In a forward genetic screen for mutants with defective responses to cVA (11-cis-vaccenyl acetate) pheromone, we recovered four mutants unresponsive to 1% cVA that also lacked normal spontaneous action potentials in the absence of cVA (Fig. 1 A–C) (1–3). Approximately half of the Or67d neurons from these four lines were insensitive to all concentrations of cVA, and those that did respond had severely impaired cVA sensitivity compared with wild-type controls (Fig. 1 A and C). Complementation analysis demonstrated that all four were alleles of a single locus we named dATP8B. The four mutant alleles were indistinguishable and have little or no variation in penetrance or mutant strength (Fig. 1A).
Fig. 1.
dATP8B mutants are defective for cVA responses and spontaneous activity in Or67d neurons. (A) Sample single sensillum recording traces to 1% cVA from control (wild-type) and the five dATP8B mutant alleles. Df is Exelixis 6162 (Bloomington stock 7641) deleted for the dATP8B locus. Bal is TM6B. (B) Spontaneous activity (spikes in the absence of cVA) is eliminated in the Or67d neurons from dATP8B1 mutants and restored by expressing a dATP8B cDNA with pOr67dGAL4 (Rescue). n ≥ 14 for each genotype. (C) Dose–response curves for wild type and dATP8B1 mutants. dATP8B1 mutants have a profound loss of responsiveness to cVA compared with wild-type controls. n = 14 for WT, n = 96 for dATP8B5 mutants. (D) Or83c misexpressed in Or67d neurons instead of the endogenous Or67d receptor gene respond to farnesol (genotype w ; UAS Or83c ; Or67dGAL4). (Top) Responses of these transgenic flies to three concentrations of the Or83c ligand, farnesol (9). (Middle) Responses are moderately reduced in these transgenic flies in the dATP8B mutant background (genotype w ; UAS Or83c ; Or67dGAL4,dATP8B5). (Bottom) Wild-type Or67d neurons do not respond to farnesol. (E) Dose–response curves to farnesol for the three genotype in D. n = 6 for each genotype.
To establish whether these mutants affect all or a subset of odorant responses, we surveyed several of olfactory neuron classes that use different receptor classes (Figs. S1 and S2). Responses to CO2, mediated by gustatory receptor family members (4, 5), and odorants detected by several variant ionotropic receptor family members (6) were not affected in the mutants (Fig. S2). By contrast, odorants detected by odorant receptor members (ORs) that multimerize with the OR coreceptor Orco had reduced odorant responses and action potential amplitudes that were 50% smaller in the mutants compared with wild-type controls (Fig. S1). Orco is a coreceptor thought to multimerize with OR receptor subunits to produce functional odorant-gated ion channels (7, 8). Orco mediates food odorant responses in most basiconic sensilla neurons and cVA responses in Or67d-expressing trichoid neurons. The cVA response defects are more strongly affected in the mutants than other Orco-dependent olfactory neuron responses (compare Fig. 1C and Fig. S1C), so we focused our studies on these neurons.
To determine whether the severity of the cVA detection phenotype is neuron-dependent, we expressed a farnesol-activated OR, Or83c, in Or67d neurons in lieu of the endogenous Or67d receptor (9). Similar to basiconic neurons, there is a moderate reduction in farnesol sensitivity in the mutant (Fig. 1 D and E) that is less severe than the dATP8B mutant cVA defects. This suggests that the severity of the response defects is receptor dependent and not neuron dependent.
We mapped the mutant locus to a single gene at position 87A on the right arm of the third chromosome (Fig. 2A). This gene encodes a member of the P4-type ATPase family similar to vertebrate ATP8B. All four mutants we recovered contain inactivating lesions in dATP8B (Fig. 2B). dATP8B1 (Z3-1778) has a 10-bp deletion at the start of exon 17, which removes the splice acceptor. This lesion is predicted to result in skipping exon 17 and producing a frameshift mutation and premature termination (Fig. 2B). dATP8B2 (Z3-3278) has a 56-bp deletion in exon 17 that is predicted to produce a frameshift mutation and a truncated product. dATP8B3 (Z3-3028) has a nonsense mutation in exon 8 that converts the Arg913 codon to a stop codon (CGA to TGA). dATP8B4 (Z3-4031) has a point mutation that introduces a premature stop codon in exon 12 at Glu1160 (CAA to TAA). A fifth allele (dATP8B5) was obtained that contains a piggyback transposable element integrated within the coding sequence of exon 11 (10). dATP8B5 mutants have cVA response defects that are indistinguishable from ethyl methanesulfonate alleles (Fig. 1A).
