Abstract
Homeobox genes constitute a large family of transcription factors that are essential during normal development and are often dysregulated in cancer. However, the molecular mechanisms by which homeobox genes influence cancer remain largely unknown. Here we show that the tissue-restricted cyclin A1 is a transcriptional target of the Six1 homeoprotein. Both genes are expressed in the embryonic but not the terminally differentiated mammary gland, and Six1-knockout mice show a dramatic reduction of cyclin A1 in the embryonic mammary gland. In addition, both genes are reexpressed in breast cancers. Six1 overexpression increases cyclin A1 mRNA levels and activity, cell proliferation, and tumor volume, whereas Six1 down-regulation decreases cyclin A1 mRNA levels and proliferation. Overexpression of Six1 in wild-type mouse embryonic fibroblasts, but not in knockout variants lacking the cyclin A1 gene, induces cell proliferation. Furthermore, inhibition of cyclin A1 in Six1-overexpressing mammary carcinoma cells decreases proliferation. Together these results demonstrate that cyclin A1 is required for the proliferative effect of Six1. We conclude that Six1 overexpression reinstates an embryonic pathway of proliferation in breast cancer by up-regulating cyclin A1.
The molecular pathways involved in oncogenesis often represent aberrations of processes that normally occur during embryogenesis (1). The homeobox superfamily of genes encodes transcription factors that are critical for normal development and are frequently inappropriately expressed in cancer (1, 2). However, because few of their in vivo transcriptional targets have been identified, there is an incomplete understanding of the molecular mechanisms by which they act or the relevance of their overexpression in cancer.
The Six family of homeobox genes has been implicated in the proliferation of progenitor populations before cell type specification (3–10). Six6 represses the cyclin-dependent kinase inhibitor p27, thereby promoting progenitor cell proliferation in the pituitary gland and the retina (3). In Six1-knockout mice, numerous organs fail to develop properly because of an increase in apoptosis and a decrease in cellular proliferation (6–8). Recently identified targets of Six1 include c-Myc and Gdnf, both which are implicated in cell growth and proliferation (7), but the role of these genes in stimulating Six1-mediated proliferation has not been determined.
Several members of the Six family have been implicated in the pathogenesis of human cancers (11–16). Because of their role in proliferation during normal development, it is possible that these genes, when aberrantly expressed, may play a role in the proliferative aspects of tumorigenesis. However, the molecular means by which the Six family members affect cancer remain unexplored.
Our previous data demonstrated that Six1 overexpression leads to an attenuation of the DNA damage-induced G2 checkpoint, suggesting a role for Six1 in the cell cycle and identifying a mechanism through which Six1 may affect tumorigenesis (15). We further demonstrated that Six1 overexpression occurs in 44% of primary breast cancers and 90% of metastatic lesions (15), and its overexpression has since been reported in Wilms' tumors (13) and rhabdomyosarcomas (14). In this study we examine additional roles of Six1 in the cell cycle and identify the molecular mechanism by which Six1 influences cellular proliferation. By directly activating cyclin A1 transcription, Six1 sets in motion a pathway for proliferation in normal development that can be aberrantly used in tumorigenesis. Thus, the transcriptional activation of cyclin A1, a tissue-restricted cyclin that is expressed in the embryonic mammary gland but not in the differentiatedadult mammary gland, has important implications for the role of Six1 in both normal development and cancer.
Materials and Methods
Generation of Adenoviral Constructs, Adenoviral Transductions, and Microarray Analysis. Human Six1 was subcloned into pShuttle-CMV (a gift from Bert Vogelstein, Johns Hopkins Medical School, Baltimore) and recombinant adenovirus was prepared as described (44). Ad-GFP (a gift from Kornelia Polyak, Harvard Medical School, Boston) or Ad-LacZ (a gift from Jerry Schaak, University of Colorado Health Sciences Center) were used as controls. Transcriptional profiles were obtained for MCF12A (immortalized mammary epithelial) cells transduced with either Ad-Six1 or Ad-GFP at a multiplicity of infection of 10–50 in two independent experiments. Microarray analysis was performed as described (45), using the Affymetrix GeneChip U133A. Intensity values were scaled such that the overall fluorescence intensity of each microarray was equivalent. Expression values below baseline were set to 20. Raw gene expression data are available at www.broad.mit.edu/cancer/pub/six1. Six1-regulated genes were defined as those whose: (i) expression level at any point after infection with Ad-Six1 was greater than 3-fold above or below that in the uninfected time-zero control, in both independent experiments; (ii) fold change at a time point adjacent to the maximum or minimum was greater than 2, in both experiments; and (iii) expression level after infection with control adenovirus did not change by more than 3-fold from the uninfected time-zero control, in either experiment. For the experiments using the mouse embryonic fibroblasts (MEFs) from cyclin A1-knockout (Ccna1-/-) mice, all transductions with Ad-Six1 or Ad-LacZ were carried out at a multiplicity of infection of 50 and were performed with cells at early passages (passage 5–7).
