Abstract
The higher and selective cytotoxicity of [Pt(O,O′-acac)(γ-acac)(DMS)] toward cancer cell in both immortalized cell lines and in breast cancer cells in primary cultures, stimulated a pre-clinical study so as to evaluate its therapeutic potential in vivo. The efficacy of [Pt(O,O′-acac)(γ-acac)(DMS)] was assessed using a xenograft model of breast cancer developed by injection of MCF-7 cells in the flank of BALB/c nude mice. Treatment of solid tumor-bearing mice with [Pt(O,O′-acac)(γ-acac)(DMS)] induced up to 50% reduction of tumor mass compared with an average 10% inhibition recorded in cisplatin-treated animals. Thus, chemotherapy with [Pt(O,O′-acac)(γ-acac)(DMS)] was much more effective than cisplatin. We also demonstrated enhanced in vivo pharmacokinetics, biodistribution and tolerability of [Pt(O,O′-acac)(γ-acac)(DMS)] when compared with cisplatin administered in Wistar rats. Pharmacokinetics studies with [Pt(O,O′-acac)(γ-acac)(DMS)] revealed prolonged Pt persistence in systemic blood circulation and decreased nefrotoxicity and hepatotoxicity, major target sites of cisplatin toxicity. Overall, [Pt(O,O′-acac)(γ-acac)(DMS)] turned out to be extremely promising in terms of greater in vivo anticancer activity, reduced nephrotoxicity and acute toxicity compared with cisplatin.
Keywords: anticancer agents, breast cancer, pharmacokinetic, cisplatin, Pt(II)-analogs
Despite the ubiquitous use of cisplatin in oncology, this drug is associated with significant dose-limiting toxicities including nephrotoxicity and neurotoxicity. There is correspondingly a clear incentive to develop new strategies for safer and more effective cisplatin therapy.
In this context, new platinum(II) complexes containing acetylacetonate (acac) in the coordination sphere of the metal have been designed and synthesized by some of us: [PtCl(O,O'-acac)(DMSO)] with only one oxygen-bonded (O,O'-acac) acac, [Pt(O,O'-acac)(γ-acac)(DMSO)] containing both an O,O'-acac and a σ-bonded (γ-carbon bonded) acac, their dimethylsulphide (DMS) analogs having the same key structures.1, 2 The ability of these new Pt(II) compounds to induce cell death in human cervical carcinoma HeLa cells, in human breast cancer cells MCF-7 and in primary cultured human breast epithelial cells has been characterized and compared with the well-established anticancer drug, cisplatin.3, 4, 5 Among them, [Pt(O,O'-acac)(γ-acac)(DMS)] exhibited the highest in vitro activity from the panel of platinum(II) complexes evaluated.3 In addition, the reactivity of these novel complexes with nucleobases and sulfur ligands suggests that the mechanisms that underlie their cytotoxic activity may not necessarily require reaction with DNA.3 Interestingly, [Pt(O,O'-acac)(γ-acac)(DMS)] passes the blood-brain barrier and reaches the central nervous system in doses much higher than cisplatin. Nevertheless, [Pt(O,O'-acac)(γ-acac)(DMS)] displayed a low cytotoxicity in normal tissue, certainly less important than the neurotoxicity caused by cisplatin.6 The higher and selective cytotoxicity of [Pt(O,O'-acac)(γ-acac)(DMS)] toward the cancer cell observed in immortalized cell lines of the breast was confirmed in breast cancer cells in primary cultures.4, 5 The [Pt(O,O'-acac)(γ-acac)(DMS)] produces apoptotic effects at concentrations lower than cisplatin, whose undesirable side effects continue to limit its effectiveness. The selectivity of [Pt(O,O'-acac)(γ-acac)(DMS)] effects, stimulates a more detailed study aimed at pre-clinical investigation of its therapeutic potential in vivo.
Results
In vivo toxicity
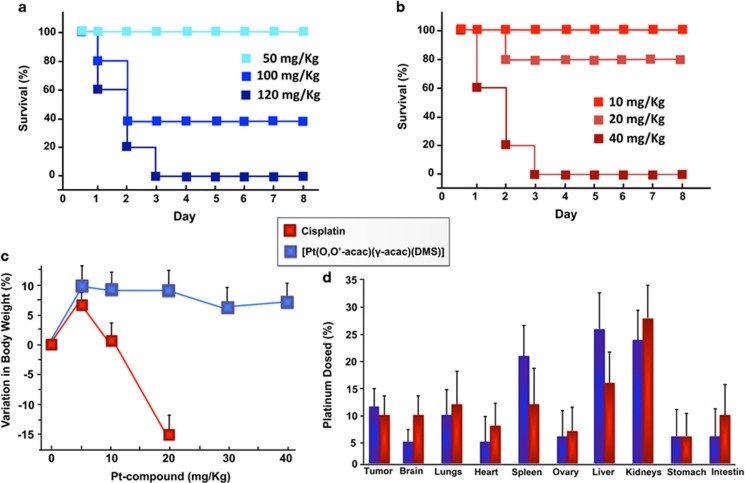
We examined the maximum tolerated dose (MTD) of various doses of cisplatin or [Pt(O,O'-acac)(γ-acac)(DMS)] after a single intravenous injection in female athymic mice. The MTD was estimated based on the threshold at which all animals survived, as shown in the Kaplan–Meier survival curve. In the [Pt(O,O'-acac)(γ-acac)(DMS)] group, mice receiving doses up to 50 mg/kg tolerated the dose, but three of five mice and all five mice, respectively, in the 100 and 120 mg/kg groups died (Figure 1a).
