Abstract
Claspin is a homolog of Mrc1, a checkpoint protein required for the DNA replication checkpoint in yeast. In Xenopus, phosphorylated Claspin binds to xChk1 and regulates xChk1 activation in response to replication stress. In this study, we have shown that the human homolog of Claspin is required for resistance to multiple forms of genotoxic stress including UV, IR, and hydroxyurea. Phosphorylation of Claspin was found to depend on the ataxia telangiectasia mutated-Rad3 related (ATR) pathway. DNA damage induces the formation of a complex between Claspin and BRCA1, a second regulator of Chk1 activation. Claspin was found to control BRCA1 phosphorylation on serine 1524, a site whose phosphorylation is controlled by the ATR pathway. These results are consistent with a model in which ATR regulates Claspin phosphorylation in response to DNA damage and replication stress resulting in recruitment and phosphorylation of BRCA1. BRCA1 and Claspin then function to activate the tumor suppressor Chk1. Unexpectedly, we found that Claspin has a second, positive role in control of the cell cycle as Claspin overexpression increased cell proliferation. These results suggest that Claspin has properties of both a tumor suppressor and an oncogene.
In response to DNA damage and replication stress, cells activate regulatory pathways to promote DNA repair and to prevent cell-cycle progression until the genotoxic stress is relieved (1, 2). In mammalian cells, two protein kinases of the phosphoinositide 3-kinase-related kinase family, ataxia telangiectasia mutated and ataxia telangiectasia mutated-Rad3 related (ATR) play central roles in sensing and responding to chromosomal insults (3, 4). These kinases phosphorylate a variety of molecules to carry out many cellular responses, including DNA synthesis inhibition, cell-cycle arrest, DNA damage repair, and, under certain circumstances, induction of apoptosis (2). Down-stream of the phosphoinositide 3-kinase-related kinase family is the Mediator family of molecules, which includes a variety of BRCA1 c-terminal (BRCT)-repeat proteins, including BRCA1, and a second family of proteins such as Claspin in mammals and Mrc1 in yeast (5–7). Downstream of the Mediators are the checkpoint kinases, Chk1 and Chk2, which have been shown to be activated by the ataxia telangiectasia mutated or ATR pathways and are required for the checkpoint responses (8–11). The phosphoinositide 3-kinase-related kinases, Mediators, and CHKs form an interacting network of signaling molecules as opposed to a simple linear pathway.
Of the Mediator class of proteins, Claspin and Mrc1 have been implicated in responding to DNA replication stress (5–7, 12, 13). Both proteins are phosphorylated in response to replication stress, and phosphorylation is required for their ability to activate their downstream CHK kinase. Xenopus Claspin controls activation of Chk1, whereas budding yeast Mrc1 controls activation of Rad53, a Chk2 kinase homolog. Mrc1 localizes to the replication fork and has been shown to have a positive function in DNA replication (14, 15) that is independent of its checkpoint function (6, 14).
Although Claspin is required for checkpoint signaling in response to replication blocks in Xenopus (5, 12, 13), its role in mammalian cells is unclear. In this study, we show human Claspin plays an important role in positively regulating cell proliferation and in the DNA damage response, where it works together with BRCA1 to regulate Chk1.
Methods
Cells. U2OS cells were purchased from the American Type Culture Collection (ATCC) and maintained in McCoy's 5A medium supplemented with 10% FBS, glutamine, penicillin, and streptomycin. All other cell lines were maintained in DMEM with 10% FBS.
Small Interfering RNA (siRNA). The siRNA duplexes were 19 base pairs with a 2-base deoxythymidine overhang (Dharmacon Research). The sequences of Claspin siRNA3 and siRNA7 oligonucleotides are CCUUGCUUAGAGCUGAGUCdTdT and GGAAAGAAAGGCAGCCAGAdTdT, respectively. The control LacZ siRNA has the sequence CGUACGCGGAAUACUUCGAdTdT. Cells were transfected with siRNA duplexes by using Oligofectamine (Invitrogen) following the manufacturer's instructions.
Vectors. The Flag-tagged Claspin vector was constructed by the blunt-ended insertion of the full-length Claspin RT-PCR product into the EcoRV site on the expression vector (p3xFLAG-CMV-10, Sigma). The Claspin viral vector was generated by inserting the same RT-PCR product into BstXI-cleaved pBabe-Puro.