Cell Culture and Transfections. All mammary epithelial cell lines were cultured as per recommendations from the American Type Cell Collection (ATCC). The 21T series of cell lines (16N, 21PT, 21NT, 21MT1, and 21MT2) were obtained from Ruth Sager (Dana–Farber Cancer Institute) and were cultured as previously described (15). With additional passages of the 21T series of cell lines, Six1 expression has altered such that all cancer cell lines currently express high levels of Six1 in approximately equal amounts. Six1 MCF7 stable cell lines were previously generated (15), and Six1 21PT transfectants were generated as previously described by using the SIXFL plasmid (15). Control lines for MCF7 transfectants constitute one cell line transfected with Six1 but not expressing the transgene (MCF7-Control1) and two lines transfected with pcDNA3.1(+)CAT (MCF7-Control2 and -Control3). Control lines for the 21PT series constitute three stable clones transfected with pcDNA3.1(+)LacZ. Transient transfections for short interfering RNA (siRNA) experiments were performed as described (19); however, the electroporator was set to pulse at 300 V with a 2-ms burst duration instead of 140 V and 1.5 ms, and 4-mm gap cuvettes were used rather than 1-mm gap cuvettes.
Quantitative Reverse Transcription Real-Time Polymerase Chain Reaction (qRT-PCR), Northern Blot, RT-PCR, Immunoprecipitation, and Western Blot Analyses. Total RNA from cell lines and primary breast tumors was isolated with TRIzol reagent according to the manufacturer's protocol (Invitrogen Life Technologies). qRT-PCR was performed with a model 7700 instrument (Applied Biosystems). Amplicons were detected by using TaqMan fluorescence probes as described elsewhere (20). Target genes were analyzed by using standard curves to determine relative levels of gene expression. Individual RNA samples were normalized according to the levels of 18S rRNA. Northern blotting and RT-PCR were performed as described (15, 21). All primers and probes used in this study are described in Table 1, which is published as supporting information on the PNAS web site. Immunoprecipitation of Six1 was performed after the methods of Sauk et al. (22). Western blot analyses using the anti-Six1 antibody were performed as described (23).
Immune-Complex Kinase Assays. Histone H1 kinase assays were performed as described (24). Cyclin A1, cyclin A2, and cdk2-associated kinases were immunoprecipitated by using the following antibodies: cyclin A1 (25), cyclin A2 (clone BF683, Santa Cruz Biotechnology), cdk2 (clone D-12, Santa Cruz Biotechnology).
Assays of Proliferation. Cell growth and flow cytometry experiments were performed as described (21, 26). Incorporation of 5-bromodeoxyuridine (BrdUrd) was quantitated by immunofluorescence performed on cells labeled with 10 μM BrdUrd for 1 or 3 h.
Tumorigenicity Assays. To assess the growth of tumors in nude mouse assays, five 8-week-old nude mice per cell line were injected s.c. in the flank with 1 × 107 cells (MCF7-SIX1 and MCF7-Control cell lines) suspended in 100 μl of medium without serum. All mice injected with MCF7 cells were supplemented with estrogen pellets (27), and tumor size was measured over a 6-week period. Volumes are reported as mm3, calculated by using the formula volume = 0.5 × length × width2.
Chromatin Immunoprecipitation (ChIP) Assays. ChIP assays were performed by using the Upstate Biotechnology ChIP procedure (see manufacturer's recommendations). Precipitated DNA was analyzed by using PCR supplemented with 0.5 μCi (1 μCi = 37 kBq) of [32P]dCTP. Primers flanking the Six1 site of activation (-207 to -18 of cyclin A1 promoter) and the negative control site (-2312 to -2107 of cyclin A1 promoter) are described in Table 1.
Statistical Analyses. Analysis of variance was used to test group effects, with post hoc comparisons based on the Tukey test. The Spearman rank correlation test (rS) was assessed to verify the association between expression levels of Six1 and cyclin A1 on log-transformed values [loge(x + 1)]. In our comparisons, P ≤ 0.05 was considered to indicate statistical significance.
Results and Discussion
Six1 Activates the Cyclin A1 Promoter. To gain insight into the molecular mechanism by which Six1 affects the cell cycle, we examined the gene expression profiles of immortalized human mammary MCF12A cells, which express low endogenous Six1 levels, transduced with either Six1-expressing or GFP-expressing adenovirus. When the criteria outlined in Materials and Methods were used, 21 Six1 up-regulated and 14 Six1 down-regulated genes were identified, some of which are known to be important in cell cycle control (Fig. 6, which is published as supporting information on the PNAS web site). In two independent experiments we found a Six1-dependent up-regulation of the tissue-restricted cyclin A1 (17, 18, 28). Cyclin A1 has previously been shown to be expressed only in early embryogenesis (18, 28), in the germ line (17), in hematopoiesis (25), and in the brain (29). Neither c-Myc nor Gdnf, previously identified Six1 targets in C2C12 cells (7), was upregulated when MCF12A mammary epithelial cells were transduced with Six1. This finding may represent a difference in the function of Six1 in different cell types, or it may be due to the nature of the experimental systems used to identify Six1 target genes. In addition to cyclin A1, p57kip2, a cyclin-dependent kinase inhibitor, was up-regulated by Six1. However, whereas cyclin A1 was reproducibly and consistently up-regulated by Six1 in all systems tested, p57kip2 was differentially regulated by Six1 in different cell types and systems, and as such we focused our attention on cyclin A1.