Figure 1.
Survival rate of Nude BALB/c mice treated with [Pt(O,O'-acac)(γ-acac)(DMS)] (a) and with cisplatin (b). (c) Body weight variations observed after 10 days from the intravenous administration of [Pt(O,O'-acac)(γ-acac)(DMS)] and cisplatin. Results represent the average weight of the six animals per group. (d) Tissue distribution of [Pt(O,O'-acac)(γ-acac)(DMS)] and cisplatin in female mice, after 30 days from the intravenous administration. Statistical analyses were performed using one-way ANOVA with a Tukey post hoc test, P<0.05 (compared with control)
Only a dose of 20 mg/kg of cisplatin was tolerated by the athymic mice, but they had significant weight loss, whereas those receiving lower doses did not (Figure 1b).
We therefore conclude that the MTD for [Pt(O,O'-acac)(γ-acac)(DMS)] is 50 mg/kg and that of cisplatin is 20 mg/kg.
To further define the MTD, in the [Pt(O,O'-acac)(γ-acac)(DMS)] group, the overall toxicity as revealed by body weights was monitored over 28 days; mice receiving 5 mg/kg, 10 and 20 mg/kg [Pt(O,O'-acac)(γ-acac)(DMS)] showed normal weight gain (Figure 1c).
During the observation period, no deterioration in health was observed in mice treated with 5 mg/kg and 10 mg/kg [Pt(O,O'-acac)(γ-acac)(DMS)], and the overall behavior was no different compared with that observed for untreated animals. The [Pt(O,O'-acac)(γ-acac)(DMS)]-treated mice did not develop significant polyuria, as in cisplatin-treated mice (0.85±0.1 ml/24 h and 2.8±0.5 ml/24 h [Pt(O,O'-acac)(γ-acac)(DMS)] versus cisplatin, P<0.05).
The comparative tissue distributions of [Pt(O,O'-acac)(γ-acac)(DMS)] and cisplatin were studied by measuring Pt content after a single intravenous dose. [Pt(O,O'-acac)(γ-acac)(DMS)] showed a very similar distribution pattern to that typically exhibited by Pt compounds including cisplatin, the maximum concentration of Pt occurring in the kidney, liver and spleen. The highest Pt concentration was found after 30 days (Figure 1d). Statistical analyses of these data were performed using one-way ANOVA with Tukey post hoc test, P<0.05 (compared with control).
[Pt(O,O'-acac)(γ-acac)(DMS)] inhibits tumor growth in a xenograft murine model
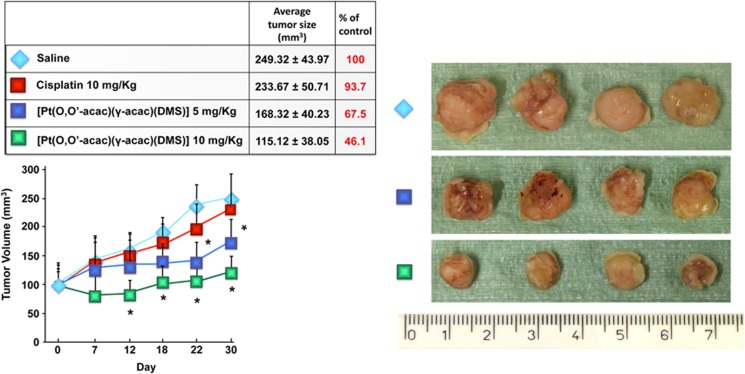
We studied the efficacy of [Pt(O,O'-acac)(γ-acac)(DMS)] using a xenograft model of breast cancer developed by injection of MCF-7 cells in the flank of BALB/c nude mice. After tumors had grown to ∼100 mm3, the mice were randomized into five groups in such a manner as to minimize weight and tumor size differences among the groups. After administering a single intravenous of saline as a control, or two doses (5 and 10 mg/kg) of [Pt(O,O'-acac)(γ-acac)(DMS)] or cisplatin, the tumor volumes of BALB/c mice were measured by a vernier calliper every 3 days for 30 days in total. The average tumor volumes of each group were calculated and tumor growth curves were drawn as usual. Statistical analysis was carried out using variance analysis and Student's t-test, and differences were considered significant where P<0.05.
Over the course of 30 days, the average tumor volume increased from 98,50 ±34.27 to 249.32±43.97 mm3 for the saline group, 233.67±50.71 mm3 for the cisplatin group (10 mg/kg; P>0.05), 168.32±40.23 mm3 for the group treated with 5 mg/kg of [Pt(O,O'-acac)(γ-acac)(DMS)] (P<0.05) and 115.12±38.05 mm3 for the group treated with 10 mg/kg of [Pt(O,O'-acac)(γ-acac)(DMS)] (P<0.05) (Figure 2). Thus, the results indicate that 10 mg/kg [Pt(O,O'-acac)(γ-acac)(DMS)] was more effective than 10 mg/kg cisplatin over the first 12 days.
Figure 2.