Antibodies. The Claspin antibody was directed against a Claspin peptide (CKHKKKEPSLESGVH) generated by Bethyl Laboratories (Montgomery, TX). Anti-chk1, anti-phospho-Chk1, and BRCA1 antibodies were purchased from Santa Cruz Biotechnology, Cell Signaling (Beverly, MA), and Oncogene Science, respectively. Anti-phospho-BRCA1 antibody was generously provided by Dr. Jun Qin (Baylor College of Medicine).
Cell-Survival Assays. U2OS cells were transfected with siRNAs two times with a 24-h interval and 48 h after the second transfection plated at low density and irradiated with various doses of ionizing radiation. Cells were incubated for 2–3 weeks to allow colonies to form. Colonies were detected by staining with 2% methylene blue/50% ethanol.
Radio-Resistant DNA Synthesis Assay. The radio-resistant DNA synthesis assay was performed as previous described (16). Briefly, U2OS cells were transfected with siRNAs twice. After the second transfection, cells were incubated in McCoy's 5A medium containing 10 nCi/ml [14C]thymidine (NEN) overnight. The medium was then replaced with normal McCoy's 5A medium and incubated for another 24 h. Cells were irradiated, incubated for 30 min at 37°C, and then pulse-labeled with 2.5 μCi/ml [3H]thymidine (NEN) for 15 min. Cells were harvested, washed twice with PBS, and fixed in 70% methanol for 30 min. After cells were transferred to Whatman filters and fixed sequentially with 70% and 95% of methanol, the filters were air-dried, and the radioactivity was assayed in a liquid scintillation counter. The resulting ratio of 3H cpm to 14C cpm, corrected for cpm that resulted from channel crossover, was a measure of DNA synthesis.
G2/M Checkpoint Assay. U2OS cells were transfected with siRNAs two times with a 24-h interval and 48 h after the second transfection was exposed to 3 Gy ionizing radiation. One hour later, cells were fixed and stained with propidium iodide and antibody against phospho-histone H3 (Cell Signaling), followed by FITC-conjugated second antibody (Jackson ImmunoResearch). The percentage of M-phase cells was determined by flow cytometry.
BrdUrd Incorporation. Cells were incubated with culture medium containing BrdUrd for 20 h. BrdUrd staining was performed by using a Zymed BrdUrd labeling kit following the manufacturer's protocol.
Results
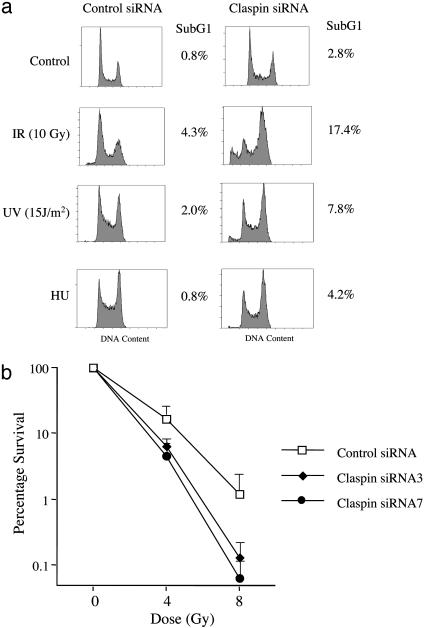
Claspin Is Required for Checkpoint Activation and Cell Survival After DNA Damage. To determine whether Claspin plays a role in the DNA damage and replication checkpoints, U2OS cells were transfected twice with two independent siRNAs against two nonoverlapping sequences on Claspin. Forty-eight hours after the second transfection, cells were challenged with different genotoxic insults: ionizing radiation (10 Gy), UV (15 J/m2), or hydroxyurea (2 mM). As judged by the increase in the sub G1, apoptotic population, Claspin-depleted cells were more sensitive to all three stresses (Fig. 1a). The increase in sensitivity to ionizing radiation was further confirmed by a colony-forming assay. Compared to control siRNA treatment, the Claspin–siRNA-treated cells were significantly more sensitive to ionizing irradiation (Fig. 1b). These results indicate that Claspin plays an important role in the cellular responses to both DNA damage and replication blocks.
Fig. 1.
Claspin deficiency increases the sensitivity to genotoxic stress. (a) U2OS cells were transfected twice with control or Claspin siRNAs. Forty-eight hours after the second transfection, cells were treated with different genotoxic agents as indicated; 72 h after treatment with the indicated agents, DNA contents were analyzed by FACS. (b) U2OS cells were transfected with siRNAs as described above. The transfected cells then were plated at low density and irradiated, and the colonies were counted after 2 weeks.