To determine whether Six1 activates the cyclin A1 promoter, MCF7 mammary carcinoma cells were transfected with a full-length human cyclin A1 promoter–luciferase reporter construct (30) in the presence of increasing concentrations of a Six1 expression plasmid. Six1 transactivated the cyclin A1 promoter in a dose-dependent manner, with activation reaching 12-fold at the highest concentration (Fig. 1A). To delineate the region within the cyclin A1 promoter through which Six1 confers its activity, cyclin A1 promoter deletion constructs (30) and Six1 expression constructs were cotransfected into MCF7 cells and luciferase assays were performed. The region between -37 and -112 of the cyclin A1 promoter was identified as necessary for activation by Six1 (Fig. 1B). This region does not contain a described consensus sequence for Six1 DNA binding (ATCCTGA) (7, 31), suggesting that Six1 may interact with additional sequences, either directly or indirectly. Binding of Six1 within the -37 to -112 region of the cyclin A1 promoter was confirmed by ChIP assays from the 21PT mammary carcinoma cell line, a line that expresses high endogenous levels of Six1 (Fig. 1C). These results demonstrate that Six1 activates cyclin A1 through an interaction with the cyclin A1 promoter.
Fig. 1.
Six1 directly activates the tissue-specific cyclin A1. (A) MCF7 cells were transfected with increasing concentrations of the human Six1 expression plasmid (SIXFL) plus the full-length human cyclin A1 promoter–luciferase construct. The relative fold increase in activity is compared with cells transfected with the empty vector plus the cyclin A1 promoter–luciferase construct and with cells transfected with SIXFL and the promotorless luciferase construct, all normalized to Renilla-luciferase activity. (B) Six1 activation of the cyclin A1 promoter in MCF7 cells occurs through the region from -112 to -37. Analysis was performed as described in A, with 5 μg of SIXFL and the promoter–luciferase constructs as depicted in the figure. (C) ChIP assays were performed in 21PT cells with the anti-Six1 antibody (23) or no antibody (control), and the immunoprecipitates were analyzed by PCR with cyclin A1 promoter-specific primers in the Six1 binding region (-207 to -18) or with primers upstream of the binding region (-2312 to -2107).
Six1 and Cyclin A1 Are Coordinately Regulated During Mammary Gland Development and Tumorigenesis. Cyclin A1 is implicated in cell cycle control in early embryogenesis and in germ cells (17, 18, 28), but its expression has not been examined during mammary gland development. To determine whether both Six1 and cyclin A1 are expressed in the developing mammary gland, we examined their expression throughout mammary gland development by qRT-PCR. Both Six1 and cyclin A1 are highly expressed in the embryonic mammary gland at day 18.5, with levels decreasing after birth (Fig. 2A). Cyclin A1 levels are dramatically reduced by the time of maturity (10 weeks), whereas Six1 levels decline more slowly. By the time the mammary gland is fully differentiated (pregnancy), very little Six1 expression is observed (Fig. 2A). Embryonic day 18.5 mammary glands from Six1-knockout mice (6) have a >90% reduction in cyclin A1 mRNA as compared with wild-type embryonic day 18.5 mammary glands (Fig. 2B), suggesting that Six1 is upstream of cyclin A1 in vivo. The restriction of cyclin A1 to the embryonic mammary gland is consistent with published data demonstrating that this A-type cyclin is relatively specific to embryogenesis and the germ line, as opposed to most adult somatic cells (17, 18, 28).
Fig. 2.
Six1 and its target cyclin A1 are reexpressed in human breast cancers. (A) qRT-PCR demonstrates that Six1 and cyclin A1 are highly expressed in the embryonic mammary gland as opposed to the fully differentiated adult pregnant and lactating mammary gland. (B) Reduction of cyclin A1 in Six1-knockout mammary glands. qRT-PCR using probes for Six1 and cyclin A1 was performed on mammary glands isolated from day 18.5 wild-type, heterozygote, and Six1-knockout embryos. Three independent samples for each condition were tested (wild type, heterozygote, and knockout), and each sample represents a pool of at least 15 animals. (C) qRT-PCR demonstrates that Six1 and cyclin A1 mRNA levels are overexpressed in numerous breast cancer cell lines as compared with immortalized normal breast lines. (D) Cyclin A1 levels correlate with Six1 in breast cancer samples as determined by qRT-PCR.