Balb/c nude mice carrying breast cancer developed by injection of MCF-7 cells (around 100 mm3) received intravenous [Pt(O,O'-acac)(γ-acac)(DMS)] (5 and 10 mg/kg) or cisplatin (10 mg/kg). The results are presented as average of six animals and are statistically different from control at *P<0.05. After killing, the tumors were collected and measured
Biodistribution and excretion in vivo
The distribution and excretion of [Pt(O,O'-acac)(γ-acac)(DMS)] and cisplatin were also investigated in male Sprague Dawley rats, following intravenous administration of the MTDs of the compounds.
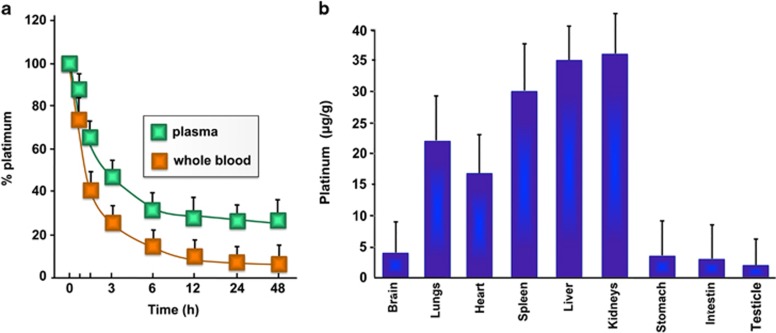
Biodistribution of [Pt(O,O'-acac)(γ-acac)(DMS)] after intravenous administration was determined by measuring platinum concentrations in plasma, whole blood and peripheral tissues at different time (Figure 3). Consistent with previous reports,7, 8 free cisplatin disappeared rapidly from circulation, with only about 9% of the injected dose detectable in plasma 5 min after dosing. However, [Pt(O,O'-acac)(γ-acac)(DMS)] showed remarkably prolonged blood circulation, with more than 65% of the injected dosage retained 1 h after dosing and about 26% of the injected dose detectable 24 h after dosing. The Pt remaining in systemic circulation 1 h post [Pt(O,O'-acac)(γ-acac)(DMS)] administration was 40% in blood, suggesting that Pt does not distribute extensively in red blood cells (Figure 3a).
Figure 3.
(a) Variation of percentage Pt dose in blood and plasma with time following the administration of [Pt(O,O'-acac)(γ-acac)(DMS)] intravenously to rat. (b) Tissue distribution of [Pt(O,O'-acac)(γ-acac)(DMS)] in male rats, after 48 h from the intravenous administration. Statistical analyses were performed using one-way ANOVA with a Tukey post hoc test, P<0.05 (compared with control)
[Pt(O,O'-acac)(γ-acac)(DMS)]-treated rats had a fourfold higher maximum Pt than cisplatin-treated rats (Cmax= 90.21± 3.1 μg/ml versus 22.62±1.8 μg/ml for [Pt(O,O'-acac)(γ-acac)(DMS)] and cisplatin, respectively P<0.001 n=6; Table 1), as well as 20-fold higher plasma AUC0–24 (868.20±9.6 h·μg/ml versus 40.38±6.2 h·μg/ml, for [Pt(O,O'-acac)(γ-acac)(DMS)] and cisplatin, respectively P<0.001 n=6; Table 1). The mean area under curve for total Pt in the blood is about 30% of that seen in plasma; the mean time to maximum serum concentration (Tmax) was 0.5 h in both whole blood and plasma (Figure 3a and Table 1).
Table 1. Pharmacokinetic parameters for [Pt(O,O'-acac)(γ-acac)(DMS)].
| Pharmacokinetic parameters | AUC0–24 (h-μg/ml) | Cmax (μg/ml) | Tmax (h) | CL (ml/h) | Vd (ml/kg) | Λ |
|---|---|---|---|---|---|---|
| In plasma | 868.20 | 90.21 | 0.5 | 0.57 | 1.8 | 0.32 |
| In whole blood | 263.33 | 68.78 | 0.5 | 2.6 | 8.4 | 0.31 |
Abbreviations: AUC, area under curve; Cmax, maximum concentration observed; CL, clearance; Tmax, time at maximum concentration; Vd, volume of distribution
Both clearance and volume of distribution (Vd) were decreased by 15-fold (0.57±0.2 ml/h versus 7.98±1.2 ml/h, for [Pt(O,O'-acac)(γ-acac)(DMS)] and cisplatin, respectively P<0.001 n=6; Table 1) and 46-fold (1.8±0.2 ml/Kg versus 82.67±2.2 ml/Kg, for [Pt(O,O'-acac)(γ-acac)(DMS)] and cisplatin, respectively P<0.001 n=6; Table 1), in the [Pt(O,O'-acac)(γ-acac)(DMS)] treatment group compared with the cisplatin-treatment group.
The rat tissue distributions of [Pt(O,O'-acac)(γ-acac)(DMS)] are shown in Figure 3b. After a single intravenous dose of [Pt(O,O'-acac)(γ-acac)(DMS)], kidney, liver and spleen showed the highest Pt concentration at 48 h. Statistical analyses of these data were performed using one-way ANOVA with a Tukey post hoc test, P<0.05 (compared with control). It is very interesting to note that the renal toxicity after treatment with [Pt(O,O'-acac)(γ-acac)(DMS)] is less than that of cisplatin as creatinine levels were not significantly different in the treated compared with the control group (Table 2). In addition, there was no effect on the total protein, the aspartate aminotransferase and alanine aminotransferase plasma levels, indicating a not obvious toxicity to the liver (Table 2).