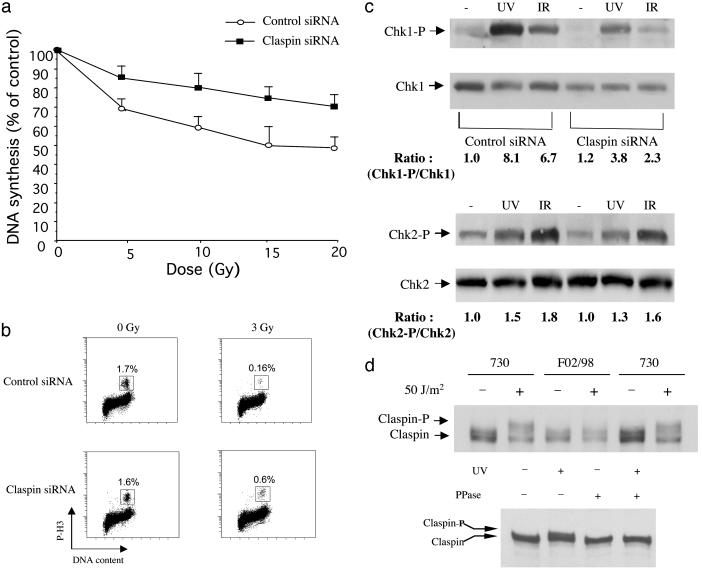
Claspin Regulates DNA Damage Checkpoints. Before massive apoptosis occurred (72 h after IR), we observed a prolonged G2 phase accumulation for Claspin-depleted U2OS cells (data not shown). A prolonged G2 accumulation after IR has previously been linked to a defective S-phase checkpoint in cells lacking BRCA1 or Nbs1 (17), suggesting a role of Claspin in the intra-S phase checkpoint. Thus, we examined DNA synthesis inhibition in response to IR (18) in cells lacking Claspin. As shown in Fig. 2a, Claspin-deficient cells showed a significant radio-resistant DNA synthesis phenotype, indicating a role for Claspin in the intra-S-phase checkpoint. We also examined whether Claspin played a role in the G2/M checkpoint. Cells treated with Claspin-specific siRNA were irradiated and labeled with anti-phospho-histone H3 antibody as a marker for M phase cells (19). In contrast to the control cells which were arrested in G2, a significantly higher population of Claspin-depleted cells entered mitosis, indicating the requirement of Claspin for the G2/M checkpoint (Fig. 2b). Together, our results indicate that human Claspin plays an important role in DNA damage checkpoints, contributing to resistance to the toxic effect of DNA damage.
Fig. 2.
Claspin is required for the IR-induced intra-S and G2/M checkpoints. (a) IR-induced intra-S phase checkpoint. DNA synthesis was assessed 30 min after various doses of IR in U2OS cells twice-transfected with Claspin siRNA or the control siRNA. (b) IR-induced G2/M checkpoint analysis. U2OS cells were either untreated or irradiated with 3 Gy and then incubated for 1 h before fixation. Cells in mitosis were determined by staining with propidium iodide and phospho-histone H3 antibody followed by FITC-conjugated secondary antibody. The percentage of M-phase cells was determined by FACS for p-H3. (c) U2OS cells were transfected with control or Claspin siRNA twice. Forty-eight hours after the second transfection, cells were either unirradiated or irradiated with UV (50 J/m2) or IR (10 Gy); 2 h after irradiation, cells were harvested for Western blotting and probed with antibodies against S345P-Chk1 or Chk1 (Upper) or antibodies against T68P-Chk2 or Chk2 (Lower). (d Upper) 730 cells, a normal human primary fibroblast line and F02/98 cells, a primary fibroblast line from a Seckel Syndrome patient, were unirradiated or irradiated with UV. Two hours after the treatment, cells were harvested for Western blotting and probed with anti-Claspin antibodies. (Lower) 730 cells were either unirradiated or irradiated with UV (50 J/m2). Two hours after the treatment, cells were harvested, and the lysates were treated with or without λ protein phosphatase for Western blotting and probed with anti-Claspin antibodies.