To determine the expression levels of Six1 and cyclin A1 in a pure mammary epithelial population, we performed qRT-PCR for both Six1 and cyclin A1 in immortalized mammary epithelial cell lines. Expression of both Six1 and cyclin A1 in human mammary epithelial cell lines MCF10A, MCF12A, and 16N is very low (Fig. 2C), consistent with our in vivo data that demonstrate that these genes are not highly expressed in the adult mammary gland (Fig. 2 A). In contrast, Six1 levels are dramatically increased in human mammary carcinoma cell lines, and this increase correlates with an increase in cyclin A1 expression (rS = 0.80, P < 0.005; Fig. 2C). Furthermore, analysis of 25 primary breast cancer samples for Six1 and cyclin A1 expression demonstrated a statistically significant correlation between the two (rS = 0.78, P < 0.001; Fig. 2D). Taken together, these facts suggest that Six1 may reinitiate an embryonic pathway in breast cancer by activating cyclin A1.
Six1 Induces Cellular Proliferation and Increases Tumor Volume in Nude Mice. Cyclin A1, like cyclin A2, can bind to and activate both cdk2 and cdk1 (32), and it has been implicated both in entrance into and progression through S phase as well as in the G2/M transition (24, 33, 34). Although we previously demonstrated that Six1 overexpression results in an attenuation of the G2 checkpoint (15), a phenotype consistent with overexpression of an A-type cyclin (35, 36), we had not determined whether overexpression of Six1 could contribute to cell cycle functions at the G1/S transition or in S phase. Examination of both Six1 mRNA and protein levels throughout the cell cycle demonstrates its presence as early as the G1/S boundary and its continued increase as cells progress through S phase and into mitosis (unpublished data). Furthermore, Six1 was recently identified as a target of E2F1, a transcription factor known to be critical for the G1/S transition (37). For these reasons, stable MCF7 transfectants overexpressing Six1 or control transfectants were examined for their proliferative potential as well as for cyclin A1 expression. Fig. 3A demonstrates overexpression of the Six1 protein in stable MCF7-SIX1 cell lines. These lines also overexpress cyclin A1 mRNA ≈2- to 3-fold, whereas cyclin A2 levels are unchanged (Fig. 3A). Consistent with the increase in cyclin A1 mRNA, cyclin A1-associated and cdk2-associated kinase activities are increased in the Six1-overexpressing cell lines, whereas cyclin A2-associated kinase activity remains unchanged (Fig. 3B). Six1-overexpressing cells have a statistically significant increase in proliferation (Fig. 3 C and D, and Fig. 7, which is published as supporting information on the PNAS web site), and an acceleration in cell cycle progression that occurs as early as the G1/S transition is observed these cells (Fig. 3E). This acceleration demonstrates that effects of Six1 on the cell cycle are not confined to the G2/M transition. Similar results were obtained in a different mammary carcinoma cell line stably overexpressing Six1 (Fig. 8, which is published as supporting information on the PNAS web site). When MCF7-SIX1 cell lines were injected into nude mice, tumors formed were significantly larger than those formed by the MCF7-Control cells (Fig. 3F). These data demonstrate that Six1 overexpression in mammary carcinoma cells results in hyperproliferation and a greater tumor burden, consistent with an increase in proliferation as the result of a direct activation of cyclin A1.
Fig. 3.
Overexpression of Six1 induces cyclin A1 expression and cellular proliferation. (A) MCF7-SIX1 transfectants have increased levels of cyclin A1 mRNA as compared with control transfectants. Upper shows a representative immunoprecipitation of Six1 in MCF7-SIX1 and MCF7-Control transfectants, whereas Lower demonstrates by qRT-PCR that cyclin A1 levels are increased in the Six1-transfectants, whereas cyclin A2 levels remain unchanged. (B) Immune-complex kinase assays demonstrate an increase in cyclin A1- and cdk2-associated kinase activities in Six1-overexpressing MCF7 cells, whereas cyclin A2-associated kinase activity remains unchanged. Histone H1 was used as a substrate for all immune complexes. (C and D) Assays measuring cell growth (C) and BrdUrd incorporation (D) demonstrate that Six1 overexpressing cell lines have a statistically significant increase in proliferation as compared with control cells. Each time point in the cell growth curve represents the mean of nine counts for each cell strain and is expressed as the mean ± SD of three cell lines per group. (E) Representative flow cytometry of one Six1-overexpressing (MCF7-SIX1–3) and one control (MCF7-Control 3) transfectant demonstrates that overexpression of Six1 accelerates cell cycle progression in MCF7 cells (similar results were obtained for the other Six1-overexpressing clones). The numbers on the bottom represent hours after release from serum starvation. (F) Expression of Six1 significantly increases tumor burden in nude mice. MCF7 cells (control and Six1-overexpressing) were injected into the flank of 8-week-old nude mice (previously implanted with estrogen pellets, four or five mice per group) at 10 × 106 cells per mouse in 100 μl of medium without serum, and tumor size was measured over a 6-week time period. Data are shown as mean ± SEM.
