Abstract
Calcineurin signalling plays a critical role in the pathogenesis of many cardiovascular diseases. Calcineurin has been proven to affect a series of signalling pathways and to exert a proapoptotic effect in cardiomyocytes. However, whether it is able to regulate autophagy remains largely unknown. Here, we report that prolonged oxidative stress-induced activation of calcineurin contributes to the attenuation of adaptive AMP-activated protein kinase (AMPK) signalling and inhibits autophagy in cardiomyocytes. Primary cardiomyocytes exhibited rapid formation of autophagosomes, microtubule-associated protein 1 light chain 3 (LC3) expression and phosphorylation of AMPK in response to hydrogen peroxide (H2O2) treatment. However, prolonged (12 h) H2O2 treatment attenuated these effects and was accompanied by a significant increase in calcineurin activity and apoptosis. Inhibition of calcineurin by FK506 restored AMPK function and LC3 expression, and decreased the extent of apoptosis caused by prolonged oxidative stress. In contrast, overexpression of the constitutively active form of calcineurin markedly attenuated the increase in LC3 induced by short-term (3 h) H2O2 treatment and sensitised cells to apoptosis. In addition, FK506 failed to induce autophagy and alleviate apoptosis in cardiomyocytes expressing a kinase-dead K45R AMPK mutant. Furthermore, inhibition of autophagy by 3-methylanine (3-MA) or by knockdown of the essential autophagy-related gene ATG7 abrogated the protective effect of FK506. These findings suggest a novel role of calcineurin in suppressing adaptive autophagy during oxidative stress by downregulating the AMPK signalling pathway. The results also provide insight into how altered calcineurin and autophagic signalling is integrated to control cell survival during oxidative stress and may guide strategies to prevent cardiac oxidative damage.
Keywords: oxidative stress, cardiomyocyte, autophagy, calcineurin, AMPK
Oxidative stress is mainly caused by a disturbance to the basal cellular redox state and has been implicated in various human diseases,1, 2 especially heart pathophysiological processes. It results in damage to subcellular organelles and nucleic acids and causes protein aggregation as well as alternations to a sequence of signalling cascades that affect cell apoptosis, ageing and autophagy.3, 4 Studying the mechanisms underlying the interaction of these signals in the context of cardiomyocyte oxidative stress would enable us to gain a deeper understanding of how to prevent oxidative damage during cardiovascular complications such as ischaemia–reperfusion injury.
Cardiomyocytes are highly metabolic and nonreplicative cells for which maintenance of homeostasis largely depends upon intracellular renewal. Autophagy refers to an evolutionarily conserved and programmed self-degrading process during which damaged organelles and aggregated proteins are enveloped by double membrane vesicles and sequestered for lysosome degradation.5, 6 Recent studies have highlighted the importance of autophagy in both physiological and pathological states. It is widely accepted that a physiological level of autophagy is crucial for maintenance of cellular homeostasis, whereas cells that undergo excessive autophagy exhibit autophagic cell death. Despite reports that autophagy can execute cell death in various scenarios, recent accumulating evidence has demonstrated that autophagy is more of a survival-prone response than an inducer of cell death.6, 7 Autophagy enables cells to recycle aggregated proteins and damaged organelles into raw materials that is essential for living cells to recover from stresses such as energy depletion and oxidative and endoplasmic reticulum stress (ER stress).8, 9, 10, 11 Some diseases, including diabetes, cancer and neurodegenerative diseases, are closely related to malfunctions in autophagy.6, 12, 13 Moreover, genetically depleting autophagy disrupts cellular functions in vivo and in vitro.14, 15, 16 These reports suggest that autophagy may be a versatile and essential survival mechanism against stressors in mammalian cells.
The nutrient sensor mTOR (mammalian target of rapamycin) is likely the core regulator of autophagy. It suppresses autophagy through inactivation of ATG1 and ATG13 that are indispensable autophagy-related proteins (ATGs) for autophagosome biogenesis.17, 18 In parallel, the energy sensor AMPK (5′ adenosine monophosphate-activated protein kinase), which is abundant in cardiomyocytes, can activate autophagy through direct activation of ULK1 or by inhibiting mTORC1 in mammalian cells.19, 20
AMPK is a kinase composed of the catalytic subunit α and the regulatory subunits β and γ. AMPK becomes activated when phosphorylation occurs at threonine-172 of the catalytic subunit.21 It modulates cell functions by regulating cell pathophysiological processes such as apoptosis, lipid metabolism, ageing and autophagy under stress.20, 22 Despite proof that AMPK protects cardiomyocytes from oxidative stress-induced injury,23, 24, 25 little is known about the role of AMPK pathway-mediated autophagy in cardiomyocytes under oxidative stress. In addition, given the various altered signalling cascades during oxidative stress, it is tempting to assume that some signals might regulate autophagy by interacting with the AMPK pathway.
Calcineurin, also known as calcium/calmodulin-dependent protein phosphatase 2B (PP2B), reportedly mediates multiple signalling cascades, including apoptosis, by dephosphorylating several substrates in cardiovascular diseases.26, 27, 28 It is still a matter of debate whether calcineurin is protective or detrimental to the heart. In addition, it is unknown whether calcineurin participates in regulating autophagy of cardiomyocytes during oxidative stress. The pharmacological inhibitor of calcineurin, cyclosporine A, has been reported to enhance AMPK phosphorylation in the rat hippocampus,29 indicating the potential role of calcineurin in autophagy regulation. Therefore, we explored whether calcineurin is capable of regulating autophagy and the underlying molecular mechanism. Our present study reveals a novel role of calcineurin as an autophagy inhibitor by repression of the AMPK/mTOR signalling pathway. Moreover, we demonstrate that AMPK-dependent autophagy is cytoprotective in the context of oxidative stress.
Results
Oxidative stress induces apoptosis and autophagy and increases calcineurin activity in cardiomyocytes
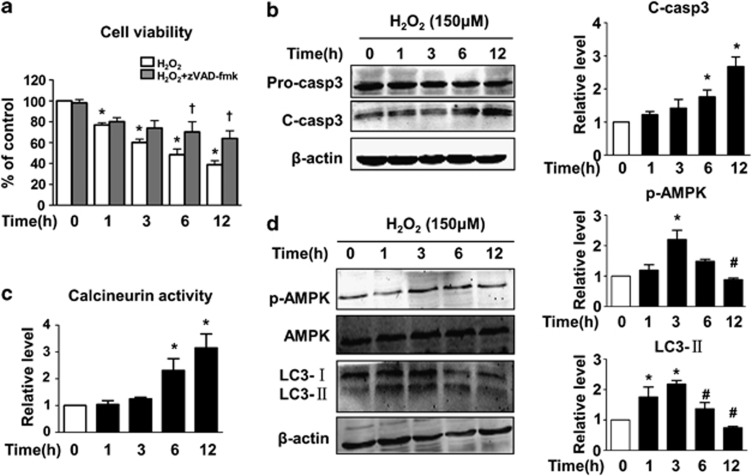
In this study, we first confirmed the presence of oxidative damage in primary cardiomyocytes. We assessed cell viability at various time points using MTT assay. As shown in Figure 1a, there was a time-dependent decrease in cell viability to 40% compared with the control group at 12 h after exposure to hydrogen peroxide (H2O2).The reduction in cell viability was significantly reversed by pan-caspase inhibitor zVAD-fmk at later time points (6 and 12 h).The cleavage of caspase-3 was also used to determine the extent of apoptosis. Consistent with the cell viability assay, H2O2 treatment increased the cleavage of pro-caspase-3 (34 kDa) to its active form (17 kDa) in a time-dependent manner, as was evident at 6 and 12 h (Figure 1b).These results suggest that long-term H2O2 treatment induces caspase-dependent apoptosis in cardiomyocytes.