Claspin Is Required for Chk1 Activation upon DNA Damage. It has been shown that disruption of Chk1 abrogates the IR-induced intra-S phase and G2 checkpoints (9, 20). Furthermore, Claspin has been implicated in Chk1 regulation in Xenopus (5, 12, 13). Therefore, we sought to determine whether Claspin depletion blocked IR-induced Chk1 activation. As shown in Fig. 2c (Upper), cells treated with Claspin siRNA showed significantly reduced Chk1 S345 phosphorylation either in response to ionizing radiation or UV. This effect was Chk1-specific because another effector kinase, Chk2, was not affected (Fig. 2c Lower). Our results, therefore, indicated that Claspin may be a general regulator for Chk1 activation in the responses to both DNA damage and replication stress. Because Chk1 activation depends on both ATR and Claspin, we sought to determine whether there was functional interactions between ATR and Claspin. Claspin is phosphorylated after replication blocks or UV irradiation, which causes the slower migration of the protein on SDS/PAGE. Recently, primary fibroblasts derived from a Seckel syndrome patient were found to contain a splicing mutation in the ATR gene that resulted in reduced expression of the wild-type ATR product (21). As shown in Fig. 2d, UV induced Claspin phosphorylation in normal fibroblasts but not in fibroblasts harboring the ATR mutation, indicating a dependency for Claspin phosphorylation on the ATR pathway. Claspin phosphorylation has been previously shown to be required for binding and activation of xChk1 in Xenopus (5), and we suspect that Claspin may be a direct target of ATR to facilitate Chk1 binding just as Mrc1 is a target of the ATR homolog Mec1 in yeast (14).
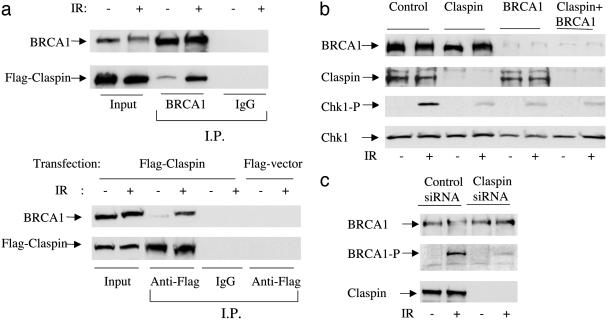
Claspin Binds to BRCA1 and Regulates BRCA1 Phosphorylation. The functions of Claspin in checkpoint regulation and Chk1 activation are very similar to the functions of another important checkpoint protein, BRCA1. BRCA1 has been shown to be involved in both intra-S phase and G2-phase checkpoints after ionizing radiation (19). In addition, its role in the G2/M check-point is believed to be through the activation of Chk1 activity (22). Because of those functional similarities, we suspected that Claspin and BRCA1 may interact in response to DNA damage. To determine whether these two proteins physically associate, we expressed Flag-tagged Claspin in HeLa cells and performed immunoprecipitation assays to assess their binding. Using an anti-BRCA1 antibody, we detected a small amount of Flag–Claspin associated with the endogenous BRCA1 protein (Fig. 3a Upper). Their coimmunoprecipitation significantly increased after cells were exposed to 10 Gy ionizing radiation. Reciprocally, when Flag–Claspin was immunoprecipitated, BRCA1 protein was coimmunoprecipitated, and the binding increased significantly after IR (Fig. 3a Lower).
Fig. 3.
Claspin interacts with BRCA1 and regulates IR-dependent Chk1 phosphorylation. HeLa cells were transfected with Flag-tagged Claspin and either unirradiated or irradiated with IR (10 Gy). One hour after treatment, cells were harvested for immunoprecipitations. (a Upper) Cell extracts were incubated with antibodies against BRCA1 or IgG (IgG, control) and protein A Sepharose. Immunoprecipitates were separated by SDS/PAGE and immunoblotted with antibodies against BRCA1 or the Flag epitope. (Lower) The same as above except anti-Flag antibodies were used instead of anti-BRCA1 antibodies for immunoprecipitation. (b) U2OS cells were either transfected twice with control siRNA, Claspin siRNA, or BRCA1 siRNA alone or in combination. Forty-eight hours after the second transfection, cells were either unirradiated or irradiated with 10 Gy ionizing radiation; 1 h after the treatments, cells were harvested for Western blotting and probed with the indicated antibodies. (c) Cell lysates from the control or Claspin-depleted cells were prepared for Western blotting and probed with antibodies against BRCA1, p-S1524-BRCA1, or Claspin.
