Abstract
Pilot trials evaluating the efficacy and safety of the first licensed hepatitis C virus (HCV) protease inhibitors (PIs), boceprevir (BOC) and telaprevir (TVR), for the treatment of genotype 1 infection in HCV/HIV co-infected patients revealed similar results as in HCV mono-infected patients. HCV liver disease progresses more rapidly in co-infected patients, particularly with advanced immunodeficiency. Therefore, HCV treatment in HIV is of great importance. However, dual therapy with pegylated interferon (PegIFN) and ribavirin (RBV) has been associated with lower cure rates and increased toxicities in co-infected subjects, thereby limiting overall HCV therapy uptake. The availability of HCV PIs opens new perspectives for HCV cure in co-infected patients, with a 70% sustained virologic response (SVR) rate in HCV treatment-naïve patients. Despite these impressive advances, the use of the new treatment options has been low, reflecting the complex issues with modern triple HCV therapy. Indeed pill burden, adverse events (AEs), drug–drug interactions (DDIs) and high costs complicate HCV therapy in HIV. So far, studies have shown no tolerability differences in mono- and co-infected patients with the early stages of liver fibrosis. Regarding DDIs between HVC PIs and antiretroviral drugs, TVR can be safely administered with efavirenz (with dose adjustment of TVR), etravirine (ETR), rilpivirine, boosted atazanavir (ATV/r) and raltegravir (RAL), while BOC can be safely administered with ETR, RAL and potentially ATV/r for treatment-naïve patients under careful monitoring. Currently, the great number of HCV molecules under development is promising substantially improved treatment paradigms with shorter treatment durations, fewer AEs, less DDIs, once-daily administration and even interferon-free regimens. The decision to treat now with the available HCV PIs or defer therapy until the second generation of HCV direct acting antivirals become available should be based on liver fibrosis staging and fibrosis progression during follow up. More data are urgently needed regarding the efficacy of triple therapy in HIV/HCV co-infected patients who previously failed PegIFN/RBV therapy as well as in patients with more advanced fibrosis stages.
Keywords: HIV, hepatitis C, DAA, pegylated interferon, ribavirin
Introduction
During the last two decades the availability of highly active antiretroviral therapy (HAART) has turned HIV infection into a treatable chronic disease. Consequently, liver disease, particularly due to chronic viral hepatitis as well as non-AIDS related malignancies have become the most important causes of non-AIDS associated morbidity and mortality in HIV-infected patients [Boesecke et al. 2012]. HIV and hepatitis C virus (HCV) are sharing similar routes of transmission, leading to a high prevalence of HCV infection among patients with HIV. Indeed, in Europe almost 25% of all HIV patients have concomitant HCV co-infection [Lacombe and Rockstroh, 2012].
The progression of liver disease is more rapid in HCV/HIV co-infected patients than in HCV mono-infected patients, particularly in advanced immunodeficiency and patients with previous AIDS diagnosis [Branch et al. 2012]. These observations are explained on the one hand by the impaired innate and adaptive immune system responses, caused by HIV infection, and on the other hand by the profibrogenic effect on the liver tissue of HIV itself [Lacombe and Rockstroh, 2012]. Recent studies have shown that the antiviral therapy for HCV in HCV/HIV co-infected patients leads to a regression of liver fibrosis compared to untreated patients [Merli et al. 2012] and that this positive effect is even greater in patients with sustained virologic response (SVR) [Fernandez-Montero et al. 2012], emphasizing the importance of HCV therapy in HCV/HIV co-infected patients.
The gold standard therapy for HCV infection until recently was the combination of pegylated interferon (PegIFN) alpha with ribavirin (RBV), with overall cure results of around 50% [Ingiliz and Rockstroh, 2012], with higher SVR values in patients with genotype 2 and 3 HCV infection and lower values in patients with genotype 1 and 4 HCV infection [Piroth, 2011; Lacombe and Rockstroh, 2012]. These results are even lower in patients with HCV/HIV co-infection and in addition treatment management in co-infection appears to be more complex due to the presence of frequent comorbidities (drug/alcohol abuse or depression), as well as tolerability issues and increased adverse events (AEs) [Piroth, 2011; Naggie and Sulkowski, 2012].
In 2011, the US Food and Drug Administration (FDA) approved the use of two direct-acting antivirals (DAAs), boceprevir (BOC) and telaprevir (TVR), for the treatment of HCV genotype 1 infection in mono-infected patients, but these drugs have not yet obtained the approval for the use in HCV/HIV co-infected patients [Naggie and Sulkowski, 2012]. Although studies have shown a good response to triple therapy in HCV/HIV co-infected patients [Rockstroh, 2012], there are still complex issues, such as high pill burden, AEs and drug–drug interactions (DDIs) that remains to be addressed. Most importantly, with the exciting news about many other promising DAAs in development, the clinical question arises about who to treat now and in whom treatment can be deferred safely.