Figure 1.
The effect of hydrogen peroxide (H2O2) on cardiomyocyte apoptosis, calcineurin activity, AMPK phosphorylation and LC3 expression. Primary cardiomyocytes were treated with H2O2 at a final concentration of 150 μM for 1, 3, 6 or 12 h. (a) The effect of H2O2 on cell viability at various time points with or without zVAD-fmk. (b) H2O2 induces time-dependent activation of caspase-3 as shown by western blotting. The statistical bar graph (right panel) shows the comparison of cleaved caspase-3 levels that have been normalised to β-actin. (c) H2O2 time dependently enhances calcineurin (Cn) activity. Cells were treated as described. Calcineurin activity in each group was normalised to the control group. (d) The effect of H2O2 on LC3 and AMPK protein expression at each time point as detected by western blotting. The levels of LC3-II and p-AMPK were normalised to β-actin and are shown in the bar graph (right panel). Data are presented as the mean±S.E.M., n≥3. *P<0.05 versus the control group, #P<0.05 versus 3 h and †P<0.05 versus H2O2 alone at 6 and 12 h
It has been documented that calcineurin transmits a pro-apoptotic signal in several apoptosis models.27, 28, 30, 31, 32 Therefore, we postulated that a calcineurin-related signal response may be activated because of aberrant calcium signalling under oxidative stress conditions. To test this hypothesis, we performed a cellular calcineurin activity assay. As expected, the calcineurin activity significantly increased by threefold in response to long-term (12 h) H2O2 treatment in a time-dependent manner (Figure 1c).
Autophagy can be induced by stressors, including reactive oxygen species (ROS). We next detected the dynamic changes in autophagy by western blot analysis of microtubule-associated protein 1 light chain 3 (LC3), a recognised autophagy marker. Cytosolic LC3-I is converted to LC3-II via lipidation and redistributed to the autophagosome membrane in response to autophagic stimuli.33 Thus, LC3-II directly reflects the number of autophagosomes. As shown in Figure 1d, H2O2 dramatically enhanced both LC3-I and LC3-II expression by threefold compared with the control group within the first 3 h of exposure. Notably, sustained treatment with H2O2 reduced the levels of both LC3-I and LC3-II.
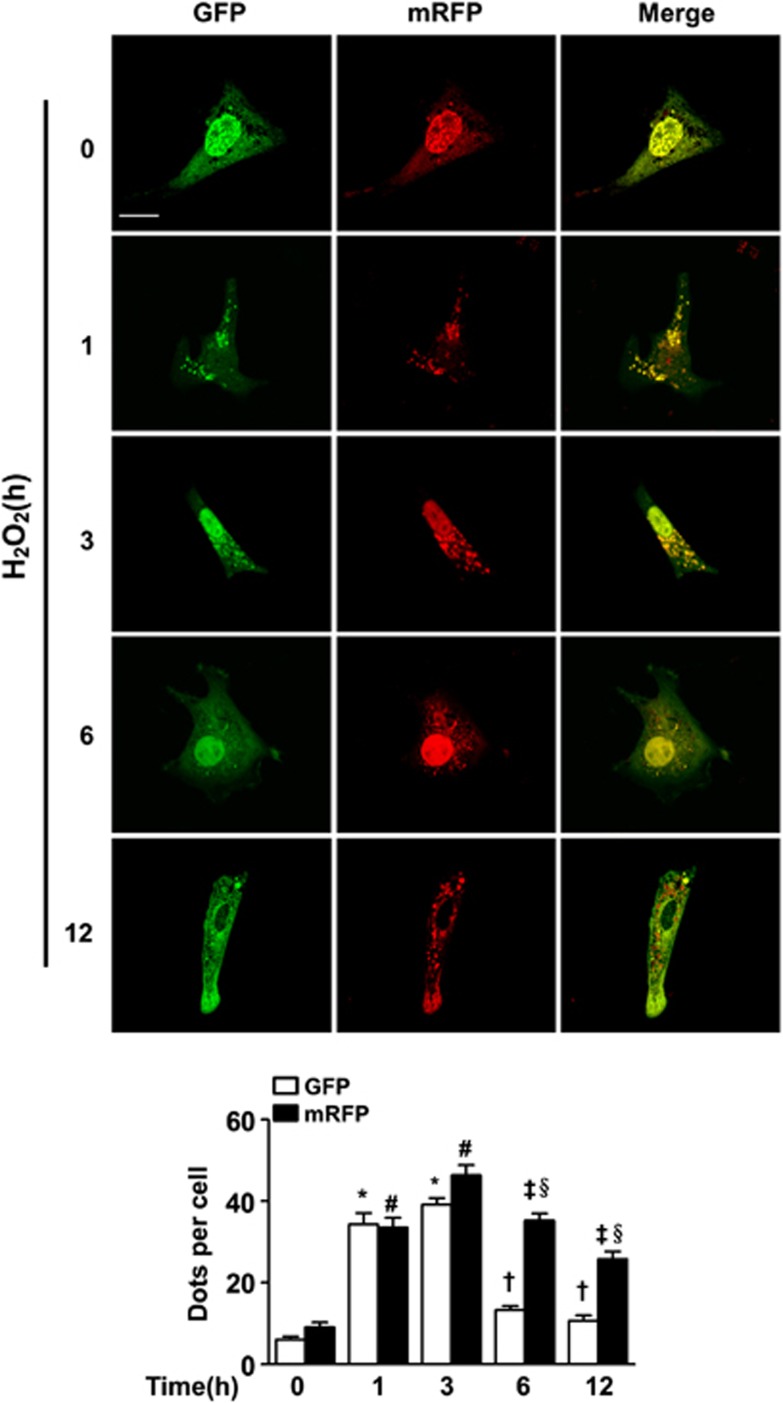
Autophagy is a dynamic process that involves autophagosome formation and lysosome degradation. To assess the influence of H2O2 on autophagosome formation and the autophagosome–lysosome fusion process, we transfected cardiomyocytes with a construct expressing monomeric red fluorescent protein/green fluorescent protein (mRFP–GFP) tandem tagged LC3 protein to examine the autophagosome maturation process. In merged images, the yellow and red puncta represent autophagosomes and autolysosomes, respectively, because mRFP but not GFP retains fluorescence in the acidic environment of lysosomes.34 Consistent with the results of western blot analysis, we observed that the number of autophagosomes rapidly increased within 3 h of H2O2 treatment. Sustained treatment resulted in a decrease in the number of yellow puncta, and the number of red puncta was also slightly reduced (Figure 2).
Figure 2.