To determine whether the endogenous function of Six1 is to promote proliferation, Six1 was knocked down in mammary carcinoma cells expressing high levels of the gene (21PT cells). By using the pSUPER system and a high-efficiency electroporation protocol (38), vectors expressing either a Six1-specific double-stranded RNA (designed to target the Six1 mRNA at base pairs 409–428) or a scrambled version of the same RNA molecule were transfected into 21PT cells. When the Six1-specific vector was used, a rapid downregulation of Six1 mRNA and protein was observed and maintained over a 7-day time course (Fig. 4A). The decrease in Six1 levels resulted in a concomitant decrease in cyclin A1 mRNA, demonstrating that endogenous Six1 regulates cyclin A1 mRNA levels (Fig. 4B). Cell growth assays and BrdUrd incorporation assays showed a statistically significant decrease in proliferation when Six1 was down-regulated with siRNA (Fig. 4 C and D). Whereas Six1 overexpression accelerated cell cycle progression, a decrease in Six1 levels slowed the progression of cells through the cell cycle (Fig. 4E).
Fig. 4.
Inhibition of Six1 by siRNA in 21PT cells decreases cyclin A1 expression and cellular proliferation. (A) siRNA against Six1 decreases Six1 levels over a 7-day time course. Northern blot (Six1 and actin) and Western blot (Six1) analysis demonstrate that Six1 levels are decreased after introduction of the siRNA-SIX1 construct. For the Western blot analysis, similar protein concentrations were loaded per pair on each day, with increasing concentrations loaded on subsequent days. (B) qRT-PCR and Northern blot analyses demonstrate a decrease in cyclin A1 expression (Upper) when Six1 is inhibited (Lower). (C and D) Cell growth (C) and BrdUrd incorporation (D) assays show a statistically significant decrease in proliferation when Six1 is downregulated by siRNA. (E) Flow cytometry on propidium iodide-stained cells demonstrates a slower progression through the cell cycle when Six1 is downregulated. Inset represents Six1 protein levels over the time course. Numbers represent hours after release from serum starvation.
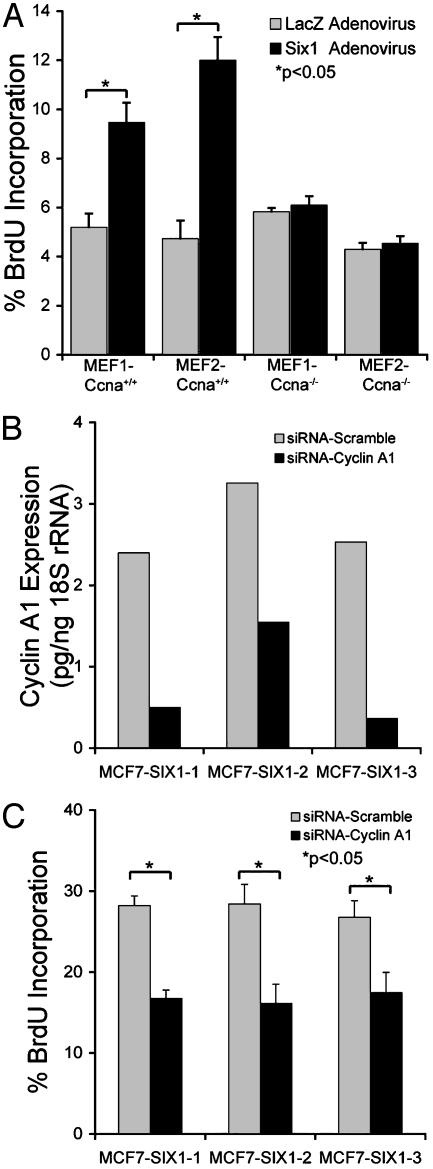
Six1 Is Dependent on Cyclin A1 to Stimulate Cellular Proliferation. To determine whether activation of cyclin A1 by Six1 is required for the proliferative effect of Six1, MEFs were established from wild type (Ccna1+/+) and cyclin A1-deficient (Ccna1-/-) mice (mice kindly provided by M. Carrington, University of Cambridge, U.K. and J. Sobczak-Thepot, Faculté de Medecine Necker, France). The presence of cyclin A1 in two independent MEF-Ccna1+/+ clones, and its absence from two independent MEF-Ccna1-/- clones, was confirmed by RT-PCR (Fig. 9A, which is published as supporting information on the PNAS web site). When Six1 was introduced into MEF-Ccna1+/+ by adenoviral transduction, cyclin A1 levels were increased, whereas no increase was observed in the MEF-Ccna1-/- clones (Fig. 9B). Transduction of Six1 into MEF-Ccna1+/+ ledtoan increase in proliferation, as measured by BrdUrd incorporation, as compared with control transduced MEFs (Fig. 5A). In contrast, introduction of Six1 into cyclin A1-deficient MEFs did not result in an increase in proliferation (Fig. 5A). This demonstrates that Six1 mediates its effects on proliferation through the induction of cyclin A1.