Fig. 2.
The dATP8B locus encodes a P4-type ATPase. (A) Polytene chromosome region encoding the dATP8B locus (modified from ref. 43). Df3128 (longer thick bar) failed to complement cVA sensitivity in the dATP8B1 mutant allele. Smaller deficiencies further restricted the dATP8B locus to a small region of 87A, defined by Df7641 (small thick bar) that failed to complement dATP8B1, and Df7642 and Df7968 that complement dATP8B1. (B) Genomic structure of the dATP8B locus. Exons are denoted by bars, introns by lines. The gray bar denotes exon 7 that is absent from most dATP8B cDNAs isolated from antennal tissues. The position and molecular lesions of the five dATP8B alleles are depicted. (Scare bar, 1 kb.) (C) Predicted transmembrane structure of the dATP8B protein. Aspartate 597 is the first residue in the conserved sequence DKTGTLT and glutamate 351 in the conserved DGETN domain. The position of the engineered Glu to Gln mutant allele is depicted. Numbers represent relative location of the different mutant alleles and exon 7. (D) Structural similarity between dATP8B and human ATP8B1. dATP8B (Upper) and ATP8B1 are more than 40% identical, sharing well-conserved domains. The numbers below each domain represent sequence identity and similarity.
The dATP8B locus is predicted to encode several large, alternately spliced transcripts (11). To determine which transcripts were expressed in the antenna, we isolated dATP8B cDNAs from reverse-transcribed mRNA purified from wild-type antennas (Materials and Methods). Most of the dATP8B cDNAs lacked exon 7, corresponding closely to predicted transcript D (Fig. 2B) (11). This shorter splice variant is enriched in antennas, whereas dATP8B transcripts with and without exon 7 are widely distributed in heads and bodies and throughout development (Fig. S3). The protein predicted for the major antenna transcript is a 10-transmembrane segment protein with amino acid sequence similar to human ATP8B and ATP8A isoforms of the P4-type ATPase family (Fig. 2 C and D). P4-type ATPases constitute a large, conserved family essential for creating and maintaining lipid asymmetry in membranes (12–15). In humans, ATP8B1 regulates the lipid composition of the canaliculi cells that transport bile acids from the liver. ATP8B1 is thought to transfer phosphatidylserine (PS) from the outer to the inner leaflet of the plasma membrane, making these cells resistant to bile acids (16). Defects in ATP8B1 expression cause progressive familial intrahepatic cholestasis type 1 (12, 17). Lesions in ATP8A1 are associated with abnormalities in the developing nervous system (12, 16, 18–20). Sixteen P4-type ATPases are encoded in the human genome, and six are present in the Drosophila genome, but the function of the majority of these proteins is unknown.
To determine whether dATP8B is required in olfactory neurons or in the nonneuronal support cells that secrete the sensillum lymph and odorant binding proteins, we expressed a dATP8B cDNA in either the Or67d olfactory neurons or the associated support cells in the dATP8B5 mutant background. The cDNA fails to rescue when expressed in support cells (21) (Fig. 3A). dATP8B cDNA expressed in the Or67d neurons with Or67dGAL4 fully rescues the spontaneous activity and cVA sensitivity defects (22) (Figs. 1B and 3A). cDNAs that include exon7 also rescue the dATP8B5 mutant defects (Fig. 3C).
Fig. 3.
dATP8B is required for normal function in Or67d neurons. (A) dATP8B1 homozygous mutants (open circles) have defective cVA responses compared with wild-type controls (open squares). Expression of a dATP8B cDNA regulated by the Or67d promoter (rescue Or67d, genotype w ; UAS Or67d /+ ; Or67dGAL4, dATP8B1/dATP8B1) restores dATP8B1 mutants to wild-type cVA sensitivity (closed squares), but expression of dATP8B in support cells of dATP8B1 mutants using the lush promoter (rescue LUSH, closed circles; genotype w ; UAS dATP8B/LUSH GAL4 ; dATP8B1) fails to rescue cVA sensitivity. (B) Sample traces showing rescue of normal spontaneous activity and 1% cVA sensitivity to Or67d neurons from dATP8B5 mutants by expressing a dATP8B cDNA with a single copy of Or67dGAL4. (C) cVA dose–response curves for wild-type (open boxes), dATP8B5 mutants (open circles), and transgenic flies expressing wild-type (filled boxes) or dATP8BE351Q mutant dATP8B cDNAs (open stars) in the dATP8B5 mutant background. A longer dATP8B cDNA, including exon 7 also rescues (filled triangles). n = 6–8. (D) dATP8B5 mutants never exposed to cVA show typical defects. (Upper) Responses of dATP8B5 females raised in mixed cultures to 300-ms pulses of air with 1%, 10%, or 100% cVA. (Lower) Female dATP8B5 mutants isolated as larvae, raised in isolation, and never exposed to cVA before testing are not different from those raised in mixed culture.