Hydrogen peroxide (H2O2) promotes autophagic flux in cardiomyocytes. Cardiomyocytes were transfected with tandem-LC3 construct (GFP-mRFP-LC3) for 24 h, exposed to 150 μM H2O2 for 1, 3, 6 or 12 h and photographed using confocal microscopy. Representative images are shown in the upper panel. Both the GFP and mRFP dots were counted, and the statistical analyses are shown in the bottom panel. The data were obtained from 3 independent experiments, and 10 cells were scored in each experiment. Scale bar=20 μM. Data are presented as the mean±S.E.M. *P<0.05 versus control GFP, #P<0.05 versus control mRFP, †P<0.05 versus 3 h GFP, ‡P<0.05 versus 3 h mRFP, and §P<0.05 versus the corresponding GFP dots at each time point
These data indicated that H2O2 induced autophagic flux; moreover, the number of autophagosomes decreased at the late stage (6–12 h) of treatment relative to the early stage. A few studies have reported that AMPK plays critical roles in the regulation of autophagosome formation in response to ROS.35, 36 We evaluated the phosphorylation of AMPK at threonine-172 that directly reflects its enzymatic activity. The changes in the expression of phosphorylated AMPK were similar to those observed for LC3-II. The phosphorylation of AMPK rapidly increased in response to oxidative stress, whereas it declined in a time-dependent manner beyond 3 h. Given these results, we wondered whether the apoptosis induced by prolonged oxidative stress is related to the reduced autophagosome formation and whether calcineurin is implicated in the regulation of autophagy through its effect on the AMPK/mTOR axis.
Inhibition of calcineurin promotes autophagy and alleviates oxidative stress-induced apoptosis in cardiomyocytes
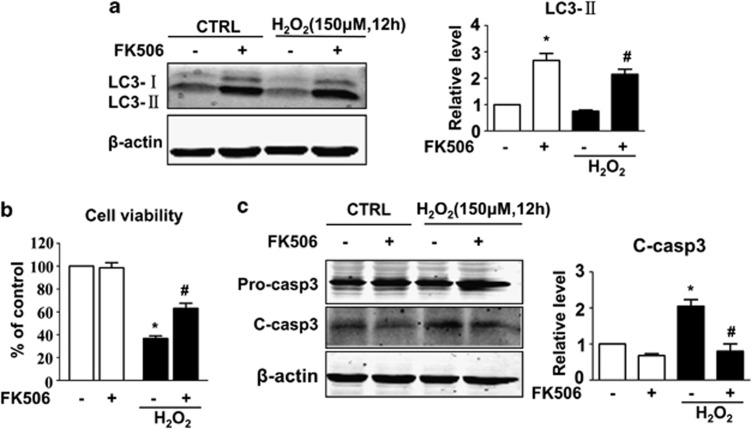
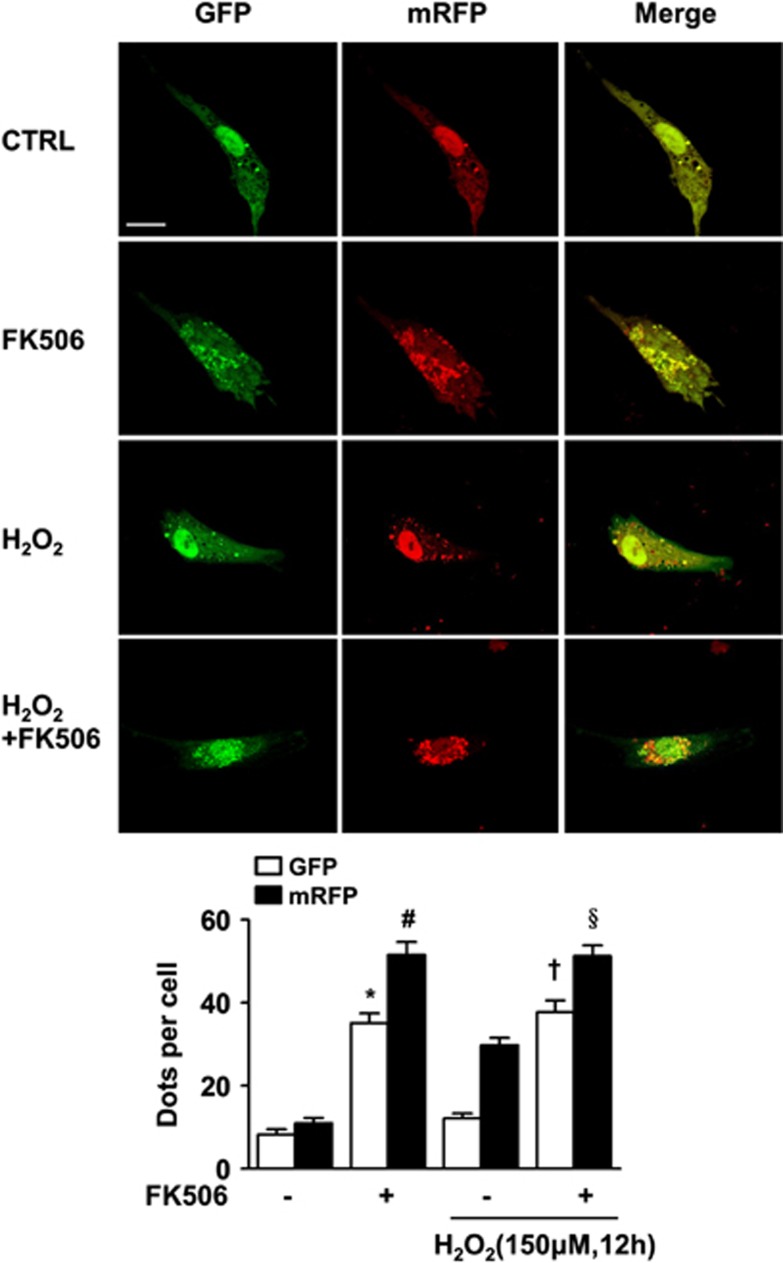
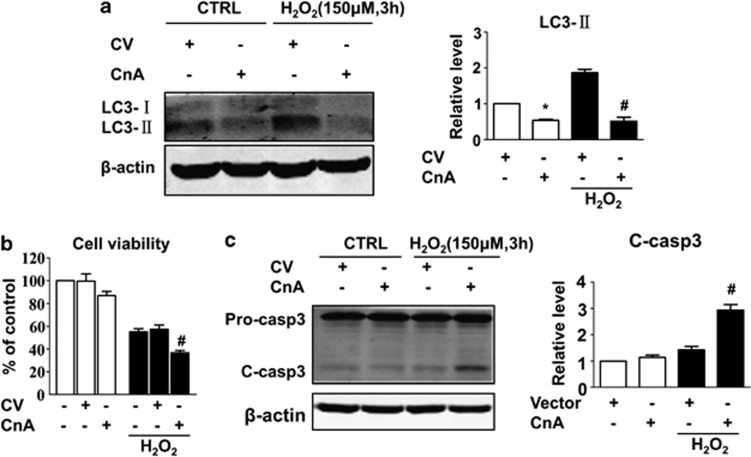
To determine the potential role of calcineurin in autophagy, cardiomyocytes were treated with FK506, a specific calcineurin inhibitor, and subjected to prolonged H2O2 exposure (12 h). Consistent with our original assumption, we observed a pronounced elevation in LC3-II immuno-reactivity in the FK506 groups with or without H2O2 (Figure 3a). Inhibition of calcineurin not only resulted in an increase in the number of autophagosomes, but also enhanced the autophagic flux, as shown by the dramatic increase in the number of both GFP and mRFP puncta (Figure 4).
Figure 3.