Table 2. Total protein, creatinine, aspartate aminotransferase, alanine aminotransferase in rats after a single intravenous administration of [Pt(O,O'-acac)(γ-acac)(DMS)].
| mg/Kg | Creatinine mg/dl | Total protein g/dl | Aspartate aminotransferase U/l (GOT) | Alanine aminotransferase U/l (GPT) |
|---|---|---|---|---|
| 0 | 0.42±0.03 | 4.77±5.5 | 85.3±7.6 | 28.4±7.5 |
| 5 | 0.40±0.02 | 5.38±2.9 | 86.7±9.2 | 31.3±6.5 |
| 10 | 0.42±0.03 | 4.31±8.5 | 86.4±6.5 | 29.7±8.3 |
| 20 | 0.45±0.06 | 5.01±5.3 | 92.3±8.6 | 28.6±5.6 |
We also determined the Pt content by atomic absorption spectroscopy, in the urine and feces after [Pt(O,O'-acac)(γ-acac)(DMS)] or cisplatin intravenous administration. Excretion of [Pt(O,O'-acac)(γ-acac)(DMS)] was much lower than that of the cisplatin. The 24-h cumulative Pt excretion after [Pt(O,O'-acac)(γ-acac)(DMS)] was about eight-times less than after cisplatin treatment (4±1.6 versus 31.6±3.4%), and 8% was eliminated after 48 h. Fecal recovery represented 2.5% of the administered dose after 24 h, and 9% was excreted in the feces after 72 h.
Effects of [Pt(O,O'-acac)(γ-acac)(DMS)] on kidney, liver and peripheral nervous system
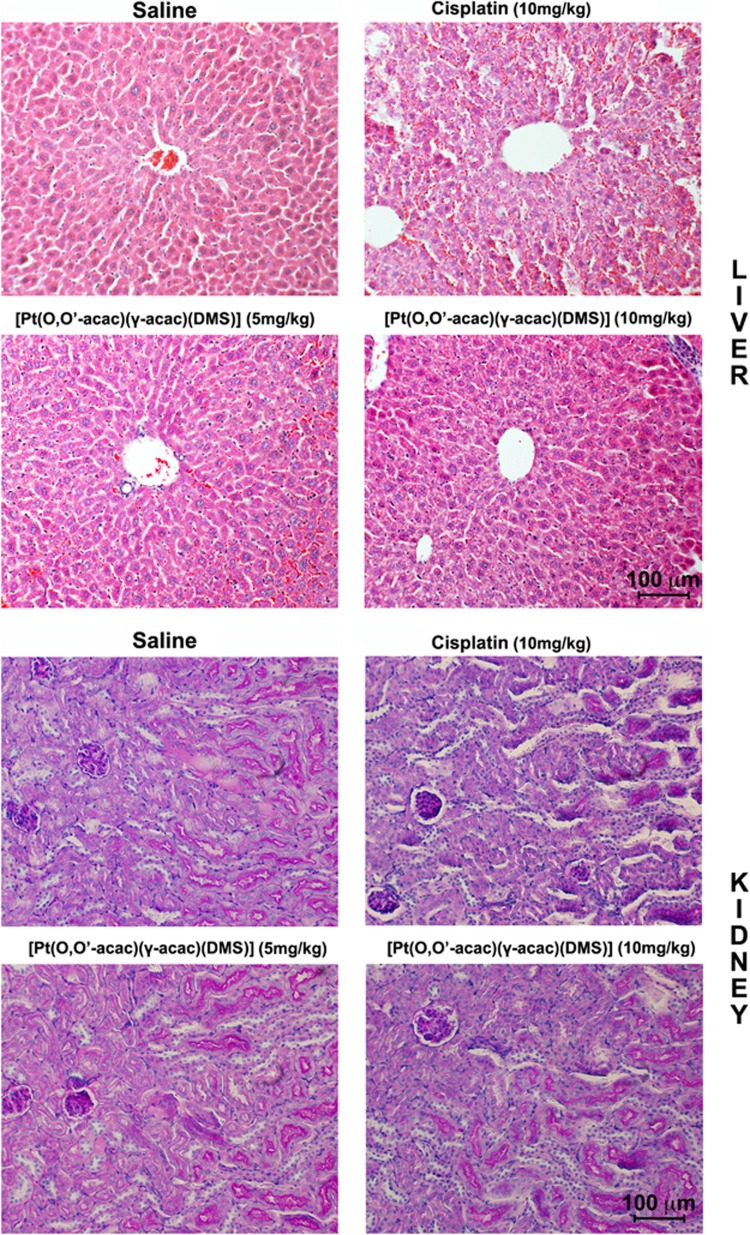
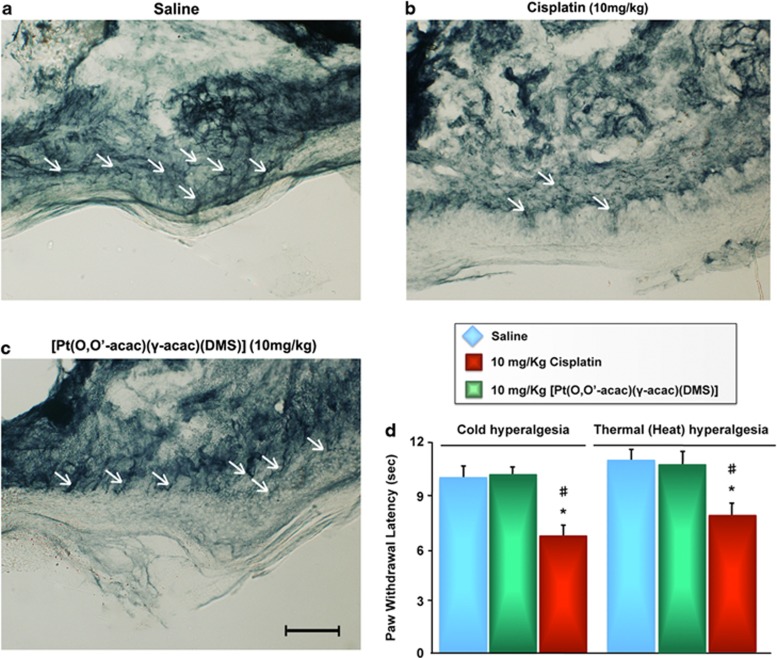
Light microscopic examination of the hematoxylin and eosin (H&E) stained sections from day-28 killed animals, indicated a focal subcapsular tubular vacuolization, dilated subcapsular space and consistent basophilia in kidneys, and also dilated intra-lobular spaces and evident edema in liver from all animals in the cisplatin-treated group (Figure 4). Interestingly, no histopathological changes were observed in liver and kidneys from [Pt(O,O'-acac)(γ-acac)(DMS)]-treated group mice, compared with the controls, despite much higher platinum accumulation in these tissues relative to the compound treatment (Figure 4). In addition, punch skin biopsies were performed on the mice hind paws, and sections were stained with a monoclonal antibody to neuron-specific ubiquitin hydrolase, PGP9.5. The number of intraepidermal sensory fibers did not decrease in the [Pt(O,O'-acac)(γ-acac)(DMS)]-treated mice group (Figure 5c).
Figure 4.
Histopathological changes in liver and kidney tissues from toxicity analysis in female Balb/c mice. Light microscopy images of hematoxylin and eosin-stained liver (top panel, day 28) and kidney (down panel, day 28) were taken at a magnification of x20. Tissue samples were collected at day 28 following intravenous administration of [Pt(O,O'-acac)(γ-acac)(DMS)] or cisplatin at different doses. Scale bar 100 μm
Figure 5.