Is the enzymatic activity of dATP8B required for olfactory function? dATP8B contains the conserved DGETN and DKTGT domains found in all P4-type ATPase family members (23) (Fig. 2C). The flipping mechanism for P4-type ATPases is thought to be analogous to that of SERCA1 (ATP2A1), a P-type ATPase that pumps calcium ions across membranes (24). Translocation involves a cycle of autophosphorylation and dephosphorylation on a conserved aspartate that converts the E1 phosphoaspartate, substrate-bound conformation to the E2 conformation that favors release of the substrate on the other side of the membrane and hydrolysis of the phosphoaspartate (25, 26). The conserved aspartic acid residue in the DKTGT domain is autophosphorylated and dephosphorylated during the substrate translocation cycle in related P-type ATPases, whereas the DGETN domain is important for the phosphatase activity of the enzyme (27, 28). We engineered a dATP8B mutant predicted to disrupt the phosphorylation cycle (29). Glutamate to glutamine substitutions in the dGETN domain in SERCA1 impair the phosphatase activity of the enzyme, locking the enzyme in the phosphoaspartyl state (29, 30). We produced transgenic flies expressing the equivalent mutation in the DGETN domain, dATP8BE351Q. The mutant protein fails to rescue the defects in the dATP8B mutant background (Fig. 3C). These findings are consistent with a requirement for the enzymatic activity of dATP8B in Or67d neurons.
Antiserum that recognizes the C terminus of the dATP8B protein was used to identify its cellular and subcellular localization (Materials and Methods). The immune serum reacts with the cilia of Drosophila olfactory neurons, and the protein is present in the cilia of Or67d neurons (Fig. 4 A, C, and D). dATP8B5 mutants lack staining (Fig. 4B), confirming the specificity of the antiserum and that dATP8B5 is a null allele. We further examined what other olfactory neurons express dATP8B. Although dATP8B expression levels vary among olfactory neuron cilia, we found that the protein colocalizes with GFP expressed with the Orco promoter (Fig. 4 E–G). Localization of dATP8B protein to the site of olfactory signal transduction in Orco-expressing olfactory cilia is consistent with the observed olfactory defects.
Fig. 4.
dATP8B protein is enriched in a subset of olfactory neuron cilia. (A) Anti-dATP8B antiserum recognizes antigen localized in the cilia of wild-type olfactory neurons. (B) Anti-dATP8B antiserum does not react to dATP8B5 mutant antenna indicating the antiserum is specific. (C) Colocalization of dATP8B in wild-type cilia expressing membrane-bound GFP in Or67d neurons. Arrow depicts colocalization of the two markers. (D) Or67d neuron cilia from dATP8B mutants expressing a dATP8B cDNA with Or67dGAL4. Arrow depicts dATP8B in the cilia. (E) Frozen tissue section through the antenna of a transgenic fly expressing membrane-tethered GFP expressed with the Orco promoter. (F) dATP8B protein localization in the same section. (G) Merged image of E and F above. Although dATP8B levels vary among the cilia of different receptor neurons, all Orco-expressing neurons express dATP8B.
Could dATP8B function as a cVA flippase, explaining the particular sensitivity of cVA responses to the loss of this factor? dATP8B might flip cVA lipids from outside to the inside of Or67d neurons, inactivating the cVA signal. Weak cVA exposure to males in mixed cultures, for example, might result in desensitization of the Or67d neurons, producing the dATP8B phenotype. To examine this possibility, we recorded responses from virgin female dATP8B mutants isolated as larvae, raised in isolation, and never exposed to cVA. Flies never exposed to cVA have the typical dATP8B phenotype, with no spontaneous activity and blunted cVA sensitivity that is indistinguishable from flies raised in mixed cultures (Fig. 3D). This suggests that the mutant phenotype does not arise from cVA desensitization.