Next, we sought to determine whether Claspin and BRCA1 belonged to the same pathway or two distinct pathways in Chk1 regulation. We either depleted individual proteins or double-depleted both proteins and analyzed their effects on Chk1 activation after IR. As shown in Fig. 3b, the depletion of either Claspin or BRCA1 repressed IR-dependent Chk1 S345 phosphorylation to the same extent. The combination of both siRNAs did not further inhibit Chk1 activation, suggesting that Claspin and BRCA1 functioned in the same signaling pathway. A recent study indicated that Claspin bound to chromatin around the time of the initial unwinding step of replication and suggested it functions to monitor the replication process like Mrc1 (13). Therefore, Claspin may help to recruit signal mediators, such as BRCA1, to transduce signals to downstream effectors. BRCA1 is phosphorylated upon DNA damage at various sites, including S1524. If Claspin functions upstream of BRCA1, depletion of Claspin should interfere with BRCA1 phosphorylation. Using antibodies against phospho-S1524-BRCA1, we demonstrated a requirement for Claspin on IR-dependent BRCA1 phosphorylation. BRCA1 was strongly phosphorylated at S1524 after IR, and this phosphorylation was significantly reduced when Claspin was depleted in cells (Fig. 3c). Therefore, Claspin acts upstream of BRCA1 or together with BRCA1 to regulate Chk1 activation.
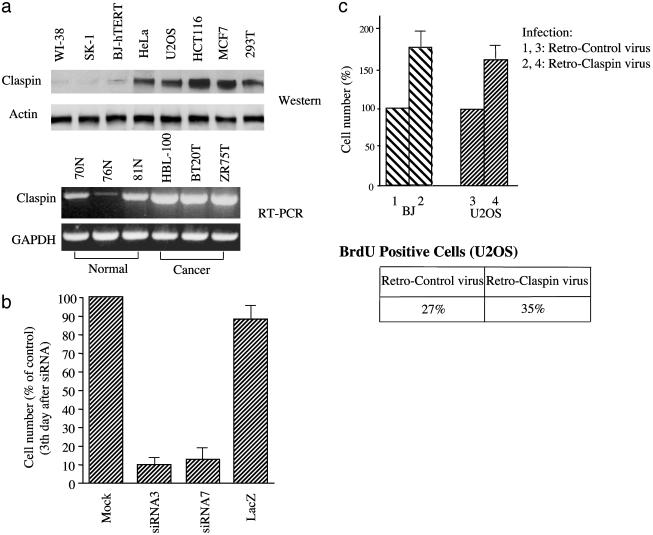
Claspin Regulates Cell Proliferation. Because Claspin is involved in maintenance of intact checkpoints and probably genomic stability, we were interested in determining whether Claspin expression was reduced in some cancer cells. Surprisingly, when we analyzed the expression levels of Claspin in different cell lines, we found that Claspin was expressed at much higher levels in cancer cells compared with the normal primary cells (Fig. 4a Upper). Claspin expression has been shown to be cell-cycle regulated, peaking at S/G2 phase (23). This raised the caveat that the increased expression might simply be a reflection of a higher S phase population in cancer cells. However, by quantitating the Claspin levels and percentage of S/G2 cells, we found this is not the case. Cancer cells showed 6- to 7-fold higher levels of Claspin but only a 2- to 3-fold increased number of S phase cells.
Fig. 4.
Claspin functions in DNA replication and proliferation. (a Upper) Claspin protein levels were analyzed in untransformed cells (WI-38, SK-1, BJ-hTERT) or cancer cell lines (all other five cell lines). (Lower) Claspin RNA levels were determined by RT-PCR in the cells derived from normal mammary glands (70N, 76N, 81N) or breast cancer cell lines (HBL-100, BT20T, ZR75T). (b) U2OS cells were mock-transfected or transfected with siRNAs against Claspin (siRNA3 and siRNA7) or LacZ (control) twice. Equal cell numbers were plated 48 h after the second transfection, and the total cell numbers were counted again 3 days later. (c Upper) BJ cells or U2OS cells were infected with retrovirus expressing Claspin (bars 2 and 4) or the vector control (bars 1 and 3). After puromycin selection, stable clones were first pooled together; equal cell numbers from the pooled population were then plated, and the total numbers of cells were counted again 3 days later. (Lower) U2OS cells described above were stained and analyzed for the percentage of BrdUrd-positive cells by immunohistochemical staining and counted under a microscope.