PGP9.5-staining shows epidermal-terminal innervation (arrows) in the hind plantar paw skin of normal mice (a) similar to those in [Pt(O,O'-acac)(γ-acac)(DMS)]-treated mice (c). Loss of PGP9.5 positive nerve fibers is evident following cisplatin treatment (b). Scale bar 100 μm. (d) Hindpaw sensitivity to cold and noxious heat was significantly increased in cisplatin- but not in [Pt(O,O'-acac)(γ-acac)(DMS)]-treated mice compared with vehicle-treated mice, as determined by reduced hindpaw withdrawal latency to immersion in cold water (4.5 °C). Bars represent the mean±S.E.M. of mice per group. *P<0.05 versus vehicle group. #P<0.05 versus [Pt(O,O'-acac)(γ-acac)(DMS)] group
No cold hyperalgesia was present in [Pt(O,O'-acac)(γ-acac)(DMS)]-treated rats, based on a significantly reduced hindlimb withdrawal latency following immersion in a cold 4.5 °C water bath. In addition, thermal response to noxious heat was unaffected in hind paws of [Pt(O,O'-acac)(γ-acac)(DMS)] infused rats (Figure 5d). All baseline measures were not significantly different between groups before treatment (data not shown).
Discussion
We evaluated the anticancer activity in vivo of [Pt(O,O'-acac)(γ-acac)(DMS)] on the murine-tumor models to confirm the promising results previously obtained in vitro toward several human-tumor cell lines and primary breast cell.3, 4, 5, 9 Chemotherapy with [Pt(O,O'-acac)(γ-acac)(DMS)] was proved to be much more effective than cisplatin. Remarkably, treatment of solid tumor-bearing mice with [Pt(O,O'-acac)(γ-acac)(DMS)], induced up to 50% reduction of tumor mass compared with an average 10% inhibition recorded in cisplatin-treated animals.
Although these in vivo experiments undoubtedly demonstrate the greater anticancer activity of [Pt(O,O'-acac)(γ-acac)(DMS)], it might be argued that these results could have been largely predictable, as the platinum content after [Pt(O,O'-acac)(γ-acac)(DMS)] treatment was notably higher than after cisplatin, as also previously shown in vitro.3 This difference in the platinum concentration is due to the characteristics of Pt compounds, which can bind to protein or other tissue compositions. Moreover, the effectiveness of this compound is also supported by the observation that chemotherapy with [Pt(O,O'-acac)(γ-acac)(DMS)] was well tolerated. In this regard, the body weight loss of the animals was recorded daily as a sign of general toxicity. Intriguingly, mice treated with [Pt(O,O'-acac)(γ-acac)(DMS)] did not lose body weight, did not suffer from anorexia and no deterioration in health was observed, whereas those treated with cisplatin looked prostrate and showed loss of weight.