Orco and Snmp1 are both essential factors required for cVA detection. Snmp1 is a CD36 homolog essential for cVA detection but not required for detection of most food odorants (1) (31). We tested whether the dendritic localization of dATP8B is affected by loss of Or67d, Snmp1, or Orco. We found normal localization of dATP8B in the cilia in Snmp1Z0429 and Or67dGAL4 mutants, but much of the dATP8B protein mislocalized to the olfactory neurons cell bodies in Orco2 mutants (Fig. S4). This suggests Orco is important for localization of dATP8B to the cilia.
Is the flippase required for localization of any cVA-sensitivity factors? Orco and Snmp1 localization are unaffected in dATP8B mutants (Fig. 5D and Fig. S4). However, there is a reduction in Or67d in the dendritic cilia relative to the cell bodies in the mutants. We quantified Or67d using GFP-tagged Or67d in lieu of untagged Or67d. We found a significant reduction in the fraction of Or67d that localized to the cilia in dATP8B mutants compared with wild-type controls (Fig. 5 A–C). This suggests that receptor trafficking may underlie part or all of the olfactory defects.
Fig. 5.
Or67d levels are reduced in dATP8B mutant cilia. (A) Example of GFP-tagged Or67 expressed in wild-type dATP8B background (genotype, w: UAS GFPOr67d ; Or67dGAL4 /+). (B) Typical example of GFP-tagged Or67d in the dATP8B mutant background (genotype w ; UAS GFPOr67d ; Or67dGAL4, dATP8B5/dATP8B5). (C) Quantification of cilia to cell body ratio GFP-tagged Or67d in wild type and dATP8B mutants. Genotypes are significantly different at P < 0.01 by Student’s 2-tailed t test. n = 20 for each bar. (D) Quantification of cilia to cell body signal ratio for Orco in Or67d neurons. Error bars represent SEM. n = 20 for each. (E) Bovine ATP8A2 expressed under control of the Or67d promoter in the dATP8B5 mutant background localizes to the proximal dendrites of the Or67d neurons (genotype w ; UAS ATP8A2 ; Or67dGAL4, dATP8B5/dATP8B5). (F) Graph depicts the cVA dose–response curves for dATP8B5 mutants expressing bovine ATP8A2 compared with wild-type controls. n ≥ 10 for each time point.
Is dATP8B protein specifically required, or is proper lipid localization the essential aspect of the dATP8B mutant phenotype? We expressed a vertebrate P4-type ATPase, bovine ATP8A2, in Or67d neurons in dATP8B5 mutant background (Fig. 5E). This isoform is selective for PS over phosphatidylethanolamine and phosphatidylcholine, according to the ATPase activity of recombinant protein in the presence of these different lipids (32). Remarkably, expression of this vertebrate enzyme completely rescued the dATP8B5 mutant phenotype (Fig. 5F). This suggests lipid translocation is critical for olfactory neuron responsiveness and not a specific requirement for dATP8B protein itself, for example as a receptor subunit.
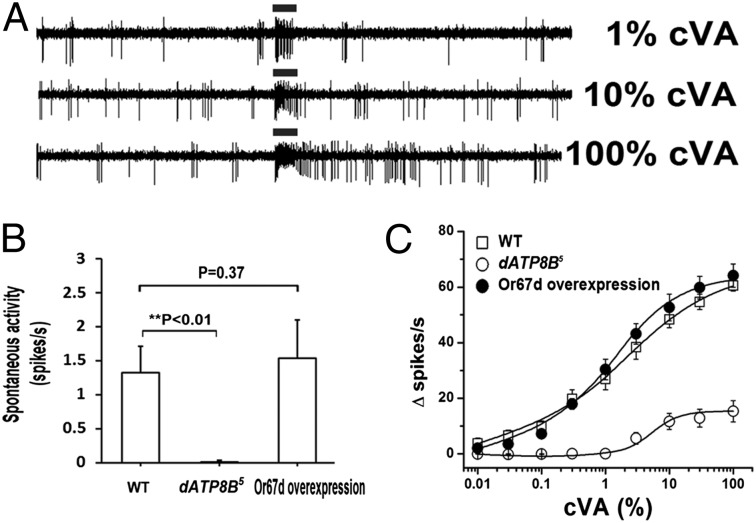
Because Or67d levels are reduced in the cilia, we tested whether this is the basis of the mutant phenotype by overexpressing Or67d subunits in these neurons to determine whether this could rescue these defects. We introduced an extra copy of UAS-Or67d expressed under control of the strong ELAV neuronal promoter in the dATP8B5 mutant background. The Or67d transgene, together with the two endogenous Or67d genes, restored spontaneous activity and normal cVA pheromone detection in the dATP8b mutant background (Fig. 6).