Direct-acting antivirals
The current HCV structure and lifecycle research have enabled the identification of several targets for DAAs. These are mostly nonstructural HCV or host proteins with an important role in the HCV replication cycle [Lange and Sarrazin, 2012]. In Table 1 the different targets are listed, as well as the inhibitors for each one of these targets and their current phase of development.
Table 1.
Direct-acting antivirals currently under development (adapted from Lange and Sarrazin [2012]).
| Drugs name | Phase | |
|---|---|---|
| NS3/4A protease inhibitors | Telaprevir | IV |
| Boceprevir | IV | |
| Simeprevir | III | |
| Faldaprevir | III | |
| Vaniprevir | III | |
| Danoprevir | III | |
| Asunaprevir | III | |
| ABT450 | III | |
| MK-5172 | II | |
| GS-9256 | II | |
| Sovaprevir | II | |
| GS-9451 | II | |
| NS5B polymerase inhibitors | Nucleoside analogue | III |
| Sofosbuvir | II | |
| Mericitabine | II | |
| INX-189 | II | |
| IDX184 | II | |
| ALS-2200 | ||
| Non-nucleoside analogue | II | |
| BI207127 | II | |
| TMC647055 | II | |
| Filibuvir | II | |
| VCH759 | II | |
| VCH916 | II | |
| VX222 | II | |
| Setrobuvir | II | |
| ABT-072 | II | |
| ABT-333 | II | |
| Tegobuvir | II | |
| VX-222 | ||
| NS5A inhibitors | Daclatasvir | III |
| GS-5885 | II | |
| Cyclophilin inhibitors | SCY-635 | III |
| NIM811 | II |
Boceprevir
Boceprevir is a linear compound which bonds reversibly to the NS3/4A protease, thereby blocking the replication of genotype 1 HCV. When boceprevir is used in monotherapy, it rapidly leads to the development of resistance, but in combination with PegIFN and RBV this problem can be overcome [Soriano et al. 2011].
A phase IIa, double-blind study in 98 HCV/HIV co-infected patients investigated the safety and efficacy of BOC in combination with PegIFN alpha 2b and weight-based dose RBV. All patients received 4 weeks of lead-in therapy with PegIFN/RBV, followed by 44 weeks of either triple therapy (BOC 800 mg TID + PegIFN/RBV) or PegIFN/RBV alone [Sulkowski, 2013]. The patients were naïve to treatment and had genotype 1 HCV infection only. Most of the patients included in the study were male (69%), white (82%), with a median age of 43 years, noncirrhotic (95%), and had a HCV viral load >800,000 IU/ml (88%). Also they were mostly under HAART, with undetectable HIV viral load and CD4 cell count >500 cells/mm3. The antiretroviral therapeutic combinations used in the study were predominately based on boosted protease inhibitors (PIs; >90%). Nonnucleoside reverse transcriptase inhibitors (NNRTIs), zidovudine or didanosine were not allowed as background HIV antiretroviral therapy (ART) in this study [Rockstroh, 2012]. Comparing the BOC + PegIFN/RBV arm with the standard therapy arm, the differences in SVR12 showed almost the same HCV cure rates in HCV/HIV co-infected patients as in HCV naïve mono-infected patients (delta of 34% and 28%, respectively) [Rockstroh, 2012; Poordad et al. 2011]. The overall results of the study are shown in Table 2.
Table 2.
Comparative results for BOC-based triple therapy versus standard therapy in HCV/HIV co-infected patients [Rockstroh, 2012].
| Undetectable HCV RNA (<50 IU/ml) | BOC + PegIFN/ RBV (n, %) |
PegIFN/ RBV (n, %) |
|---|---|---|
| n = 64 | n = 34 | |
| Week 4 | 3 (4.7%) | 3 (8.8%) |
| Week 8 | 27 (42.2%) | 5 (14.7%) |
| Week 12 | 38 (59.4%) | 8 (23.5%) |
| Week 24 | 47 (73.4%) | 11 (32.4%) |
| EOT | 42 (65.6%) | 10 (29.4%) |
| SVR12 | 37 (60.7%) | 9 (26.5%) |
BOC, boceprevir; EOT, end of therapy; HCV, hepatitis C virus; PegIFN, pegylated interferon; RBV, ribavirin; SVR12, sustained virologic response at 12 months.