FK506 induces autophagy and alleviates hydrogen peroxide (H2O2)-induced apoptosis. Cells were incubated with or without H2O2 (150 μM) in the presence or absence of FK506 (1 μM) for 12 h and subjected to MTT assay and western blotting, respectively. (a) FK506 induced LC3 expression with or without H2O2. LC3-II levels were normalised to β-actin and are shown in the bar graph on the right panel. (b) FK506 increased cell viability and (c) decreased the cleavage of caspase-3. The cleaved caspase-3 expression was normalised to β-actin and is shown in the right panel. Data are presented as the mean±S.E.M., n≥3. *P<0.05 versus the control group and #P<0.05 versus the H2O2 alone group
Figure 4.
FK506 promotes the formation of autophagosomes and autolysosomes. Cells were transfected as described in Figure 2 and were subsequently treated as described in Figure 3. The number of both the GFP and mRFP dots was significantly increased with or without H2O2 treatment as shown in the bar graph in the bottom panel. Scale bar=20 μM. Data are presented as the mean±S.E.M. *P<0.05 versus control GFP, #P<0.05 versus control mRFP, †P<0.05 versus H2O2-alone GFP and §P<0.05 versus H2O2-alone mRFP
Previous studies have demonstrated that calcineurin induces apoptosis in mammalian cells, including cardiomyocytes. The role of calcineurin in the apoptotic model we used has seldom been tested. We observed that inhibition of calcineurin exerts a protective effect. As shown in Figure 3a and b, cells treated with FK506 displayed decreased cleavage of pro-caspase-3 after long-term (12 h) H2O2 treatment. The cell viability recovered by an average of 20% as detected by MTT assay. These results showed that inhibition of calcineurin enhances autophagy and protects cardiomyocytes from oxidative stress-induced apoptosis.
Overexpression of calcineurin inhibits autophagy and exaggerates oxidative stress-induced apoptosis
To further ascertain the inhibitory role of calcineurin on autophagy, cardiomyocytes were transiently transfected with a construct expressing the constitutively active form of calcineurin A (CnA). The cells were subsequently subjected to H2O2 or control vehicle (phosphate-buffered saline (PBS)) treatment for 3 h, a time point that showed maximum autophagosome formation and mild apoptosis (Figures 1b, d and 2). Overexpression of calcineurin resulted in reduced LC3-II levels with or without H2O2 (Figure 5a). Moreover, although overexpression of calcineurin A did not markedly change caspase-3 cleavage under normal conditions, we observed increased activation of caspase-3 and decreased viability of cells after brief exposure to H2O2 (3 h) that were comparable to those observed after long-term H2O2 treatment (12 h) (Figure 5b and c). These results strongly indicate that calcineurin is a negative regulator of autophagy and exerts a pro-apoptotic effect during oxidative stress.
Figure 5.
Overexpression of calcineurin inhibits autophagy and sensitises cells to undergo H2O2-induced apoptosis. Cells were transfected with the control vector (CV) or vector expressing calcineurin A for 24 h and subsequently incubated with or without H2O2 (150 μM) for 3 h. After the treatment, the cells were harvested for MTT assay and western blotting. (a) Calcineurin inhibits LC3 expression whether H2O2 is present or not. (b) Overexpression of calcineurin results in decreased cell viability and (c) increased caspase-3 cleavage. Data are presented as the mean±S.E.M., n≥3. *P<0.05 versus the control group and #P<0.05 versus the H2O2-alone group
The AMPK/mTOR axis mediates the effect of calcineurin on autophagy and cell survival during oxidative stress
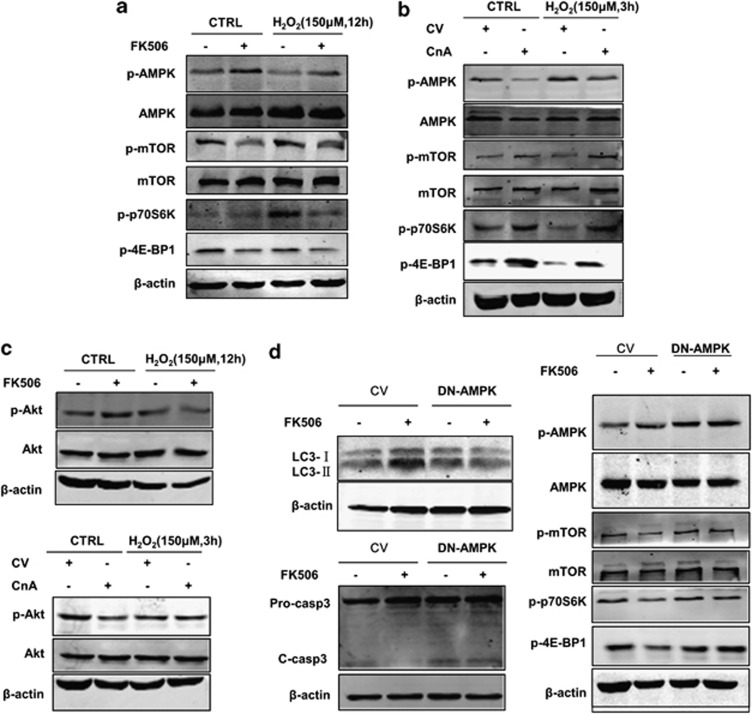
AMPK/mTOR is critical in the regulation of autophagy, and a positive correlation between phosphorylation of AMPK and LC3-II expression was observed in our cellular model. We thus simultaneously evaluated the changes in the AMPK signalling pathway during both calcineurin inhibition and overexpression. As shown in Figure 6a, inhibition of calcineurin by FK506 significantly restored the phosphorylation of AMPK at 12 h in response to H2O2 treatment. Accordingly, the activity of the mTOR signalling, which negatively modulates autophagy and is known to be inhibited by AMPK, was downregulated when treated with FK506, as represented by decreased phosphorylation of mTOR (Ser2448) and its two primary downstream targets p70 ribosomal protein S6 kinase (p70S6K; Thr389) and elongation factor 4E binding protein 1 (4E-BP1; Thr37/46). In contrast, overexpression of the active form of calcineurin attenuated AMPK signalling and enhanced mTOR signalling under both normal and oxidative stress conditions (Figure 6b).
Figure 6.
AMPK/mTOR signalling mediates the effect of calcineurin on autophagy and cell survival after H2O2 treatment. (a) Inhibition of calcineurin by FK506 enhances AMPK phosphorylation and attenuates the phosphorylation of mTOR. Cells were treated as described in Figure 3, and whole-cell lysates were subjected to western blot analysis. (b) Overexpression of calcineurin attenuates the phosphorylation of AMPK and enhances mTOR phosphorylation. Cells were treated as described in Figure 5, and whole-cell lysates were subjected to western blot analysis. (c) Calcineurin attenuates Akt signalling under normal conditions but not under oxidative stress conditions. Cells were treated as previously described. (d) FK506 showed no effect on autophagy regulation, cell protection and mTOR inhibition in DN-AMPK-expressing cells. Cells were transfected with the control vector (CV) and the DN-AMPK vector for 24 h and subsequently incubated with 150 μM H2O2 in the presence or absence of FK506 (1 μM) for 12 h. Representative images are from three or more independent experiments
Because calcineurin has been shown to dephosphorylate protein kinase B (Akt),37 which is also a potent regulator of mTOR, we examined whether Akt is involved in the regulation of mTOR by calcineurin. As shown in Figure 6c, inhibition of calcineurin by FK506 significantly increased the phosphorylation of Akt under normal conditions. However, this effect was not observed after H2O2 treatment. Similarly, calcineurin overexpression resulted in a significant decrease in the phosphorylation of Akt that was not observed with H2O2. These results indicate that Akt is not involved in the regulation of mTOR by calcineurin under oxidative stress conditions.