Although cisplatin is one of the most effective anticancer drugs in use today, it conveys considerable toxicity to patients;10 on the other hand, very few toxicity data are available in the literature concerning anticancer agents with β-diketonate ligand as leaving groups.11, 12
Despite the much higher accumulation of platinum in kidney, liver and spleen in the [Pt(O,O'-acac)(γ-acac)(DMS)]-treated group (indicated by pharmacokinetics and biodistribution), there was no significant change in toxicity markers of blood chemistry, indicating no obvious toxicity to the kidney and liver. Consistent with a previous report for cisplatin,13 the greater part of Pt appears to be excreted by the kidney. Increased levels of urinary total protein and creatinine in treated animals are widely recognized as a sign of general kidney injury.14 Nevertheless, [Pt(O,O'-acac)(γ-acac)(DMS)] did not cause changes in the urinary profile compared with control rats, even at a higher dose (20 mg/kg). This is a significant result considering that renal toxicity is the most severe limitation of cisplatin treatment.15 Despite the very little information available on cisplatin-induced liver injury, hepatotoxicity is also a dose-limiting side effect in cisplatin-based chemotherapy.16, 17, 18 In fact, high doses of cisplatin can alter the clinical situation of patients.19, 20, 21 Generally, liver toxicity of cisplatin is characterized by apoptotic lesions in the liver parenchyma22 and elevation of serum transaminases. [Pt(O,O'-acac)(γ-acac)(DMS)]-treated animals had normal serum levels of hepatic markers and no changes in tissue histopathology. In fact, histological investigations have confirmed the favorable toxicity profile of the tested [Pt(O,O'-acac)(γ-acac)(DMS)]. Microscopic evaluations of liver and renal tissues examined were considered similar with physiological conditions when compared with control animals. In addition, cisplatin-induced peripheral neuropathy can influence the quality of life and survivorship in patients. Here, mice receiving [Pt(O,O'-acac)(γ-acac)(DMS)] did not show an alteration in the sensory fibers ascending vertically between the keratinocytes to reach the stratum corneum of the epidermis and did not develop peripheral neuropathy as resulted by the hot/cold hyperalgesia test.
Most small-molecule anticancer drugs currently in clinical use, including cisplatin, doxorubicin and paclitaxel, are inherently associated with a lack of tumor selectivity and short blood circulation time, which causes various toxic side effects.23 In particular, cisplatin has an unfavorable pharmacokinetic profile, and all Pt-based anticancer drugs with a low molecular weight have a short half-life in the blood circulatory system.24 Nevertheless, pharmacokinetic studies with nanoparticles encapsulating Pt revealed prolonged persistence in systemic blood circulation and decreased accumulation of Pt in the kidneys, a major target site of cisplatin toxicity.25
Significantly prolonged circulation of [Pt(O,O'-acac)(γ-acac)(DMS)] was observed. In addition, [Pt(O,O'-acac)(γ-acac)(DMS)] is more localized in the plasma compartment, a favorable indication for Pt being available to reach the target-tumor sites.
In conclusion, [Pt(O,O'-acac)(γ-acac)(DMS)] turned out to be extremely promising in terms of greater in vivo anticancer activity, reduced nephrotoxicity and acute toxicity compared with cisplatin, thus providing a solid starting point for its validation as a suitable candidate for further pharmacological testing.
Materials and Methods
Reagents
[Pt(O,O'-acac)(γ-acac)(DMS)] was prepared according to previously reported procedures.1, 2
Tumor cells
MCF-7 human breast cancer cells were cultured in DMEM (Celbio, Pero, Milan, Italy) supplemented with 10% fetal bovine serum, penicillin (100 U/ml) and streptomycin (100 mg/ml). Cells were routinely passed by treatment with trypsin (0.05%)/EDTA.
Ethics statement
All animals received care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, published by the National Institutes of Health (NIH Publication No. 86-23, revised 1985), as well as in accordance with the Italian laws on animal experimentation (art. 4 and 5 of D.L. 116/92).
Evaluation of maximum tolerated dose
Nude BALB/c mice were randomly allocated to seven groups (five animals per group) and treated with a single intravenous injection of [Pt(O,O'-acac)(γ-acac)(DMS)] (50, 100 and 120 mg/kg), cisplatin (10, 20 and 40 mg/kg) or saline as a control. The weight and physical states of all the animals were monitored for a period of 8 days.
Biodistribution and excretion study
The blood persistence properties of [Pt(O,O'-acac)(γ-acac)(DMS)] and cisplatin were determined using male Wistar rats weighing ∼150 g. The animals, five per group, were injected in the tail vein with [Pt(O,O'-acac)(γ-acac)(DMS)] (10 mg/kg), cisplatin (10 mg/kg) or saline. At predetermined time intervals, blood samples were collected in pre-weighed heparinized tubes and centrifuged to get the plasma. The percentage of Pt was calculated by taking into consideration that blood constitutes 7% of the body weight and plasma constitutes 55% of the blood volume.26 Collective urine samples were accumulated over 72 h and stored frozen until directly analyzed by the flameless atomic absorption spectrometry. Tissue samples required prior digestion in concentrated nitric acid. Gentle heating was required to complete the digestion. Following evaporation to near dryness, the digests were taken up in 1 N HCl (2 ml) and again heated to near dryness to remove excess nitric acid. After repeating this last stage with 0.1 N HCl (2 ml), the digests were dissolved in 1 ml of 0.1 N HCl for Pt analysis.