Fig. 6.
Overexpression of Or67d rescues dATP8B mutant defects. (A) Sample traces from flies overexpressing Or67d in the olfactory neurons with ELAV Gal4 (genotype ELAV Gal4/ + ; UAS Or67d/+ ; dATP8B5. Note restoration of spontaneous activity and cVA responses. (B) Quantification of spontaneous activity in flies overexpressing Or67d. n = 8 for each genotype. (C) Dose–response curves for wild-type (open squares), dATP8B5 (open circles), and dATP8B5 mutants overexpressing GFP-tagged Or67d with ELAV Gal4 (filled circles). n = 8 for each genotype and time point.
Discussion
We present evidence that an aminophospholipid transferase plays a role in olfactory sensitivity and receptor trafficking in Orco-expressing olfactory neurons. Because we can rescue the mutant phenotype by overexpressing Or67d receptor subunits, we think the most likely role for dATP8B is to facilitate the proper localization of Orco-associated tuning receptor subunits. How lipid translocation mediates this process remains a question. In yeast, the major phenotype associated with loss of P4-ATPase function is abnormal transport vesicle budding from the transgolgi network (19, 33, 34). Similar defects in cargo sorting have been observed in Caenorhabditis elegans Tat-1 mutants (35), and other C. elegans P4-ATPase mutants are defective for phagocytosis of dead cells and regulation of ectosome production (35–38). Proper lipid localization may be required for vesicle docking or receptor recycling leading to reduced receptor levels in the cilia. Reduced receptor density could explain the observed right-shift in the odorant and pheromone dose–response curves and the reduced maximal activation of Or67d neurons. This could also explain the absent spontaneous activity, although it is puzzling that there is a complete loss of these events and not reduced frequency. It will be of great interest in the future to determine whether dATP8B is a competence factor important for receptor expression, or whether it actively regulates receptor density and odorant sensitivity through a lipid translocation mechanism. It will also be of interest to establish whether PS or phosphatidylethanolamine or both are mislocalized in dATP8B mutant Or67d cilia, and to understand why levels of Orco in the cilia seem to be unaffected.
Malaria-transmitting mosquitoes Anopheles gambiae and Aedes aegypti harbor genes with 69% and 77% amino acid identity with dATP8B. If these homologs perform a similar role, they are potential targets to manipulate mosquito behavior. Finally, there are four additional P4-type ATPase genes in the fly genome, and dATP8B splicing variants are widely expressed in the head and body. It will be important to determine what roles these additional flippase enzymes play in Drosophila biology.
Materials and Methods
Fly Stocks.
All animal work was done in strict accordance with University of Texas Southwestern Animal Resource Center policies and the American Association for Accreditation for Laboratory Animal Studies guidelines, and the animal care and use program conforms to all current National Institutes of Health standards for the care and use of animals in research.
Drosophila melanogaster strains were provided by the Bloomington Stock Center and raised on standard cornmeal agar molasses medium. dATP8B mutants were identified in a screen for cVA defective mutants (1). The wild-type strain used in this study was w1118. Or67d2 mutants (Or67dGAL4) were generously provided by B. J. Dickson (Howard Hughes Medical Institute, Janelia Farm Research Campus, Ashburn, VA) (22), the UAS GFP-tagged Or67d was provided by Richard Benton (University of Lausanne, Lausanne, Switzerland) (31), and UAS CD4tdGFP was from Chun Han (Cornell University, Ithaca, NY) (39). To test for rescue of dATP8B mutant cVA responses by dATP8B cDNAs, we crossed w ; UAS-dATP8B cDNA ; Or67dGAL4, dATP8B5 flies to w ; UAS-dATP8B cDNA ; dATP8B5 and tested the F1 progeny. Similarly, to test for rescue of cVA responses using engineered mutant forms of dATP8B, UAS- dATP8BE351Q or bovine ATP8A2 was substituted for the wild-type dATP8B cDNA in the cross. (Fig. S5 shows complete genotypes used in this work.)
Single Sensillum Recordings and Preparation of cVA.