To further address the mechanisms by which calcineurin inhibits autophagy, we manipulated AMPK activity by expressing a dominant negative mutant of AMPK (DN-AMPK), thereby inhibiting endogenous AMPK activity. Cells expressing the kinase-dead K45R DN-AMPKα2 mutant exhibit greater basal phosphorylation levels as previously described.38 As shown in Figure 6d, treatment with FK506 resulted in increased levels of LC3-II in cells expressing the control vector, whereas this effect was abolished in cells expressing DN-AMPK as shown by the unchanged LC3-II levels. FK506 failed to blunt mTOR signalling in cells expressing the DN-AMPK mutant. The activation of caspase-3 was also analysed by western blotting. As shown in Figure 6d, inhibition of calcineurin by FK506 decreased the level of cleaved caspase-3 in cells transfected with the control vector, whereas this effect was abolished in cells expressing the DN-AMPK mutant. These results indicate that the AMPK/mTOR axis is responsible for the induction of autophagy caused by calcineurin inhibition and to a certain extent mediates a protective effect against oxidative damage.
Calcineurin induces apoptosis through suppression of autophagy during oxidative stress
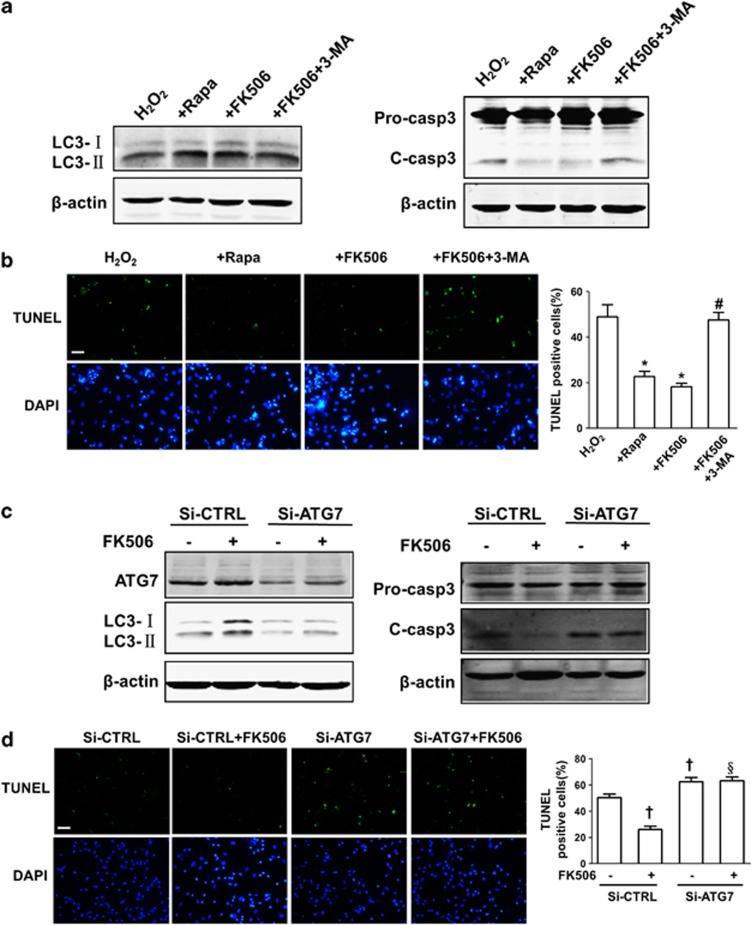
To investigate the role of autophagy in cell survival under oxidative stress conditions, we manipulated autophagy activity using rapamycin and 3-methyladenine (3-MA) to inhibit the activity of mTOR and class III PI3K, respectively, and to promote or suppress autophagy, respectively. As expected, western blotting and TUNEL (terminal deoxynucleotidyl transferase dUTP nick-end labelling) assays both showed that rapamycin attenuated the activation of caspase-3 and reduced the number of TUNEL-positive cells while inducing autophagy. FK506 mimicked the effects of rapamycin; however, 3-MA cancelled the protective effect of FK506 (Figure 7a and b).
Figure 7.
Autophagy protects cells from apoptosis induced by hydrogen peroxide (H2O2). (a) The effect of rapamycin, FK506 and FK506 combined with 3-MA on LC3-II and cleaved caspase-3 expression as detected by western blotting. (b) The effect of rapamycin, FK506 and FK506 combined with 3-MA on H2O2-induced apoptosis as determined by TUNEL staining. Cells were incubated with H2O2 (150 μM) in the presence of rapamycin (Rapa, 500 nM), FK506 (1 μM) or FK506 (1 μM) combined with 3-MA (5 mM) for 12 h. (c) The effect of ATG7 knockdown on LC3-II and cleaved caspase-3 expression detected by western blotting. (d) The effect of ATG7 knockdown on H2O2-induced apoptosis as determined by TUNEL staining. Cells were transfected with negative control or ATG7 siRNA for 48 h and were subsequently subjected to H2O2 (150 μM) for 12 h with or without FK506 (1 μM). Scale bar=100 μM, the data for statistical analysis were from three independent experiments and the percentage of TUNEL-positive cells was scored in four non-overlapping microscopic fields in each experiment. Data are presented as the mean±S.E.M. *P<0.05 versus H2O2 alone and #P<0.05 versus H2O2+FK506, †P<0.05 versus si-CTRL and §P<0.05 versus si-CTRL+FK506
In view of the fact that 3-MA and rapamycin have pleiotropic effects besides autophagy modulation, we synthesised small interfering RNA (siRNA) against essential autophagy gene ATG7 to inhibit autophagy more specifically. Knockdown of ATG7 significantly inhibited the autophagy marker LC3-II (Figure 7c). Consistent with the results obtained from pharmacological modulation of autophagy, gene-specific inhibition of autophagy by ATG7 knockdown not only increased H2O2-induced apoptosis but also markedly blocked the protective effects of calcineurin inhibitor FK506 (Figure 7c and d). These results confirmed our hypothesis that autophagy is an adaptive response that protects cardiomyocytes under oxidative stress from apoptosis and that increased autophagic activity at least partially confers the protective effects of calcineurin inhibition.
Discussion
In this study, we demonstrated that calcineurin confers oxidative stress damage on cardiomyocytes by negatively regulating cardiomyocyte autophagy through the AMPK/mTOR axis. The following evidence supports this conclusion: (1) inhibition of calcineurin dramatically enhanced AMPK/mTOR signalling, increased autophagy and partially rescued cardiomyocytes from cell death; (2) overexpression of calcineurin attenuated AMPK/mTOR signalling, decreased autophagy and exacerbated oxidative stress damage; (3) the induction of autophagy caused by calcineurin inhibition was abolished in cardiomyocytes expressing dominant negative mutant AMPK; and (4) inhibition of autophagy by 3-MA and ATG7 knockdown abrogated the protective effects of FK506.