Treatment failure occurred less frequently in the BOC + PegIFN/RBV arm (9%) compared with the standard therapy arm (53%), but more patients receiving triple combination had to discontinue the therapy due to AEs (20% in BOC + PegIFN/RBV arm versus 9% in standard therapy arm) [Rockstroh, 2012]. The most frequent AEs were anaemia, dysgeusia, decrease of appetite, vomiting, and asthenia. Almost half of the patients (41%) in the BOC + PegIFN/RBV study arm presented with anaemia, compared with only a quarter (26%) in the control arm. Yet, severe anaemia was observed in only a small number of patients and the percentages were comparable in both arms (5% BOC + PegIFN/RBV arm and 3% PegIFN/RBV arm) [Sulkowski, 2013]. Overall, anaemia rates in HCV/HIV co-infected patients treated with BOC-based triple therapy were similar to those observed in HCV mono-infected patients (SPRINT-2 study) [Poordad et al. 2011]. In summary, the overall safety profile showed no major differences between HCV mono- and co-infected patients [Rockstroh, 2012].
One disadvantage of BOC-based triple therapy might be the pill burden. BOC is formulated in 200 mg capsules and the daily dose is 800 mg every 8 hours, so there are 12 pills for daily administration, which need to be taken with food for better absorption. In case of AEs or toxicity the drug should be continued at the same doses, or completely stopped, as dose reductions are increasing the risk of resistance occurrence and virologic failure [Schaefer and Mauss, 2012].
Other issues that should be taken into account in HCV/HIV co-infected patients are the DDIs, as BOC, although being primarily metabolized by aldoketoreductase, is also an inhibitor as well as a substrate for the CYP3A4 enzyme [Naggie and Sulkowski, 2012; Wilby et al. 2012]. Studies of co-administration of BOC and efavirenz (EFV) in healthy volunteers showed that EFV decreases BOC Cmin by 44%, a reduction which could impact the efficacy of the regimen [Wilby et al. 2012]. Therefore, the use of HAART regimens containing EFV are contraindicated in HCV/HIV co-infected patients, when a BOC triple therapy for HCV is started. Another NNRTI drug which was tested together with BOC was etravirine (ETR). ETR area under the curve (AUC), Cmin, Cmax were slightly decreased by 23%, 29% and 24%, respectively, in the presence of BOC, whereas BOC AUC showed an increase of 10% [Hammond et al. 2012]. The magnitude of these interactions, however, has been classified as not meaningful and no dose adjustment is required when co-administering the two drugs. Another study has investigated the co-administration of BOC and raltegravir (RAL), and no significant interactions were found between the two drugs, making RAL the best option for concomitant ART, when BOC-based triple HCV therapy is being considered [De Kanter et al. 2013]. Regarding the co-administration of BOC and maraviroc, there are no data about the DDIs between these two molecules, but studies are ongoing.
There were no significant interactions between BOC and NRTI [Wilby et al. 2012], but zidovudine, didanosine and stavudine are not recommended to be used together with BOC, PegIFN and RBV, because of overlapping toxicities with IFN/RBV [Piroth, 2011]. Although in a phase IIa study in HCV/HIV co-infected patients, most of the patients were on a HIV-1 boosted PI regimen and the few HIV breakthroughs could not be related to DDIs, recent data on healthy volunteers raised concerns regarding the levels of boosted atazanavir (ATV), lopinavir (LPV) and darunavir (DRV) when administrated together with BOC [Hulskotte et al. 2012]. This study showed that, even though there were no significant interactions between BOC and low-dose ritonavir, that the Cmin of boosted ATV, LPV and DRV were decreased by co-administration of BOC by 49%, 43% and 59%, respectively [Hulskotte et al. 2012]. In addition BOC levels were also significantly reduced by LPV/r and DRV/r by 45% and 32%, respectively. Boosted ATV, however, did not change BOC levels significantly [Hulskotte et al. 2012]. In light of these results, both the FDA as well as the European Medicines Agency (EMA) stated that the use of HIV-1 PIs and BOC together are not recommended, because of the possible risk for HIV virologic breakthrough [Rockstroh, 2012]. The only exception stated by the EMA is a potential consideration of boosted ATV in patients with no prior PI resistance.
Telaprevir
TVR is also a HCV NS3/4A protease inhibitor, with an impressive antiviral activity against HCV genotype 1 infection. Like BOC, TVR monotherapy is associated with an increased risk for resistance development and HCV virologic breakthrough [Lange and Sarrazin, 2012].