Extracellular electrophysiological recordings were carried out according to ref. 40. Flies (2–7 d old, males and females) were under a constant stream of charcoal-filtered air (36 mL/min, 22–25 °C) to prevent any potential environmental odors from inducing activity during these studies. cVA was diluted in paraffin oil, and 30 μL was applied to a filter paper, inserted in a Pasteur pipette, and air was passed over the filter and presented as the stimulus. The purity of the cVA was confirmed both by NMR and by mass spectroscopy. Signals were amplified 1,000× and fed into a computer via a 16-bit analog-to-digital converter and analyzed offline with AUTOSPIKE software (USB-IDAC system; Syntech). Low cutoff filter setting was 200 Hz, and the high cutoff was 3 kHz. Action potentials were recorded by inserting a glass electrode in the base of a sensillum. Data analysis was performed according to de Bruyne et al. (41). Signals were recorded for 20 s or 30 s, starting 10 s before cVA stimulation. Action potentials were counted 1 s before cVA stimulation and for 1 s after cVA stimulation. All recordings were performed from separate sensilla, with a maximum of two sensilla recorded from any single fly.
RT-PCR Analysis.
Dissected whole antennae, heads, or bodies were disrupted using a Polytron homogenizer in TRIzol (Invitrogen) as recommended by the manufacturer. Total RNA (1 μg) was used for RT reactions using SuperScript III according to the manufacturer. One twentieth of this material was used as template for individual PCR reactions. For the CG14741 isoform assays, primers were designed that spanned several exons, including the alternatively spliced exon 7. Primer sequences are 5′-CAATACCTATGCCAAAGCCCG and 5′-ATCCGCCAGTTCATCCGTAAGC. RT products were amplified with Accuprime (Invitrogen). The predicted products are 2.72 kb for genomic DNA, 1.67 kb for long isoform variants, and 0.98 kb for the short isoforms lacking exon 7.
DNA Sequencing.
PCR was used to isolate genomic DNA from dATP8B mutants for sequencing analysis. The entire CG14741 locus was sequenced for each mutant to unambiguously define the relevant lesions. Two independent PCR reactions were performed for each genomic fragment and sequenced to eliminate potential PCR artifacts. Sequencing was performed by the sequencing core facility at University of Texas Southwestern using Applied Biosystems Inc. (ABI) Big Dye Terminator 3.1 chemistry, and analyzed on ABI capillary instruments.
Generation of dATP8B Antiserum.
A peptide corresponding to the C-terminal 160 amino acids of the dATP8B isoform D (amino acids 1322–1482) with an N-terminal 6-histidine tag was produced in bacteria using pET28a (Novagen). The polypeptide was purified by nickel chromatography (Qiagen), eluted, and dialyzed extensively with water and lyophilized. Purified polypeptide (1 mg) was mixed in 1 mL PBS and 1 mL complete Fruends adjuvant, emulsified, and 0.2 mL was injected s.c. into rabbits prescreened for lack of antibodies that cross-react with Drosophila tissue sections. Three boosts were performed as above every 3 wk but using incomplete Fruends adjuvant. Serum was used without further purification at 1:300–1:1,000 for immunofluorescence experiments.
Immunocytochemistry and Image Quantification.
Rabbit anti-dATP8B serum was heat-inactivated at 50 °C for 30 min and used without further purification. Alexa 543-conjugated goat anti-rabbit antibodies (Invitrogen Molecular Probes) were used at 1:500 dilutions. Anti-bovine ATP8A2 was reported previously (32) and was used at 1:300–1:1,000 dilutions. Images were captured on a Zeiss LSM 510 confocal microscope. For image quantification, 20 images of frozen tissue sections from flies expressing UAS GFP-tagged Or67d in wild-type or mutant background (genotypes: wild type, w; UAS GFPOr67d; Or67dGAL4, w ; UAS GFPOr67d; Or67dGAL4, dATP8B5) were stained with anti-GFP antiserum (Life Technologies, A-11122). For Orco quantification, flies expressing GFP under control of the pOr67d GAL4 (to identify at1 neurons) either in the dATP8B5 mutant background or dATP8B5 heterozygous background (carrying TM6 as the wild-type control) were stained with rabbit anti-Orco antiserum (gift of Leslie Vosshall, The Rockefeller University, New York). GFP and Orco signal intensities were quantified using a Zeiss LSM510 confocal microscope and NIH ImageJ (downloaded from http://rsbweb.nih.gov/ij/download.html). Images were obtained using identical settings between genotypes. A coworker blinded to the genotypes then analyzed the images. Pixel values were obtained for identical area rectangles (for cilia) or circles (cell bodies) between genotypes. Background was subtracted for each value (background was determined by collecting pixels from an identical area in the same image with no fluorescence signal). The ratio of cilia and cell body of was calculated, and statistical analysis was performed using Student’s t test.