Fig. 5.
Six1 is dependent on cyclin A1 to stimulate cellular proliferation. (A) BrdUrd incorporation assays reveal the dependency of Six1 on cyclin A1 for inducing cellular proliferation, as wild-type cells (MEF-Ccna1+/+) can be stimulated to increase proliferation when Six1 is introduced by adenoviral transduction, whereas cyclin A-deficient cells (MEF-Ccna1-/-) cannot be induced to proliferate by the introduction of Six1. (B) Cyclin A1 mRNA is reduced in Six1-overexpressing MCF7 cells after introduction of a cyclin A1 siRNA to levels at or slightly below levels observed in CAT transfected controls (see Fig. 3A). Cyclin A1 siRNA or a scrambled vector control were introduced into MCF7-SIX1 cells by electroporation. Approximately 36 h after introduction of the siRNA vectors, RNA was isolated from each condition and expression of cyclin A1 was measured by qRT-PCR. (C) Proliferation is decreased in MCF-SIX1 cells when cyclin A1 levels are reduced by means of siRNA technology. Cells were treated as described in B, after which proliferation was measured by BrdUrd incorporation. The experiment was performed twice in triplicate.
To demonstrate that proliferation of Six1-overexpressing mammary carcinoma cells depends on cyclin A1, cyclin A1 was knocked down by siRNA in MCF7-SIX1 cells to levels similar to the level observed in MCF-CAT cells. Fig. 5B demonstrates the levels of cyclin A1 mRNA in MCF7-SIX cells treated with a cyclin A1 scrambled siRNA vector as a control, or an siRNA vector targeting cyclin A1 (designed to target the cyclin A1 mRNA at base pairs 939–958). When cyclin A1 levels were diminished in Six1-overexpressing mammary carcinoma cells, proliferation, as measured by BrdUrd incorporation, significantly decreased (Fig. 5C). This result was recapitulated in the Six1-overexpressing 21PT mammary carcinoma cells (Fig. 10, which is published as supporting information on the PNAS web site). Together, these results demonstrate the dependence of Six1 on cyclin A1 for mediating proliferation in mammary carcinoma cells.
Members of the Six family have been implicated in the proliferation of pluripotent precursor cells during development (3, 5–10). Loss-of-function of Six1 in mice results in a reduction in size or the absence of various organs, because of a decrease in proliferation and an increase in apoptosis (6–8, 39, 40). We have shown that Six1 enhances proliferation through the direct activation of the tissue-specific cyclin A1, and we propose that one developmental function of Six1 is to stimulate the proliferation of progenitor cells by means of cyclin A1. It should be noted that whereas Six1-null animals have numerous defects (40), cyclin A1-knockout mice exhibit defects only in spermatogenesis (17), and therefore any role of cyclin A1 in the normal development of other organs in which it is expressed is likely compensated by the presence of other cyclins. A similar phenomenon is observed in the cdk2-knockout mice, where a defect in spermatogenesis and oogenesis is observed, but the mice remain viable (41), and in the cyclin E1/E2-null mice, in which some embryos that developed in the presence of a wild-type placenta were viable (42). As was demonstrated for cyclins E1/E2, various cyclins and cdks may not be essential for cell cycle progression in normal development because of redundancy, but they may still play a critical role in tumorigenesis (42). Interestingly, cyclin A1 has previously been implicated in other tumor types arising from tissues in which it may play a functional role, including acute promyelocytic leukemias and testicular tumors (25, 29, 43). Thus, we propose that the misexpression of Six1 in cancers causes an inappropriate reactivation of the cyclin A1-mediated proliferative pathway in adult somatic cells, promoting cell cycle progression and tumor growth. Together these findings provide a specific mechanism for the expansion of cells both in development and cancer, which is likely used in organ systems in addition to the breast.
Most cancer therapies currently in use suffer from the fact that they do not exclusively target cancer cells, leading to unwanted and frequently severe side effects. The elucidation of developmental pathways, such as the Six1–cyclin A1 pathway, which are inappropriately activated in tumors, but relatively silent in most normal adult cells, will not only yield an improved understanding of the mechanisms of tumorigenesis but may also lead to the development of novel, more tumor-specific cancer therapies.