The efficacy and safety of TVR-based triple therapy in HCV/HIV co-infected patients were investigated in the double-blind Study 110. The investigators enrolled 60 HCV/HIV co-infected patients, either without HAART or with HAART regimens containing EFV or boosted ATV plus tenofovir (TDF) plus either emtricitabine (FTC) or lamivudine (3TC) [Sulkowski et al. 2012; Naggie and Sulkowski, 2012]. The patients were randomized 2:1 to receive either TVR 750 mg TID (1125 mg TID when patients on EFV) + PegIFN alpha 2a + RBV 800 mg daily for 12 weeks, followed by 36 weeks of PegIFN/RBV alone, or to 48 weeks PegIFN/RBV. The patients were infected with genotype 1 HCV and were naїve to HCV therapy. As in the BOC study, most of the patients were male (88%), white (53%), with a median age of 46 years, with high HCV-RNA (83%), without advanced liver fibrosis (90%) and with a good immunological status (CD4 cell count >500/mm3) [Piroth, 2011]. The SVR12 and SVR24 results showed a notable increase in cure rate among patients with TVR-based HCV triple therapy compared with patients receiving standard therapy (71% and 74% versus 41% and 45%, respectively) [Sulkowski et al. 2012]. The results are summarized in Table 3. The overall SVR rates observed in HCV/HIV co-infected patients was quite close to those observed in studies in HCV mono-infected patients. [Jacobson et al. 2011]. SVR12 and SVR24 cure rates were similar in the arms with HAART (74% and 70%, respectively) and without HAART (71% at both endpoints), respectively. Choice of HAART regimen (either EFV or boosted ATV) showed no significant influence on treatment outcome results [Sulkowski et al. 2012].
Table 3.
Comparative results for TVR-based triple therapy versus standard therapy in HCV/HIV co-infected patients [Sulkowski et al. 2012].
| Undetectable HCV RNA (<50 IU/ml) | TVR + PegIFN/ RBV (n, %) |
PegIFN/ RBV (n, %) |
|---|---|---|
| n = 38 | n = 22 | |
| Week 4 | 26 (68%) | 0 (0%) |
| Week 12 | 30 (79%) | 6 (27%) |
| SVR12 | 28 (74%) | 10 (45%) |
| SVR24 | 27 (71%) | 9 (41%) |
HCV, hepatitis C virus; PegIFN, pegylated interferon; RBV, ribavirin; SVR12, sustained virologic response at 12 months; SVR24, sustained virologic response at 24 months; TVR, telaprevir.
The relapse rate observed in the study was 3% in the TVR + PegIFN/RBV arm and 15% in the standard therapy arm. Overall, there were only two patients on HAART who experienced HCV virologic breakthrough while on TVR therapy [Sulkowski et al. 2012]. In the TVR + PegIFN/RBV arm, two patients discontinued therapy due to AEs versus zero patients in the standard therapy arm [Piroth, 2011]. The most frequent AEs were: rash, pruritus, pyrexia, nausea and depression and neither of them were reported as being serious [Sulkowski et al. 2012]. Overall, Study 110 showed that the efficacy and safety of TVR-based triple therapy in HCV/HIV co-infected patients are comparable to the results obtained in HCV mono-infected patients [Sulkowski et al. 2012].
TVR is formulated in 375 mg film-coated tables and the daily dose is 750 mg every 8 hours, taken as 6 tablets daily, which should be taken with a fat-containing meal to increase absorption. The dose needs to be adapted when the patients are on HAART based on EFV: 1125 mg every 8 hours, taken as nine tablets daily, for 12 weeks. As for BOC therapy, TVR doses should not be decreased due to AEs or toxicity, because this would lead to lower drug concentrations, risk of resistance and virologic failure [Schaefer and Mauss, 2012].
As TVR is a strong inhibitor, as well as substrate, for the CYP3A enzyme, it has important DDIs with the main antiretroviral HIV drugs. The co-administration of TVR and EFV leads to a decrease in TVR plasma concentration, due to the EFV inducer effect on CYP3A. This effect can be counterbalanced by increasing TVR doses to 1125 mg TID [Wilby et al. 2012]. Regarding the co-administration of TVR, with ETR and rilpivirine (RPV), the observations in healthy volunteers illustrated that ETR levels remained stable and RPV AUC and Cmax increased by 79% and 47%, respectively, while TVR showed only a small decrease (Cmin 25% and 11% decrease when administrated together with ETR and RVP, respectively) [Kakuda et al. 2012]. Therefore, ETR can be used in HAART regimens of HCV/HIV co-infected patients, as the slightly decreased levels of TVR are not considered to have an impact on the outcome of treatment [Kakuda et al. 2012]. Regarding TVR and RPV co-administration, the authors of the study concluded that the increased levels of RPV should have no clinical significance [Kakuda et al. 2012]. However, as prolongations of QT interval in healthy volunteers at doses threefold higher than the regular dose have been reported [Fletcher, 2012], co-administration should be made with caution [Fletcher, 2012]. For the HIV-1 PIs, boosted LPV decreased TVR Cmin, Cmax and AUC by >50%, while the levels of LPV remained stable [Piroth, 2011]. Co-administration of boosted DRV and fosamprenavir (FPV) with TVR had an impact on the levels of both HIV-1 PI and HCV PI. DRV AUC decreased by 47% and FPV AUC by 40% [Ingiliz and Rockstroh, 2012], while TVR AUC decreased by 30–36% [Piroth, 2011]. Boosted ATV AUC showed a decrease of only 20% and therefore is the only HIV-1 PI recommended for the use in combination with TVR-based HCV triple therapy [Piroth, 2011; Ingiliz and Rockstroh, 2012]. RAL can also be used as a partner for TVR-based triple therapy, as it is not metabolized through the CYP pathway and did not exhibit any DDIs with TVR [Piroth, 2011; Ingiliz and Rockstroh, 2012]. There are no studies on the possible DDIs between TVR and maraviroc so far.