In the case of oxidative stress, interaction of ROS and Ca2+ as inducer and effector alters several signalling pathways that are critical to cell survival. As a calcium-activated phosphatase, calcineurin is abundantly expressed in the heart and plays pivotal roles in many cardiovascular diseases involving oxidative stress.26, 39, 40 It is still unknown whether calcineurin delivers pro-survival or pro-death signals in the heart. Transgenic mice that constitutively activate calcineurin A exhibit less apoptosis than WT littermates.41 In contrast, calcineurin induces apoptosis through dephosphorylation of ASK1 and ARC in cardiomyocytes exposed to H2O2 and isoprenaline, respectively.27, 30 A more recent study revealed a novel role of calcineurin in transmitting the apoptotic signal during heart ischaemia–reperfusion by regulating mitochondrial dynamics.28 Although it is well known that calcineurin directly triggers apoptosis or mediates the pro-apoptotic effect in cardiomyocytes and other cells, the link between calcineurin, autophagy and apoptosis remains obscure. Our present study suggests for the first time that apoptosis is induced at least partially by the retardation of autophagy caused by calcineurin activation. Consistent with other studies,9, 42, 43 we demonstrated that AMPK/mTOR-dependent autophagy is a mechanism that protects against oxidative stress.
Excessive ROS has the potential to cause protein misfolding and mitochondrial damage44, 45 that are detrimental to proper mitochondrial function and cause energy and nutrient depletion. Autophagy is evolutionarily utilised by eukaryotic cells to recycle cellular waste into raw materials and thereby maintain homeostasis.5, 6 Given that a cardiomyocyte is a nonreplicating cell with a high metabolism, it is of great importance for cardiomyocytes to maintain high autophagic activity in response to oxidative stress. Because ROS cause various types of cell damage, such as energy deprivation, DNA damage, calcium abnormalities and mitochondrial aggregation, autophagy may be induced by perturbations to a series of signals. The role of AMPK, which senses energy status and regulates autophagy, has been well established. AMPK is able to induce autophagy through direct activation of Ulk1 (mammalian homologue of ATG1 in yeast)19 and inhibition of mTORC1.46, 47 Given that calcium is one of the most common and effective signal transducers and plays key roles in oxidative stress,48 calcium signals may tune autophagy. Recent studies implicate calcium signalling in the induction of autophagy via calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ)-dependent activation of AMPK.49 In our study, however, we demonstrated that the calcineurin signal suppresses autophagy by counteracting the AMPK/mTOR axis that is consistent with previous studies in calcineurin-null mutants of C. elegans and in the cyclosporine A-treated rat hippocampus.29, 50
In our study, autophagosomes were rapidly assembled at an early stage after oxidative stress stimulus, but their numbers declined remarkably after continued stimulation. By utilising the tandem fluorescence LC3 microscopy assay, we verified the autophagic flux induced by oxidative stress. It is worth noting that the attenuation of the AMPK signal at late stages was observed in our experiments. Because AMPK functions as a potent autophagy regulator that affects autophagosome formation through a mTOR- and Ulk1-dependent mechanism, we still cannot rule out the contributions of attenuated AMPK signalling to the decrease in the number of autophagosomes at the late stage relative to the early stage, even though the decrease can be explained by the degradation of LC3 by the autophagosome clearance mechanism when it fuses with the lysosome. By either inhibiting or overexpressing calcineurin, we discovered that calcineurin regulates autophagy through the AMPK pathway. We have unveiled the role of AMPK-mediated autophagy on cell survival. Collectively, we proposed the following schematic model for the role of calcineurin in the regulation of oxidative stress damage. At early stages, the adaptive response is triggered and leads to AMPK activation, causing a higher autophagy level to maintain homeostasis. Despite modest increases in calcineurin activity, the adaptive signals activating AMPK are strong enough to offset the antagonistic effect of calcineurin on AMPK. However, with long-term exposure, a sustained increase in calcineurin activity beyond a certain threshold is sufficient to inhibit the AMPK pathway, thereby inhibiting adaptive autophagy with resultant cell death.
Although we identified AMPK as a main mediator of autophagy that is controlled by calcineurin, we did not exclude other mechanisms. To present studies, mTOR is at the convergence of multiple signals that modulate autophagy and is precisely regulated by upstream kinase cascades. Stressed cells often exhibit alternations of several different signals concurrently. Oxidative stress may affect mTOR at different levels. For example, the PI3K/Akt/mTOR axis is extensively studied in the regulation of autophagy. PI3K/Akt signalling negatively regulates autophagy by activating mTOR.51, 52 Calcineurin has been reported to directly dephosphorylate Akt.37 Indeed, our results are in agreement with this study when cells are under normal conditions. However, we did not observe that calcineurin has a significant effect on Akt signalling in the context of oxidative stress. Inhibition of AMPK by overexpressing the DN-AMPK construct completely abolished the effect of FK506 on mTOR and the outcome of autophagy. These results strongly suggest that calcineurin regulates mTOR in an AMPK-dependent, Akt-independent manner. In addition to regulation through the AMPK/mTOR axis, calcineurin might affect autophagy by transcriptional modulation. Recent studies have shed some light on the relationship between calcineurin and forkhead transcriptional factors (FOXO).53, 54 FOXO1 and FOXO3 are able to regulate autophagy-related gene expression in cardiomyocytes.55 Whether calcineurin regulation of cardiomyocyte autophagy involves a FOXO-dependent mechanism under oxidative stress conditions remains to be seen.
Despite that we have found that calcineurin attenuates AMPK-dependent autophagy, our understanding of how AMPK is regulated by calcineurin is still rudimentary. AMPK activity is precisely controlled by kinases that lead to phosphorylation at the Thr-172 residue. We speculate that calcineurin attenuates AMPK signalling through indirect regulation, because the dephosphorylation of AMPK is primarily under the control of PP2Cα and PP2A.56, 57 TGF-β-activated kinase 1 (TAK1), liver kinase B1 (LKB1) and CaMKKβ are upstream kinases of AMPK. In a study of the rat hippocampus,29 cyclosporine A enhanced the phosphorylation of activation residues of TAK1 and LKB1. As a protein phosphatase, calcineurin may have the potential to dephosphorylate these kinases under calcium perturbation conditions. Although TAK1 has been identified as a target of calcineurin,58 it will be interesting in our future work to determine whether calcineurin dephosphorylates these three kinases in this cellular model.
The current study is important because our findings define a novel interaction between calcineurin and the AMPK/mTOR pathway for the control of autophagy. We demonstrated that autophagy is a critical biologic process for the survival of cardiomyocytes undergoing oxidative stress. Because oxidative stress is involved in a series of cardiovascular complications, inhibition of calcineurin and activation of autophagy may be a novel therapeutic approach with a broad clinical potential to prevent oxidative stress damage to cardiomyocytes.
Materials and Methods
Cell culture and treatment
Primary rat cardiomyocytes were prepared as previously described.59 Briefly, cardiac tissues were digested by pancreatin, and the isolated cells were resuspended in Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA) containing 10% fetal bovine serum FBS; Hyclone). Cardiomyocytes were purified by differential plating, and 0.1 mmol/l BrdU (5-bromo-2-deoxyuridine) was added to exclude the cardiac fibroblasts. Cells were cultured in a 5% CO2 and 37°C humidified atmosphere, and subsequent experiments were performed 48 h after plating. To simulate oxidative stress in cardiomyocytes, H2O2 was used at a final concentration of 150 μM. ZVAD-fmk (40 μM), FK506 (1 μM) and rapamycin (500 nM) were dissolved in dimethyl sulphoxide (DMSO), and 3-MA (5 mM) was dissolved in the culture medium. All the chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell viability assay
An MTT assay was used to determine cell viability. Adherent cardiomyocytes were washed and placed in fresh medium. The cells were treated as designated and were subsequently incubated with 20 μl MTT (0.5 mg/ml) for 4 h. The culture medium was carefully removed, and 200 μl DMSO was added to each well to dissolve the formazan. After the cells were rocked for 10 min, the absorbance values were read at 570 nm using an Infinite m200pro microplate spectrophotometer (Tecan, Salzburg, Austria).