Generation of Bovine ATP8A2 and dATP8BE351Q Transgenes.
dATP8BE351Q was generated by introducing the GAG to CAG mutation in two PCR primers and amplifying the regions upstream and downstream, spanning unique restriction sites in the dATP8B cDNA (Aat2 upstream and Sfi1 downstream). These products were mixed and amplified to produce a 920-bp fragment that was confirmed for the correct sequence and cloned into the Aat2-Sfi digested wild-type cDNA and cloned into pUASt (42) for production of transgenic animals. Bovine ATP8A2 (ATPase II) was the gift of Xiao-Song Xie and was amplified, cloned into PCR2.1, sequenced and digested with Xho1 and Xba1, and cloned into pUASt.
Supplementary Material
Acknowledgments
We thank Xiao-Song Xie for the gift of the bovine ATPaseA2 isoform clone and anti-peptide antibodies (32), Leslie Vosshall for anti-Orco antiserum, Richard Benton and Barry Dickson for fly stocks, Francesca Bissman for generating transgenic flies, Charles Zuker for the Zuker mutant collection (2), and Coral Warr and Konrad Zinsmaier for sharing results before publication. This work was supported by National Institutes of Health (NIH) Grant DC002539 and NIH Grant R01 DCD011751 (to D.P.S.) and National Research Foundation of Korea Grant (NRF) funded by the Ministry of Education Grant NRF 2010-0024652 and Ministry of Science, ICT and Future Planning Grant 2009-0066616 (to T.S.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401938111/-/DCSupplemental.
References
- 1.Jin X, Ha TS, Smith DP. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci USA. 2008;105(31):10996–11001. doi: 10.1073/pnas.0803309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koundakjian EJ, Cowan DM, Hardy RW, Becker AH. The Zuker collection: A resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics. 2004;167(1):203–206. doi: 10.1534/genetics.167.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26(34):8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445(7123):86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 5.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104(9):3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136(1):149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4(2):e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Ronderos DS, Lin CC, Potter CJ, Smith DP. Farnesol-detecting olfactory neurons in Drosophila. J Neurosci. 2014;34(11):3959–3968. doi: 10.1523/JNEUROSCI.4582-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellen HJ, et al. The BDGP gene disruption project: Single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167(2):761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drysdale RA, Crosby MA. FlyBase Consortium FlyBase: Genes and gene models. Nucleic Acids Res. 2005;33(Database issue):D390–D395. doi: 10.1093/nar/gki046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folmer DE, Elferink RP, Paulusma CC. P4 ATPases—lipid flippases and their role in disease. Biochim Biophys Acta. 2009;1791(7):628–635. doi: 10.1016/j.bbalip.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 13.López-Marqués RL, Holthuis JC, Pomorski TG. Pumping lipids with P4-ATPases. Biol Chem. 2011;392(1-2):67–76. doi: 10.1515/BC.2011.015. [DOI] [PubMed] [Google Scholar]
- 14.Paulusma CC, Elferink RP. P4 ATPases—the physiological relevance of lipid flipping transporters. FEBS Lett. 2010;584(13):2708–2716. doi: 10.1016/j.febslet.2010.04.071. [DOI] [PubMed] [Google Scholar]
- 15.van der Velden LM, van de Graaf SF, Klomp LW. Biochemical and cellular functions of P4 ATPases. Biochem J. 2010;431(1):1–11. doi: 10.1042/BJ20100644. [DOI] [PubMed] [Google Scholar]
- 16.Groen A, et al. Complementary functions of the flippase ATP8B1 and the floppase ABCB4 in maintaining canalicular membrane integrity. Gastroenterology. 2011;141(5):1927–1937.e1–4. doi: 10.1053/j.gastro.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2(72):re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cacciagli P, et al. Disruption of the ATP8A2 gene in a patient with a t(10;13) de novo balanced translocation and a severe neurological phenotype. Eur J Hum Genet. 2010;18(12):1360–1363. doi: 10.1038/ejhg.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onat OE, et al. Missense mutation in the ATPase, aminophospholipid transporter protein ATP8A2 is associated with cerebellar atrophy and quadrupedal locomotion. Eur J Hum Genet. 2013;21(3):281–285. doi: 10.1038/ejhg.