In light of the good outcome of HCV PI (BOC/TVR)-based triple therapy in HCV treatment-naïve patients with HCV/HIV co-infection, and the improved treatment responses in experienced (relapsers or partial responders) HCV mono-infected patients [Soriano et al. 2011], there are now ongoing studies to evaluate the efficacy of triple therapy in HCV/HIV co-infected patients who have previously failed to standard IFN-based dual therapy. Moreover, the new studies are also examining response-guided therapy and shorter treatment durations as well as efficacy and safety of DAA-based therapy in more advanced liver fibrosis stages. Table 4 summarizes the current DAA studies in HIV/HCV co-infected individuals including the studies with either BOC or TVR or other new DAAs in development.
Table 4.
Undergoing or future HCV DAA-based therapy studies in HCV/HIV co-infected patients.
| Study drug | Study population | HCV genotype | HAART admitted in the study | Study phase | |
|---|---|---|---|---|---|
| Boceprevir ACTG Study | BOC + PegIFN/RBV RVR guided therapy | Naïve / previous failure | 1 | EFV RAL LPVr 400/100 BID ATVr DRVr 600/100 BID | III |
| Vertex 115 | TVR + PegIFN/RBV RVR guided therapy | Naïve / relapsers / partial responders / null responders | 1 | EFV / ATVr / RAL + TDF/FTC or ABC/3TC | III |
| Vertex Study in cirrhosis and HIV/HCV Coinfection | TVR + PegIFN/RBV | Cirrhosis | 1 | EFV / ATVr/ RAL / ETR / RPV + TDF/FTC or ABC/3TC | III |
| 1220.19 study | Faldaprevir + PegIFN/RBV RVR guided therapy | Naïve / relapsers | 1 | EFV / ATVr / DRVr / RAL / MVC + TDF/FTC or ABC/3TC | III |
| C212 study | Simeprevir + PegIFN/RBV RVR guided therapy | Naïve / previous failure | 1 | RPV / RAL / MVC + TDF/FTC or ABC/3TC | III |
| COMMAND-HIV | Daclatasvir + PegIFN/RBV RVR guided therapy | Naïve | 1 | EFV / NVP / ATVr / DRVr / LPVr / RAL / MVC + TDF/FTC or ABC/3TC | III |
| PHOTON 1 Study: | Sofosbuvir + RBV | Naïve Previous failure | 1,2, 3 and 4 2 and 3 | EFV / ATVr / DRVr / RAL / RPV + TDF/FTC | III |
| QUADRIH Study | Asunaprevir + Daclatasvir + PegIFN/RBV | Null-responders | 1 and 4 | RAL + TDF/FTC | II |
| Abbott IFN-free treatment trial | ABT-450/r + ABT-267 + ABT-333 + RBV | Naïve / previous failure | 1 | Stable HAART | II |
| BMS-914143 Interferon study | Interferon Lambda + Daclatasvir + RBV | Naïve Intolerant / ineligible | 1b and 4 2 and 3 | Stable HAART | III |
TC, lamivudine; ABC, abacavir; ATV, atazanavir; BOC, boceprevir; DAA, direct-acting antiviral; DRV, darunavir; EFV, efavirenz; FTC, emtricitabine; HAART, highly active antiretroviral virus; HCV, hepatitis C virus; LPV, lopinavir; MVC, maraviroc; PegIFN, pegylated interferon; RAL, raltegravir; RBV, ribavirin; RVR, rapid virological response; TDF, tenofovir; TVR, telaprevir.
Other DAAs in HCV/HIV co-infected patients
Simeprevir, another NS3/4A protease inhibitor active against genotype 1 HCV, has completed the pharmacokinetics studies in healthy volunteers and the results showed that simeprevir could be administrated together with RPV, RAL and TDF, without any dose adjustments [Ouwerkerk-Mahadevan et al. 2012a]. However, the co-administration of simeprevir with EFV needs to be avoided, due to the resulting decrease in simeprevir levels [Ouwerkerk-Mahadevan et al. 2012b]. The co-administration of this DAA and boosted DRV should also be avoided, due to the increased levels of simeprevir, even with lower doses (50 mg QD instead of 150 mg QD) [Ouwerkerk-Mahadevan et al. 2012a]. The authors suggested that the increased levels of simeprevir in the presence of boosted DRV is due to the inhibitor effect of ritonavir on the CYP3A enzyme and that this interaction is most likely also to be expected in the presence of other HIV-1 boosted PI [Ouwerkerk-Mahadevan et al. 2012a].