Cellular calcineurin activity assay
Cellular calcineurin activity was measured by calcineurin cellular assay kit (ENZO Life Sciences, Plymouth Meeting, PA, USA) according to the manufacturer's protocol. Briefly, treated cells were collected using the lysis buffer supplied in the kit. The extracts were then subjected to desalting resin to remove free phosphate. The desalted lysates were incubated with the calcineurin substrate RII phosphopeptide in assay buffer and EGTA buffer at 30°C for 30 min to determine total phosphatase activity and phosphatase activity without calcineurin, respectively. Then, 100 μl BIOMOL GREEN was added to each well to visualise the phosphate release. The colour was allowed to develop at room temperature for 20 min before the detection of the absorbance value at 620 nm on the microplate spectrophotometer. The absorbance value A620, total –A620, EGTA buffer was converted to phosphate release using the standard curve that was prepared in each assay. The phosphate release was divided by protein concentration and normalised to the control group to obtain relative calcineurin activity.
TUNEL assay
Cells were plated on coverslips in 24-well culture plates and treated as described. To evaluate the extent of apoptosis, a TUNEL assay was performed using an in situ cell death detection kit (Roche, Mannheim, Germany) according to the manufacturer's protocol. The TUNEL-stained slides were washed with PBS and counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Beyotime, Shanghai, China). A laser confocal microscope (Olympus, Tokyo, Japan) was used to acquire the images. Nuclei that were double labelled with TUNEL and DAPI were considered to be TUNEL positive. The TUNEL-positive cells were counted in four non-overlapping microscopic fields of three independent experiments.
RNA interference
The siRNA targeting rat ATG7 and nontargeting negative control siRNA were designed and synthesised by Invitrogen (Shanghai, China). The siRNAs were transfected into cells using the Xtreme GENE siRNA Transfection Reagent (Roche) at a concentration of 100 nM according to the manufacturer's instructions. Subsequent experiments were performed 48 h after the transfection.
Plasmid transfections
The tandem tagged GFP–mRFP–LC3 plasmid was generously provided by Dr. Tamotsu Yoshimori (Addgene plasmid, 21074, Addgene, Cambridge, MA, USA), the pcDNA3.0 plasmid that encodes DN-AMPKα2 (K45R) was generously provided by Dr. Morris J Birnbaum (Addgene plasmid, 15992, Addgene) and the pEGFP-C3 plasmid that encodes the constitutively active form of CnA was generously provided by Dr. Oliver Ritter (Würzburg, Germany). The control vectors pEGFP-C3 and pcDNA3.0 were obtained from Clontech (Palo Alto, CA, USA) and Invitrogen (San Diego, CA, USA) respectively. Primary cardiomyocytes at 70–80% confluence were transfected with 1.0–2.0 μg plasmid using Xtreme GENE HP (Roche) according to the manufacturer's instructions. The cells were subjected to the different designated treatments after 36 h of transfection.
Tandem mRFP–GFP fluorescence microscopy
Cells transiently expressing GFP–mRFP–LC3 were treated as designated and were then observed by laser microscopy. The number of GFP and mRFP puncta per cell was quantified manually. At least 10 cells in 3 independent experiments were analysed randomly.
Western blotting
After the treatment described above, cells were lysed in RIPA buffer. The protein concentration was determined with a BCA kit (Beyotime) according to the manufacturer's instructions. For western blot analysis, 20–40 μg denatured protein was separated on SDS-PAGE gels and transferred onto nitrocellulose membranes. Before incubation with the primary antibody, the membranes were blocked with 5% skim milk in TBS for 1 h at room temperature on a rocker. The membranes were incubated with diluted antibody in TBS containing 5% BSA and 0.1% Tween-20 at 4°C overnight. The membranes were washed 3 times with TBST (TBS containing 0.5% Tween-20) for 5 min each wash, followed by re-probing with fluorescence-conjugated secondary antibodies at room temperature for 1 h. Membranes were washed again 3 times with TBST for 5 min each before antibody detection using the Odyssey infrared scanning system (LI-COR Biosciences, Lincoln, NE, USA). The western blot bands were quantified using Odyssey 3.0 software and normalised with respect to the loading control. The antibody sources and dilutions used are as follows:
Antibodies against LC3B (2775, 1 : 1000), p-AMPKα (2535, 1 : 500), AMPKα (2531, 1 : 1000), p-mTOR (5536, 1 : 1000), mTOR (2983, 1 : 1000), p-p70S6K (9208, 1 : 500), p-4EBP1 (2855, 1 : 1000), caspase-3 (9662, 1 : 1000), Akt (9272, 1 : 1000), and ATG7 (8558, 1 : 1000) were purchased from Cell Signaling Technology (Danvers, MA, USA).
The antibody against p-Akt (sc-7985-R, 1 : 200) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
The antibody against β-actin (ab8226, 1 : 2000) was purchased from Abcam (Cambridge, MA, USA).
The fluorescence-conjugated secondary IRDye700/800 mouse and rabbit antibodies (1 : 10 000) were purchased from LI-COR.
Statistical analysis
Quantitative data were reported as the mean±S.E.M., and one-way analysis of variance (ANOVA) was used to examine the difference between groups. Pairwise comparisons were performed using the Bonferroni post hoc test. A two-tailed value of P<0.05 was considered statistically significant.
Acknowledgments
We thank Professor O Ritter and Dr. Melanie Mühlfelder (Würzburg, Germany) for providing the calcineurin plasmid. This work was supported in part by National Basic Research Program of China (973 Program) (2013CB531104), the Major Program (81230081) of National Natural Science Foundation of China and the National Nature Science Foundation of China (No. 31171094 and 81100122).
Glossary
- AMPK
AMP-activated protein kinase
- LC3
microtubule-associated protein 1 light chain 3
- CnA
calcineurin subunit A
- ATG
autophagy-related proteins
- mTOR
mammalian target of rapamycin
- Akt
protein kinase B
- p70S6K
p70 ribosomal protein S6 kinase
- 4E-BP1
elongation factor 4E binding protein 1
- FOXO
Forkhead box
- TAK1
TGF-β-activated kinase 1
- LKB1
liver kinase B1
- CaMKKβ
calcium/calmodulin-dependent protein kinase kinase β
- GFP
green fluorescent protein
- mRFP
monomeric red fluorescent protein
- DN
dominant negative
- ROS
reactive oxygen species
- PBS
phosphate-buffered saline
- DMSO
dimethyl sulphoxide
- 3-MA
3-methyladenine
- FBS
fetal bovine serum
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labelling
- DAPI
4′,6-diamidino-2-phenylindole
- BrdU
5-Bromo-2-deoxyUridine
- siRNA
small interfering RNA
The authors declare no conflict of interest.