Similar expression patterns were also observed when we compared Claspin RNA expression between cells derived from normal mammary glands and three different breast cancer cell lines (Fig. 4a Lower). These results suggested that Claspin may have a cell growth-promoting function distinct from its role in DNA damage responses. In fact, in our previous studies we have already discovered a dual role of Mrc1 in yeast (14). Like Mrc1 mutants, which show a slower S phase progression, we observed a significant growth defect and reduced replication in Claspin-deficient cells. When U2OS cells were transfected with either control siRNA or two different Claspin siRNAs, we found that the Claspin-depleted populations had many fewer cells 3 days later than the control (Fig. 4b), reflecting lower growth rates in Claspin-depleted cells. Also, when we performed thymidine incorporation to assess DNA synthesis, we found that Claspin-depleted cells had a 30–40% reduction of thymidine incorporation, indicating a reduction of replication (data not shown). Because Mrc1 and Xenopus Claspin were previously reported to either move along with the replication fork or bind to replicating chromatin, human Claspin may promote DNA replication. Consistent with this supposition, when we infected either BJ cells or U2OS cells with the retroviral vector encoding wild-type Claspin, we observed significant increase of proliferation rates in both cell lines (Fig. 4c Upper), which was consistent with an increase in the number of cells staining positive for BrdUrd in cells ectopically expressing Claspin (Fig. 4c Lower).
Discussion
In this report, we investigated the role of human Claspin in both DNA damage checkpoints and cellular proliferation. Previous studies on Xenopus Claspin were mainly focused on its role in response to replication stress during S phase. Here, we showed that Claspin status affected sensitivity to both replication stress and DNA damage induced by either ionizing radiation or UV. In addition, we demonstrated that Claspin was required for the IR-induced intra-S and G2/M checkpoints in U2OS cells. Therefore, in addition to associating with replicating chromatin to ensure proper replication processes, Claspin may regulate check-points through replication-independent mechanisms, probably in part through its regulation of Chk1 activity.
Chk1 is one of the key effector kinases which inhibits CDC25A to prevent S phase progression and the G2/M transition when cells countered replication stress, UV, or ionizing radiation (9, 20, 24–26). Chk1 is believed to be activated by the ATR–ATR-interacting protein (ATRIP) kinase (9, 25, 27). How ATR–ATRIP controls phosphorylation and activation of Chk1, however, is still unclear. In Xenopus egg extracts, Claspin was shown to be required for ATR-dependent Chk1 activation (5). In this study, we demonstrated that Claspin itself was a downstream target of ATR and functioned with BRCA1 in the same signal transduction pathway to activate Chk1. Double depletion of Claspin and BRCA1 has no additional effects on Chk1 phosphorylation compared to depletion of individual proteins. Furthermore, Claspin depletion impaired BRCA1 phosphorylation at Ser-1524, a site mainly phosphorylated by the ATR/ATRIP complex. Therefore, our results placed Claspin upstream of BRCA1 in the ATR–Chk1 pathway, and its function may be to recruit BRCA1 and Chk1 to ATR at the damaged sites on chromatin.
In addition to its roles in DNA damage responses, Claspin has a separate function involved in cell proliferation. We found that overproduction of Claspin stimulated cell proliferation. Conversely, Claspin depletion slowed down proliferation, consistent with the role of its budding yeast homolog Mrc1, which is thought to act to tether DNA polymerases to sites of replication (15). However, a function for Claspin in control of a rate-limiting step in cell proliferation is unexpected based on any of its known functions and may indicate that it positively regulated a critical cell-cycle transition such as G1/S. If it has such an oncogenic role, it might prove to be an antiproliferation target for cancer cells.
Note. While this report was in preparation, a study by Chini and Chen (23) showed that Claspin was required for Chk1 activation in response to hydroxyurea, consistent with our studies.
Acknowledgments
We thank K. Keyomarsi for providing RNA samples from normal human mammary cells and breast cancer cell lines, J. Qin for the phospho-BRCA1 antibody, and P. Jeggo for the Seckel cell line. This work was supported by National Institutes of Health Fellowship 5 F32 CA093043 (to S.-Y.L.) and a European Molecular Biology Organization fellowship (to G.S.S.). S.J.E. is supported by National Institutes of Health Grant GM 44664 and is an Investigator with the Howard Hughes Medical Institute.