Daclatasvir is a NS5A inhibitor molecule, with pan-genotypic activity and less pronounced DDIs. There were no evident DDIs between daclatasvir and TDF and although there were some interactions with EFV and boosted ATV, these could be compensated through doses adjustments: increased dose (90 mg) in the presence of EFV and decreased dose in the presence of boosted ATV (30 mg) [Bifano et al. 2012].
Another DDIs study, involved sofosbuvir, a nucleoside analogue and NS5B polymerase inhibitor. The study evaluated the co-administration of sofosbuvir together with EFV, RPV, boosted DRV, RAL, TDF and FTC and no significant clinically relevant interactions could be found between sofosbuvir and the before mentioned antiretroviral drugs [Kirby et al. 2012].
It is important to keep in mind that most DDIs have been evaluated in healthy volunteers and that it may be difficult to estimate whether these results are completely valid in patients with more advanced liver fibrosis stages and HIV infection [Rockstroh, 2012]. Indeed, more recently pharmacokinetic results from two patients with HCV-associated liver cirrhosis and HIV receiving BOC-based HCV triple therapy and on either DRV/r- or FPV/r-based ART showed completely normal drug levels of their corresponding HIV protease inhibitor again underlining that in clinical practice in HIV patients with more advanced liver disease pharmacokinetic interactions may be different than in healthy volunteers [Schwarze-Zander et al. 2012]
Apart from the pharmacokinetics studies, there are also studies in HCV/HIV co-infected patients evaluating the safety and efficacy of these new drugs in this particular patient population. In genotype 1 HCV naïve patients the second-wave HCV PIs simeprevir and faldaprevir as well as the first NS5A inhibitor daclatasvir are being evaluated. In genotype 1 HCV experienced patients (relapsers, partial responders and null responders) two triple therapies are currently being investigated: simeprevir + PegIFN/RBV and faldaprevir + PegIFN/RBV; in addition, quadruple therapy with daclatasvir, asunaprevir plus PegIFN/RBV started in December 2012 (Table 4). The advantages of these drugs are a shorter treatment period, QD administration, fewer AEs and good antiviral activity. Clearly, these results are of utmost interest, as they may promise an increasingly better tolerated therapy with shorter treatment durations and higher cure rates. For the first time in HCV/HIV co-infected patients there will be a study on an interferon-free combination, consisting of a NS5B polymerase inhibitor (sofosbuvir) and RBV. This combination is currently studied in treatment-naïve as well as treatment-experienced genotype 2 and 3 patients as well as naïve genotype 1 or 4 patients.
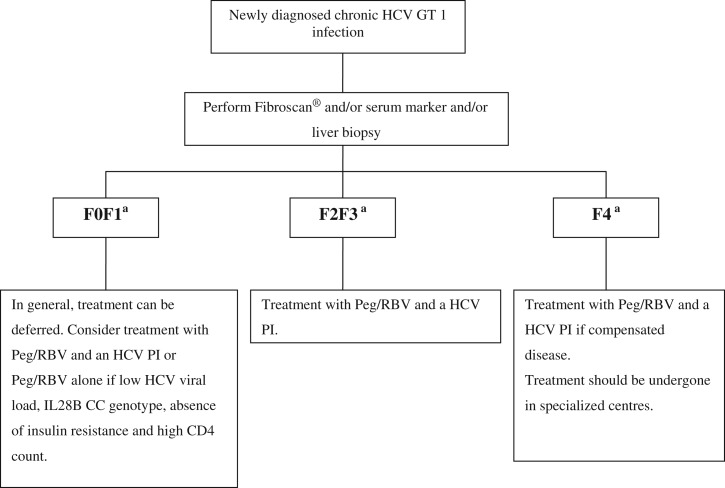
Who should we treat now?