Platinum content in the samples was determined by atomic absorption spectrometry on a Varian SpectrAA-880Z spectrometer (Varian, Inc Vacuum Technologies, Palo Alto, CA, USA).
In vivo xenograft experiments
Nude BALB/c mice (6 wk old, female, 20 to 30 g body weight) were purchased from Harlan (Carezzana, Italy) and maintained under pathogen-free conditions. The mouse MCF-7 xenograft tumor model was developed by injecting 7.5 × 106 cells of a 0.15 ml MCF-7 cell suspension into the right flank of a BALB/c mouse. Tumor cells were allowed to grow for 4 weeks without hormone support, thereafter the mice received weekly s.c. injections of 17 β-oestradiol valerate (0.1 mg/kg body weight), in sesame oil.27 Animals were monitored daily for general health and body weights were measured twice weekly. Tumor size was measured with slide callipers and volumes were calculated as (LxW2)/2, where L and W are the major and minor diameters, respectively. Once tumor volumes reached ∼100 mm3, mice were randomized to treatment and control groups of five per group. Test animals received a single intravenous of saline as a control, or two doses (5 and 10 mg/kg) of [Pt(O,O'-acac)(γ-acac)(DMS)] or cispatin, the tumor volumes of BALB/c mice were measured by a vernier calliper every 3 days for 30 days in total.
Histopathology
The tissues of all experimental groups of animals were dissected out and immediately placed in a paraformaldehyde solution, (4%) and 20 h later it was placed in 70% ethanol until paraffin inclusion. The liver and renal tissues were included and cut into 8 μm serial sections (through the medio-lateral extent) and used for histological analysis. Sections were deparaffinized and processed for staining with H&E.
Punch skin biopsies from the plantar surface of the hind paws were performed and fixed in a Zamboni fixative for 24 h. Biopsies were cryoprotected with 20% sucrose in PBS overnight at 4 °C. Immunohistochemistry procedures were performed as previously described, using PGP9.5 antibody.28
Behavioral measurements of induced neuropathy
Cold sensitivity/hyperalgesia was assessed by immersion of the rats hindpaw into a water bath containing cold (4.5 °C) water, and latency to paw withdrawal was measured using a 1/100th second digital timer. Only one hindpaw was tested during each immersion, with the maximum cutoff time limited to 20 s.
Sensitivity to noxious heat was measured using a Thermal Paw Stimulator (University of California, San Diego, CA, USA) after a 15 min acclimation period. The intensity of radiant heat was adjusted so that the vehicle-treated rats responded to the heat by elevating or licking the hindpaw approximately 10 s after the heat was initiated. A cutoff latency of 20 s was used to prevent tissue damage.
Acknowledgments
The work presented was supported by the Italian Ministry of Research and University (PRIN. 2009).
Glossary
- AUC
area under curve
- CL
clearance
- Cmax
maximum concentration
- Tmax
time at maximum concentration
- Vd
volume of distribution
- FBS
fetal bovine serum
The authors declare no conflict of interest.
Footnotes
Edited by G Ciliberto
References
- De Pascali SA, Papadia P, Ciccarese A, Pacifico C, Fanizzi FP. First examples of β-diketonate platinum II complexes with sulfoxide ligands. Eur J Inorg Chem. 2005;5:788–796. [Google Scholar]
- De Pascali SA, Papadia P, Capoccia S, Marchiò L, Lanfranchi M, Ciccarese A, et al. Hard/soft selectivity in ligand substitution reactions of β-diketonate platinum(II) complexes. Dalton Trans. 2009;37:7786–7795. doi: 10.1039/b909209a. [DOI] [PubMed] [Google Scholar]
- Muscella A, Calabriso N, De Pascali SA, Urso L, Ciccarese A, Fanizzi FP, et al. New platinum(II) complexes containing both an O,O'-chelated acetylacetonate ligand and a sulfur ligand in the platinum coordination sphere induce apoptosis in HeLa cervical carcinoma cells. Biochem Pharmacol. 2007;74:28–40. doi: 10.1016/j.bcp.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Muscella A, Calabriso N, Fanizzi FP, De Pascali SA, Urso L, Ciccarese A, et al. [Pt(O,O'-acac)(γ-acac)(DMS)], a new Pt compound exerting fast cytotoxicity in MCF-7 breast cancer cells via the mitochondrial apoptotic pathway. Br J Pharmacol. 2008;153:34–49. doi: 10.1038/sj.bjp.0707576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscella A, Vetrugno C, Fanizzi FP, Manca C, De Pascali SA, Marsigliante S. A new platinum(II) compound anticancer drug candidate with selective cytotoxicity for breast cancer cells. Cell Death Dis. 2013;4:e796. doi: 10.1038/cddis.2013.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri S, Piccolini VM, Santin G, Bottone MG, De Pascali SA, Migoni D, et al. The developmental neurotoxicity study of platinum compounds. Effects of cisplatin versus a novel Pt(II) complex on rat cerebellum. Neurotoxicol Teratol. 2011;33:273–281. doi: 10.1016/j.ntt.2010.09.005. [DOI] [PubMed] [Google Scholar]