Supplementary Material
Acknowledgments
We thank Drs. M. Neville, A. Gutierrez-Hartman, L. Sussel, and P. Jedlicka for helpful comments and critical reading of the manuscript. We thank Dr. G. Mutter for supplying the human breast tumor samples. This work was supported by grants from the National Institutes of Health (1R01CA095277-01), the Susan G. Komen Breast Cancer Foundation (9862), the American Cancer Society/University of Colorado Cancer Center, and the Avon Foundation (to H.L.F). R.D.C. was supported by fellowships from the Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES), Brazil, the University of Colorado Cancer Center, the Cancer League of Colorado, and the W. M. Thorkildsen Foundation. K.J.R. was supported by an institutional fellowship from the Department of Defense Breast Cancer Program.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ad-X, adenovirus containing gene for protein X; MEF, mouse embryonic fibroblast; siRNA, short interfering RNA; qRT-PCR, quantitative real-time RT-PCR; ChIP, chromatin immunoprecipitation.
References
- 1.Abate-Shen, C. (2002) Nat. Rev. Cancer 2, 777-785. [DOI] [PubMed] [Google Scholar]
- 2.Ford, H. L. (1998) Cell Biol. Int. 22, 397-400. [DOI] [PubMed] [Google Scholar]
- 3.Li, X., Perissi, V., Liu, F., Rose, D. W. & Rosenfeld, M. G. (2002) Science 297, 1180-1183. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami, K., Sato, S., Ozaki, H. & Ikeda, K. (2000) BioEssays 22, 616-626. [DOI] [PubMed] [Google Scholar]
- 5.Relaix, F. & Buckingham, M. (1999) Genes Dev. 13, 3171-3178. [DOI] [PubMed] [Google Scholar]
- 6.Ozaki, H., Nakamura, K., Funahashi, J., Ikeda, K., Yamada, G., Tokano, H., Okamura, H. O., Kitamura, K., Muto, S., Kotaki, H., et al. (2004) Development (Cambridge, U.K.) 131, 551-562. [DOI] [PubMed] [Google Scholar]
- 7.Li, X., Oghi, K. A., Zhang, J., Krones, A., Bush, K. T., Glass, C. K., Nigam, S. K., Aggarwal, A. K., Maas, R., Rose, D. W. & Rosenfeld, M. G. (2003) Nature 426, 247-254. [DOI] [PubMed] [Google Scholar]
- 8.Zheng, W., Huang, L., Wei, Z. B., Silvius, D., Tang, B. & Xu, P. X. (2003) Development (Cambridge, U.K.) 130, 3989-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuber, M. E., Perron, M., Philpott, A., Bang, A. & Harris, W. A. (1999) Cell 98, 341-352. [DOI] [PubMed] [Google Scholar]
- 10.Goudreau, G., Petrou, P., Reneker, L. W., Graw, J., Loster, J. & Gruss, P. (2002) Proc. Natl. Acad. Sci. USA 99, 8719-8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laflamme, C., Filion, C., Bridge, J. A., Ladanyi, M., Goldring, M. B. & Labelle, Y. (2003) Cancer Res. 63, 449-454. [PubMed] [Google Scholar]
- 12.Winchester, C., Robertson, S., MacLeod, T., Johnson, K. & Thomas, M. (2000) J. Clin. Pathol. 53, 212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, C. M., Guo, M., Borczuk, A., Powell, C. A., Wei, M., Thaker, H. M., Friedman, R., Klein, U. & Tycko, B. (2002) Am. J. Pathol. 160, 2181-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan, J., Bittner, M. L., Saal, L. H., Teichmann, U., Azorsa, D. O., Gooden, G. C., Pavan, W. J., Trent, J. M. & Meltzer, P. S. (1999) Proc. Natl. Acad. Sci. USA 96, 13264-13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford, H. L., Kabingu, E. N., Bump, E. A., Mutter, G. L. & Pardee, A. B. (1998) Proc. Natl. Acad. Sci. USA 95, 12608-12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu, Y., Khan, J., Khanna, C., Helman, L., Meltzer, P. S. & Merlino, G. (2004) Nat. Med. 10, 175-181. [DOI] [PubMed] [Google Scholar]
- 17.Liu, D., Matzuk, M. M., Sung, W. K., Guo, Q., Wang, P. & Wolgemuth, D. J. (1998) Nat. Genet. 20, 377-380. [DOI] [PubMed] [Google Scholar]
- 18.Yam, C. H., Fung, T. K. & Poon, R. Y. (2002) Cell. Mol. Life Sci. 59, 1317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agami, R. & Bernards, R. (2000) Cell 102, 55-66. [DOI] [PubMed] [Google Scholar]