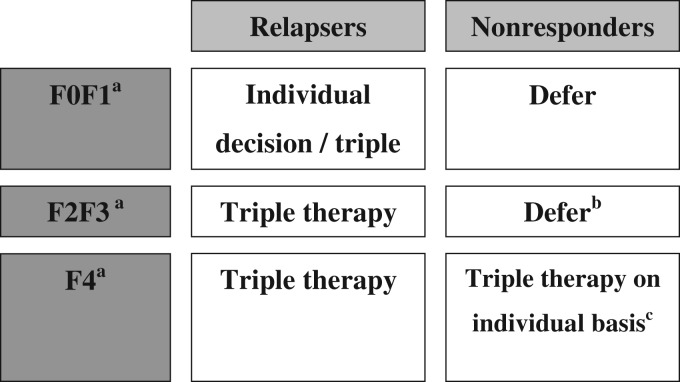
The fast development of anti-HCV therapies emphasizes the necessity of an algorithm for HCV treatment in HCV/HIV co-infected patients, as it is important to select patients who will benefit the most from the currently available HCV triple therapies and those for whom the therapy can be safely deferred until the second wave DAAs will become available. The treatment decision should be based on liver fibrosis stage and rapidity of progression, as well as on HIV disease stage. In patients with CD4 cell count <500/mm3, HAART therapy should be initiated first, as concomitant immunodeficiency lowers SVR rates [Ingiliz and Rockstroh, 2012]. The treatment can also be deferred in patients with no or mild fibrosis (F0 or F1 metavir). If significant liver fibrosis is detected (F2 or F3 metavir) or cirrhosis (without any decompensation) the HCV triple therapy should be considered for treating these patients (Figure 1) [Ingiliz and Rockstroh, 2012]. If patients already have been previously treated with PegIFN and RBV and did not achieve a SVR, the decision of treating with triple therapy should be made again based on liver fibrosis stage (Figure 2) [Ingiliz and Rockstroh, 2012].
Figure 1.
Management of newly diagnosed HCV/HIV co-infected genotype 1 patients (EACS Guidelines, version November 2012) [Ingiliz and Rockstroh, 2012].
aMetavir fibrosis score: F0 = no fibrosis; F1 = portal fibrosis, no septae; F2 = portal fibrosis, few septae, F3 = bridging fibrosis, F4 = cirrhosis;
HCV, hepatitis C virus; Peg, pegylated interferon; PI, protease inhibitor; RBV, ribavirin.
Figure 2.
Management of HIV-HCV co-infected genotype-1 patients according to fibrosis stage and prior treatment outcome (adapted from EACS Guidelines, version November 2012) [Ingiliz and Rockstroh, 2012].
aMetavir fibrosis score: F0 = no fibrosis; F1 = portal fibrosis, no septae; F2 = portal fibrosis, few septae, F3 = bridging fibrosis, F4 = cirrhosis.
bMonitor fibrosis stage annually, preferably with two established methods. Treat with triple therapy, if rapid progression.
cAs the overall cure rates in patients with cirrhosis and previous nonresponse is estimated to be very low (<15%) triple therapy needs to be discussed on an individual basis balancing low probability of cure rate, increased risk for adverse events and further disease progression.
Conclusions
A new era has opened for the treatment of chronic HCV infection, with more than 30 antiviral compounds being studied currently, which are promising greater than 70% infection cure rates, shorter therapy duration, fewer side effects, easy to take treatment and even interferon-free combinations in the near future. HCV/HIV co-infected patients remain a population which is difficult to treat due to faster liver disease progression, high baseline HCV viral loads, impaired immune status, associated comorbidities and last but not least challenging DDIs. The HCV triple therapy studies, with BOC and TVR, conducted in HCV/HIV co-infected patients showed for the first time improved cure rates, but they were performed with relatively easy to treat patients (i.e. without cirrhosis, naïve to previous HCV therapy). Also, so far no data are available for the efficacy and safety of DAA-based triple therapy in HCV treatment experienced HCV/HIV co-infected patients. Clearly, these results are urgently needed to improve the management of liver disease and to decrease the morbidity and mortality associated with HIV co-infection.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
DM declares no conflict of interest in preparing this article. JR also has no conflict of interest in preparing this article but has received honoraria for consulting or lectures from Abbott, BMS, Boehringer, Bionor, Gilead, GSK, Janssen, Merck, Novartis, Pfizer, Tobira, Vertex and ViiV.
References
- Bifano, M., Hwang, C., Oosterhuis, B., Hartstra, J., Tiessen, R. and Velinova-Donga, M. (2012) Assessment of HIV ARV drug interactions with the HCV NS5A replication complex inhibitor BMS-790052 demonstrates a pharmacokinetic profile which supports co-administration with tenofovir disoproxil fumarate, efavirenz, and atazanavir/ritonavir. CROI, Poster presentation 618.