Abbreviations: ATR, ataxia telangiectasia mutated-Rad3 related; siRNA, small interfering RNA.
References
- 1.Melo, J. & Toczyski, D. (2002) Curr. Opin. Cell Biol. 14, 237-245. [DOI] [PubMed] [Google Scholar]
- 2.Osborn, A., Elledge, S. & Zou, L. (2002) Trends Cell Biol. 12, 509-516. [DOI] [PubMed] [Google Scholar]
- 3.Abraham, R. (2001) Genes Dev. 15, 2177-2196. [DOI] [PubMed] [Google Scholar]
- 4.Shiloh, Y. (2001) Curr. Opin. Genet. Dev. 11, 71-77. [DOI] [PubMed] [Google Scholar]
- 5.Kumagai, A. & Dunphy, W. (2000) Mol. Cell 6, 839-849. [DOI] [PubMed] [Google Scholar]
- 6.Alcasabas, A., Osborn, A., Bachant, J., Hu, F., Werler, P., Bousset, K., Furuya, K., Diffley, J., Carr, A. & Elledge, S. (2001) Nat. Cell Biol. 3, 958-965. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka, K. & Russell, P. (2001) Nat. Cell Biol. 3, 966-972. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez, Y., Wong, C., Thoma, R., Richman, R., Wu, Z., Piwnica-Worms, H. & Elledge, S. (1997) Science 277, 1497-1501. [DOI] [PubMed] [Google Scholar]
- 9.Liu, Q., Guntuku, S., Cui, X. S., Matsuoka, S., Cortez, D., Tamai, K., Luo, G., Carattini-Rivera, S., DeMayo, F., Bradley, A., et al. (2000) Genes Dev. 14, 1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka, S., Huang, M. & Elledge, S. (1998) Science 282, 1893-1897. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka, S., Rotman, G., Ogawa, A., Shiloh, Y., Tamai, K. & Elledge, S. (2000) Proc. Natl. Acad. Sci. USA 97, 10389-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumagai, A. & Dunphy, W. (2003) Nat. Cell Biol. 5, 161-165. [DOI] [PubMed] [Google Scholar]
- 13.Lee, J., Kumagai, A. & Dunphy, W. (2003) Mol. Cell 11, 329-340. [DOI] [PubMed] [Google Scholar]
- 14.Osborn, A. & Elledge, S. (2003) Genes Dev. 17, 1755-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katou, Y., Kanoh, Y., Bando, M., Noguchi, H., Tanaka, H., Ashikari, T., Sugimoto, K. & Shirahige, K. (2003) Nature 424, 1078-1083. [DOI] [PubMed] [Google Scholar]
- 16.Wang, B., Matsuoka, S., Carpenter, P. & Elledge, S. (2002) Science 298, 1435-1438. [DOI] [PubMed] [Google Scholar]
- 17.Xu, B., Kim, S., Lim, D. & Kastan, M. (2002) Mol. Cell. Biol. 22, 1049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Painter, R. & Young, B. (1980) Proc. Natl. Acad. Sci. USA 77, 7315-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu, B., Kim, S. & Kastan, M. (2001) Mol. Cell. Biol. 21, 3445-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao, H., Watkins, J. & Piwnica-Worms, H. (2002) Proc. Natl. Acad. Sci. USA 99, 14795-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Driscoll, M., Ruiz-Perez, V., Woods, C., Jeggo, P. & Goodship, J. (2003) Nat. Genet. 33, 497-501. [DOI] [PubMed] [Google Scholar]
- 22.Yarden, R., Pardo-Reoyo, S., Sgagias, M., Cowan, K. & Brody, L. (2002) Nat. Genet. 30, 285-289. [DOI] [PubMed] [Google Scholar]
- 23.Chini, C. & Chen, J. (2003) J. Biol. Chem. 278, 30057-30062. [DOI] [PubMed] [Google Scholar]
- 24.Walworth, N. (2001) Curr. Opin. Genet. Dev. 11, 78-82. [DOI] [PubMed] [Google Scholar]
- 25.Guo, Z., Kumagai, A., Wang, S. & Dunphy, W. (2000) Genes Dev. 14, 2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin, J., Shirogane, T., Xu, L., Nalepa, G., Qin, J., Elledge, S. J. & Harper, J. W. (2003) Genes Dev. 17, 3062-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou, L. & Elledge, S. (2003) Science 300, 1542-1548. [DOI] [PubMed] [Google Scholar]