- 20.Lie, Y. S. & Petropoulos, C. J. (1998) Curr. Opin. Biotechnol. 9, 43-48. [DOI] [PubMed] [Google Scholar]
- 21.Coletta, R. D., Almeida, O. P., Graner, E., Page, R. C. & Bozzo, L. (1998) J. Periodontal. Res. 33, 469-475. [DOI] [PubMed] [Google Scholar]
- 22.Sauk, J. J., Smith, T., Norris, K. & Ferreira, L. (1994) J. Biol. Chem. 269, 3941-3946. [PubMed] [Google Scholar]
- 23.Ford, H. L., Landesman-Bollag, E., Dacwag, C. S., Stukenberg, P. T., Pardee, A. B. & Seldin, D. C. (2000) J. Biol. Chem. 275, 22245-22254. [DOI] [PubMed] [Google Scholar]
- 24.Liu, D., Liao, C. & Wolgemuth, D. J. (2000) Dev. Biol. 224, 388-400. [DOI] [PubMed] [Google Scholar]
- 25.Yang, R., Nakamaki, T., Lubbert, M., Said, J., Sakashita, A., Freyaldenhoven, B. S., Spira, S., Huynh, V., Muller, C. & Koeffler, H. P. (1999) Blood 93, 2067-2074. [PubMed] [Google Scholar]
- 26.Coletta, R. D., Almeida, O. P., Ferreira, L. R., Reynolds, M. A. & Sauk, J. J. (1999) Connect. Tissue Res. 40, 237-249. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, L., Kharbanda, S., McLeskey, S. W. & Kern, F. G. (1999) Cancer Res. 59, 5023-5029. [PubMed] [Google Scholar]
- 28.Howe, J. A., Howell, M., Hunt, T. & Newport, J. W. (1995) Genes Dev. 9, 1164-1176. [DOI] [PubMed] [Google Scholar]
- 29.Yang, R., Morosetti, R. & Koeffler, H. P. (1997) Cancer Res. 57, 913-920. [PubMed] [Google Scholar]
- 30.Muller, C., Yang, R., Beck-von-Peccoz, L., Idos, G., Verbeek, W. & Koeffler, H. P. (1999) J. Biol. Chem. 274, 11220-11228. [DOI] [PubMed] [Google Scholar]
- 31.Spitz, F., Demignon, J., Porteu, A., Kahn, A., Concordet, J. P., Daegelen, D. & Maire, P. (1998) Proc. Natl. Acad. Sci. USA 95, 14220-14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweeney, C., Murphy, M., Kubelka, M., Ravnik, S. E., Hawkins, C. F., Wolgemuth, D. J. & Carrington, M. (1996) Development (Cambridge, U.K.) 122, 53-64. [DOI] [PubMed] [Google Scholar]
- 33.Yang, R., Muller, C., Huynh, V., Fung, Y. K., Yee, A. S. & Koeffler, H. P. (1999) Mol. Cell. Biol. 19, 2400-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romanowski, P., Marr, J., Madine, M. A., Rowles, A., Blow, J. J., Gautier, J. & Laskey, R. A. (2000) J. Biol. Chem. 275, 4239-4243. [DOI] [PubMed] [Google Scholar]
- 35.Guo, N., Faller, D. V. & Vaziri, C. (2000) J. Biol. Chem. 275, 1715-1722. [DOI] [PubMed] [Google Scholar]
- 36.Goldstone, S., Pavey, S., Forrest, A., Sinnamon, J. & Gabrielli, B. (2001) Oncogene 20, 921-932. [DOI] [PubMed] [Google Scholar]
- 37.Young, A. P., Nagarajan, R. & Longmore, G. D. (2003) Oncogene 22, 7209-7217. [DOI] [PubMed] [Google Scholar]
- 38.Brummelkamp, T. R., Bernards, R. & Agami, R. (2002) Science 296, 550-553. [DOI] [PubMed] [Google Scholar]
- 39.Laclef, C., Hamard, G., Demignon, J., Souil, E., Houbron, C. & Maire, P. (2003) Development (Cambridge, U.K.) 130, 2239-2252. [DOI] [PubMed] [Google Scholar]
- 40.Laclef, C., Souil, E., Demignon, J. & Maire, P. (2003) Mech. Dev. 120, 669-679. [DOI] [PubMed] [Google Scholar]
- 41.Ortega, S., Prieto, I., Odajima, J., Martin, A., Dubus, P., Sotillo, R., Barbero, J. L., Malumbres, M. & Barbacid, M. (2003) Nat. Genet. 35, 25-31. [DOI] [PubMed] [Google Scholar]
- 42.Geng, Y., Yu, Q., Sicinska, E., Das, M., Schneider, J. E., Bhattacharya, S., Rideout, W. M., Bronson, R. T., Gardner, H. & Sicinski, P. (2003) Cell 114, 431-443. [DOI] [PubMed] [Google Scholar]
- 43.Muller-Tidow, C., Diederichs, S., Schrader, M. G., Vogt, U., Miller, K., Berdel, W. E. & Serve, H. (2003) Cancer Lett. 190, 89-95. [DOI] [PubMed] [Google Scholar]
- 44.He, T.C., Zhou, S., da Costa, L. T., Yu, J., Kinzler, K. W. & Vogelstein, B. (1998) Proc. Natl. Acad. Sci. USA 95, 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golub, T. R., Slonim, D. K., Tamayo, P., Huard, C., Gaasenbeek, M., Mesirov, J. P., Coller, H., Loh, M. L., Downing, J. R., Caligiuri, M. A., et al. (1999) Science 286, 531-537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.