Footnotes
Edited by RA Knight
References
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- Ansley DM, Wang B. Oxidative stress and myocardial injury in the diabetic heart. J Pathol. 2013;229:232–241. doi: 10.1002/path.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongara S, Karantza V. The interplay between autophagy and ROS in tumorigenesis. Front Oncol. 2012;2:171. doi: 10.3389/fonc.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Dutta D, Xu J, Kim JS, Dunn WA, Jr, Leeuwenburgh C. Upregulated autophagy protects cardiomyocytes from oxidative stress-induced toxicity. Autophagy. 2013;9:328–344. doi: 10.4161/auto.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, Li YB, Wang Y, Liu GD, Wang J, Zhu XO, et al. The role of autophagy in endoplasmic reticulum stress-induced pancreatic beta cell death. Autophagy. 2012;8:158–164. doi: 10.4161/auto.8.2.18807. [DOI] [PubMed] [Google Scholar]
- Ciechomska IA, Gabrusiewicz K, Szczepankiewicz AA, Kaminska B. Endoplasmic reticulum stress triggers autophagy in malignant glioma cells undergoing cyclosporine a-induced cell death. Oncogene. 2013;32:1518–1529. doi: 10.1038/onc.2012.174. [DOI] [PubMed] [Google Scholar]
- Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XC, Yu JT, Jiang T, Tan L. Autophagy modulation for Alzheimer's disease therapy. Mol Neurobiol. 2013;48:702–714. doi: 10.1007/s12035-013-8457-z. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- Kang HT, Lee KB, Kim SY, Choi HR, Park SC. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS One. 2011;6:e23367. doi: 10.1371/journal.pone.0023367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Heidrich F, Schotola H, Popov AF, Sohns C, Schuenemann J, Friedrich M, et al. AMPK - activated protein kinase and its role in energy metabolism of the heart. Curr Cardiol Rev. 2010;6:337–342. doi: 10.2174/157340310793566073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva MA, Rutter-Locher Z, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, et al. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300:H2123–H2134. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SS, Liu JJ, Yu XJ, Lu Y, Zang WJ. Protection against ischemia-induced oxidative stress conferred by vagal stimulation in the rat heart: involvement of the AMPK-PKC pathway. Int J Mol Sci. 2012;13:14311–14325. doi: 10.3390/ijms131114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WQ, Wang JX, Lin ZQ, Li YR, Lin Y, Li PF. Novel cardiac apoptotic pathway: the dephosphorylation of apoptosis repressor with caspase recruitment domain by calcineurin. Circulation. 2008;118:2268–2276. doi: 10.1161/CIRCULATIONAHA.107.750869. [DOI] [PubMed] [Google Scholar]
- Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- Park HG, Yi H, Kim SH, Yu HS, Ahn YM, Lee YH, et al. The effect of cyclosporine A on the phosphorylation of the AMPK pathway in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1933–1937. doi: 10.1016/j.pnpbp.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wilkins BJ, Lee YJ, Ichijo H, Molkentin JD. Direct interaction and reciprocal regulation between ASK1 and calcineurin-NFAT control cardiomyocyte death and growth. Mol Cell Biol. 2006;26:3785–3797. doi: 10.1128/MCB.26.10.3785-3797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Nottingham SA, Kennedy SE. Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury. J Neurosci. 2000;20:7246–7251. doi: 10.1523/JNEUROSCI.20-19-07246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chang JH, Paik SY, Tang Y, Eisner W, Spurney RF. Calcineurin (CN) activation promotes apoptosis of glomerular podocytes both in vitro and in vivo. Mol Endocrinol. 2011;25:1376–1386. doi: 10.1210/me.2011-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liang B, Shirwany NA, Zou MH. 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS One. 2011;6:e17234. doi: 10.1371/journal.pone.0017234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sid B, Verrax J, Calderon PB. Role of AMPK activation in oxidative cell damage: implications for alcohol-induced liver disease. Biochem Pharmacol. 2013;86:200–209. doi: 10.1016/j.bcp.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci USA. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Feng X, Li J, Liu J, Jin M, Liu X, Du H, et al. Protective effect of FK506 on myocardial ischemia/reperfusion injury by suppression of CaN and ASK1 signaling circuitry. Cardiovasc Toxicol. 2011;11:18–27. doi: 10.1007/s12012-010-9095-6. [DOI] [PubMed] [Google Scholar]
- Felkin LE, Narita T, Germack R, Shintani Y, Takahashi K, Sarathchandra P, et al. Calcineurin splicing variant calcineurin Abeta1 improves cardiac function after myocardial infarction without inducing hypertrophy. Circulation. 2011;123:2838–2847. doi: 10.1161/CIRCULATIONAHA.110.012211. [DOI] [PubMed] [Google Scholar]
- Bousette N, Chugh S, Fong V, Isserlin R, Kim KH, Volchuk A, et al. Constitutively active calcineurin induces cardiac endoplasmic reticulum stress and protects against apoptosis that is mediated by alpha-crystallin-B. Proc Natl Acad Sci USA. 2010;107:18481–18486. doi: 10.1073/pnas.1013555107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Pan XY, Xu Y, Xiao Y, An Y, Tie L, et al. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2012;8:812–825. doi: 10.4161/auto.19471. [DOI] [PubMed] [Google Scholar]
- Harhaji-Trajkovic L, Vilimanovich U, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumour cells. J Cell Mol Med. 2009;13:3644–3654. doi: 10.1111/j.1582-4934.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Lipton SA. Cell death: protein misfolding and neurodegenerative diseases. Apoptosis. 2009;14:455–468. doi: 10.1007/s10495-008-0301-y. [DOI] [PubMed] [Google Scholar]
- Fischer F, Hamann A, Osiewacz HD. Mitochondrial quality control: an integrated network of pathways. Trends Biochem Sci. 2012;37:284–292. doi: 10.1016/j.tibs.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Ghislat G, Patron M, Rizzuto R, Knecht E. Withdrawal of essential amino acids increases autophagy by a pathway involving Ca2+/calmodulin-dependent kinase kinase-beta (CaMKK-beta) J Biol Chem. 2012;287:38625–38636. doi: 10.1074/jbc.M112.365767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi M, Song HO, Ahnn J. Autophagy genes mediate the effect of calcineurin on life span in C. elegans. Autophagy. 2009;5:604–607. doi: 10.4161/auto.5.5.8157. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Wu YT, Tan HL, Huang Q, Ong CN, Shen HM. Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy. 2009;5:824–834. doi: 10.4161/auto.9099. [DOI] [PubMed] [Google Scholar]
- Tao L, Xie Q, Ding YH, Li ST, Peng S, Zhang YP, et al. CAMKII and Calcineurin regulate the lifespan of Caenorhabditis elegans through the FOXO transcription factor DAF-16. Elife. 2013;2:e00518. doi: 10.7554/eLife.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Pezzi E, Winn N, Paul A, McCullagh K, Slominsky E, Santini MP, et al. A naturally occurring calcineurin variant inhibits FoxO activity and enhances skeletal muscle regeneration. J Cell Biol. 2007;179:1205–1218. doi: 10.1083/jcb.200704179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284:28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Shan Q, et al. Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. J Pathol. 2010;222:199–212. doi: 10.1002/path.2754. [DOI] [PubMed] [Google Scholar]
- Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- Liu Q, Busby JC, Molkentin JD. Interaction between TAK1-TAB1-TAB2 and RCAN1-calcineurin defines a signalling nodal control point. Nat Cell Biol. 2009;11:154–161. doi: 10.1038/ncb1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Li J, Wang X, Chen N, Cai B, Wang G, et al. Tanshinone IIA protects against cardiac hypertrophy via inhibiting calcineurin/NFATc3 pathway. Int J Biol Sci. 2011;7:383–389. doi: 10.7150/ijbs.7.383. [DOI] [PMC free article] [PubMed] [Google Scholar]