- McIntosh DP, Cooke RJ, McLachlan AJ, Daley-Yates PT, Rowland M. Pharmacokinetics and tissue distribution of cisplatin and conjugates of cisplatin with carboxymethyldextran and A5B7 monoclonal antibody in CD1 mice. J Pharm Sci. 1997;86:1478–1483. doi: 10.1021/js960282u. [DOI] [PubMed] [Google Scholar]
- Newman MS, Colbern GT, Working PK, Engbers C, Amantea MA. Comparative pharmacokinetics, tissue distribution, and therapeutic effectiveness of cisplatin encapsulated in long-circulating, pegylated liposomes (SPI-077) in tumor-bearing mice. Cancer Chemother Pharmacol. 1999;43:1–7. doi: 10.1007/s002800050855. [DOI] [PubMed] [Google Scholar]
- Muscella A, Calabriso N, Vetrugno C, Fanizzi FP, De Pascali SA, Marsigliante S. The signalling axis mediating neuronal apoptosis in response [Pt(O,O'-acac)(γ-acac)(DMS)] Biochem Pharmacol. 2011;81:1271–1285. doi: 10.1016/j.bcp.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Galanski M, Jakupec MA, Keppler BK. Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr Med Chem. 2005;12:2075–2094. doi: 10.2174/0929867054637626. [DOI] [PubMed] [Google Scholar]
- Caruso F, Rossi M, Tanski J, Sartori R, Sariego R, Moya S, et al. Synthesis, structure, and antitumor activity of a novel tetranuclear titanium complex. J Med Chem. 2000;43:3665–3670. doi: 10.1021/jm990539b. [DOI] [PubMed] [Google Scholar]
- Wilson JJ, Lippard SJ. In vitro anticancer activity of cis-diammineplatinum(II) complexes with β-diketonate leaving group ligands. J Med Chem. 2012;55:5326–5336. doi: 10.1021/jm3002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–422. doi: 10.1200/JCO.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- Trevisan A, Cristofori P, Fanelli G. Glutamine synthetase activity in rat urine as sensitive marker to detect S3 segmentspecific injury of proximal tubule induced by xenobiotics. Arch Toxicol. 1999;73:255–262. doi: 10.1007/s002040050614. [DOI] [PubMed] [Google Scholar]
- Marzano C, Bettio F, Baccichetti F, Trevisan A, Giovagnini L, Fregona D. Antitumor activity of a new platinum(II) complex with low nephrotoxicity and genotoxicity. Chem Biol Interact. 2004;148:37–48. doi: 10.1016/j.cbi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Tang H, Jin YP. Study of toxic effects on hearing, kidney and liver of mice induced by anticancer agent of cisplatin and their mechanisms. Chin Pharmacol Bull. 2004;20:82–85. [Google Scholar]
- Pratibha R, Sameer R, Rataboli PV, Bhiwgade DA, Dhume CY. Enzymatic studies of cisplatin induced oxidative stress in hepatic tissue of rats. Eur J Pharmacol. 2006;532:290–293. doi: 10.1016/j.ejphar.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Iseri S, Ercan F, Gedik N, Yuksel M, Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230:256–264. doi: 10.1016/j.tox.2006.11.073. [DOI] [PubMed] [Google Scholar]
- Cersosimo RJ. Hepatotoxicity associated with cisplatin chemotherapy. Ann Pharmacother. 1993;27:438–441. doi: 10.1177/106002809302700408. [DOI] [PubMed] [Google Scholar]
- Cavalli F, Tschopp L, Sonntag RW, Zimmermann A. A case of liver toxicity following cis-dichlorodiammineplatinum(II) treatment. Cancer Treat Rep. 1978;62:2125–2126. [PubMed] [Google Scholar]
- Pollera CF, Meglio F, Nardi M, Vitelli G, Marolla P. Cisplatin-induced hepatic toxicity. J Clin Oncol. 1987;5:318–319. doi: 10.1200/JCO.1987.5.2.318. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Habeebu SS, Klaassen CD. Metallothionein (MT)-null mice are sensitive to cisplatin-induced hepatotoxicity. Toxicol Appl Pharmacol. 1998;149:24–31. doi: 10.1006/taap.1997.8325. [DOI] [PubMed] [Google Scholar]
- Wang S, Mi JB, Li YZ, Chang WB, Ci YX, Zhao MZ, et al. Pharmacokinetics and tissue distribution of i.v. injection of polyphase liposome-encapsulated cisplatin (KM-1) in rats. Acta Pharmacol Sin. 2003;24:589–592. [PubMed] [Google Scholar]
- Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG, Ithakissios DS. PLGA-mPEG nanoparticles of cisplatin: In vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J Control Release. 2002;79:123–135. doi: 10.1016/s0168-3659(01)00530-2. [DOI] [PubMed] [Google Scholar]
- Dhara S, Kolishettib N, Lippard SJ, Farokhzadb OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. PNAS. 2010;108:1850–1855. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- Kasukabe T, Okabe-Kado J, Kato N, Sassa T, Honma Y. Effects of combined treatment with rapamycin and cotylenin a, a novel differentiation-inducing agent, on human breast carcinoma MCF-7 cells and xenografts. Breast Cancer Res. 2005;7:1097–1110. doi: 10.1186/bcr1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, et al. Cutaneous innervation in sensory neuropathies:evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]