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman JA, et al. Phospholipid flippase ATP8A2 is required for normal visual and auditory function and photoreceptor and spiral ganglion cell survival. J Cell Sci. 2014;127(Pt 5):1138–1149. doi: 10.1242/jcs.145052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MS, Repp A, Smith DP. LUSH odorant-binding protein mediates chemosensory responses to alcohols in Drosophila melanogaster. Genetics. 1998;150(2):711–721. doi: 10.1093/genetics/150.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446(7135):542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 23.Fagan MJ, Saier MH., Jr P-type ATPases of eukaryotes and bacteria: Sequence analyses and construction of phylogenetic trees. J Mol Evol. 1994;38(1):57–99. doi: 10.1007/BF00175496. [DOI] [PubMed] [Google Scholar]
- 24.Olesen C, et al. The structural basis of calcium transport by the calcium pump. Nature. 2007;450(7172):1036–1042. doi: 10.1038/nature06418. [DOI] [PubMed] [Google Scholar]
- 25.Post RL, Hegyvary C, Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972;247(20):6530–6540. [PubMed] [Google Scholar]
- 26.Albers RW. Biochemical aspects of active transport. Annu Rev Biochem. 1967;36:727–756. doi: 10.1146/annurev.bi.36.070167.003455. [DOI] [PubMed] [Google Scholar]
- 27.Toyoshima C, Inesi G. Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 2004;73:269–292. doi: 10.1146/annurev.biochem.73.011303.073700. [DOI] [PubMed] [Google Scholar]
- 28.Toyoshima C, Mizutani T. Crystal structure of the calcium pump with a bound ATP analogue. Nature. 2004;430(6999):529–535. doi: 10.1038/nature02680. [DOI] [PubMed] [Google Scholar]
- 29.Anthonisen AN, Clausen JD, Andersen JP. Mutational analysis of the conserved TGES loop of sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 2006;281(42):31572–31582. doi: 10.1074/jbc.M605194200. [DOI] [PubMed] [Google Scholar]
- 30.Clausen JD, Vilsen B, McIntosh DB, Einholm AP, Andersen JP. Glutamate-183 in the conserved TGES motif of domain A of sarcoplasmic reticulum Ca2+-ATPase assists in catalysis of E2/E2P partial reactions. Proc Natl Acad Sci USA. 2004;101(9):2776–2781. doi: 10.1073/pnas.0400122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007;450(7167):289–293. doi: 10.1038/nature06328. [DOI] [PubMed] [Google Scholar]
- 32.Ding J, et al. Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. Effect of isoform variation on the ATPase activity and phospholipid specificity. J Biol Chem. 2000;275(30):23378–23386. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- 33.Natarajan P, Wang J, Hua Z, Graham TR. Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc Natl Acad Sci USA. 2004;101(29):10614–10619. doi: 10.1073/pnas.0404146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alder-Baerens N, Lisman Q, Luong L, Pomorski T, Holthuis JC. Loss of P4 ATPases Drs2p and Dnf3p disrupts aminophospholipid transport and asymmetry in yeast post-Golgi secretory vesicles. Mol Biol Cell. 2006;17(4):1632–1642. doi: 10.1091/mbc.E05-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen B, et al. Endocytic sorting and recycling require membrane phosphatidylserine asymmetry maintained by TAT-1/CHAT-1. PLoS Genet. 2010;6(12):e1001235. doi: 10.1371/journal.pgen.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darland-Ransom M, et al. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science. 2008;320(5875):528–531. doi: 10.1126/science.1155847. [DOI] [PubMed] [Google Scholar]
- 37.Wehman AM, Poggioli C, Schweinsberg P, Grant BD, Nance J. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr Biol. 2011;21(23):1951–1959. doi: 10.1016/j.cub.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sebastian TT, Baldridge RD, Xu P, Graham TR. Phospholipid flippases: Building asymmetric membranes and transport vesicles. Biochim Biophys Acta. 2012;1821(8):1068–1077. doi: 10.1016/j.bbalip.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han C, Jan LY, Jan YN. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc Natl Acad Sci USA. 2011;108(23):9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Bruyne M, Clyne PJ, Carlson JR. Odor coding in a model olfactory organ: The Drosophila maxillary palp. J Neurosci. 1999;19(11):4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30(2):537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 42.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 43.Bridges CB. Salavary chromosome maps: With a key to the banding of the chromosomes of Drosophila melanogaster. J Hered. 1935;26:60–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.