- Boesecke C., Mauss S., Rockstroh J. (2012) Management of HCV/HIV coinfection. In Mauss, Berg, Rockstroh, Sarrazin, Wedemeyer Hepatology 2012, third edition. Berlin: Flying Publisher [Google Scholar]
- Branch A., Van Natta M., Vachon M., Dieterich D., Meinert C., Jabs D. for the Studies of the Ocular Complications of AIDS Research Group (2012) Mortality in hepatitis C virus-infected patients with a diagnosis of AIDS in the era of combination antiretroviral therapy. Clin Infect Dis. 55: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kanter C., Blonk M., Colbers A., Schouwenberg B., Burger D. (2013) Lack of a clinically significant drug–drug interaction in healthy volunteers between the hepatitis C virus protease inhibitor boceprevir and the HIV integrase inhibitor raltegravir. Clin Infect Dis. 56: 300–306 [DOI] [PubMed] [Google Scholar]
- Fernandez-Montero J., Barreiro P., Vispo E., Labarga P., Rick F., Arredondo M., et al. (2012) Baseline liver stiffness and achievement of sustained virological response predict liver complications and death in HIV/HCV coinfected patients receiving peginterferon/ribavirin therapy. Hepatology. 56(4 Suppl.): 650A–651A [Google Scholar]
- Fletcher, C. (2012) Clinical Pharmacology at the 13th Workshop on Clinical Pharmacology of HIV Therapy. http://www.natap.org/2012/pharm/Pharm_19.htm.
- Hammond, H., Wolfe, P., Burton, J., Predhomme, J., Ellis, C., Ray, M., et al. (2012) Pharmacokinetic interaction between boceprevir and etravirine in HIV/HCV seronegative volunteers. Rev Antiviral Ther Infectious Dis 3: 17. [DOI] [PubMed]
- Hulskotte, E., Feng, H., Xuan, F., Van Zutven, M., Treitel, M., Hughes, E., et al. (2012) Pharmacokinetic interactions between the HCV protease inhibitor boceprevir and ritonavir-boosted HIV-1 protease inhibitors atazanavir, lopinavir and darunavir. Clin Infect Dis 56: 718–726. [DOI] [PubMed]
- Ingiliz P., Rockstroh J. (2012) HIV–HCV co-infection facing HCV protease inhibitor licensing: implications for clinicians. Liver Int. 32: 1194–1199 [DOI] [PubMed] [Google Scholar]
- Jacobson I., McHutchison J., Dusheiko G., Di Bisceglie A., Rajender Reddy K., Bzowej N. (2011) Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 364: 2405–2416 [DOI] [PubMed] [Google Scholar]
- Kakuda T., Leopold L., Nijs S., Vandevoorde A., Crauwels H., Bertelsen K., et al. (2012) Pharmacokinetic interaction between etravirine or rilpivirine and telaprevir in healthy volunteers: a randomised, two-way crossover trial. Rev Antiviral Ther Infectious Dis. 3: 20–20 [DOI] [PubMed] [Google Scholar]
- Kirby B., Mathias A., Rossi S., Moyer C., Shen G., Kearney B. (2012) No clinically significant pharmacokinetic interactions between sofosbuvir (GS-7977) and HIV antiretrovirals atripla, rilpivirine, darunavir/ritonavir, or raltegravir in healthy volunteers. Hepatology. 56(4 Suppl.): 1067A–1067A [Google Scholar]
- Lacombe K., Rockstroh J. (2012) HIV and viral hepatitis coinfections: advances and challenges. Gut. 61(Suppl. 1): i47–i58 [DOI] [PubMed] [Google Scholar]
- Lange C., Sarrazin C. (2012) Hepatitis C: new drugs. In Mauss, Berg, Rockstroh, Sarrazin, Wedemeyer Hepatology 2012, third edition. Berlin: Flying Publisher [Google Scholar]
- Merli M., Carbone A., Messina E., Galli L., Bagaglio S., Morsica G., et al. (2012) Evolution of liver fibrosis in HIV/HCV-coinfected patients: impact of anti-HCV therapy in patients with different grades of liver stiffness. Hepatology. 56(4 Suppl.): 1031A–1031A [Google Scholar]
- Naggie S., Sulkowski M. (2012) Management of patients coinfected with HCV and HIV: a close look at the role for direct-acting antivirals. Gastroenterology. 142: 1324–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwerkerk-Mahadevan, S., Sekar, V., Simion, A., Peeters, M. and Beumont-Mauviel, M. (2012a) The pharmacokinetic interactions of the HCV protease inhibitor simeprevir (TMC435) with HIV antiretroviral agents in healthy volunteers. IDweek, poster 1618.
- Ouwerkerk-Mahadevan, S., Sekar, V., Peeters, M. and Beumont-Mauviel, M. (2012b) The pharmokinetic interactions of HCV protease inhibitor TMC435 with RPV, TDF, EFV, or RAL in health volunteers. CROI, oral paper 49.
- Poordad F., McCone J., Bacon B., Bruno S., Manns M., Sulkowski M., et al. (2011) Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 364: 1195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piroth L. (2011) Direct-acting antivirals for hepatitis C virus infections in patients co-infected with human immunodeficiency virus. Clin Res Hepatol Gastroenterol. 35: S75–S83 [DOI] [PubMed] [Google Scholar]
- Rockstroh, J. (2012) HCV direct acting antivirals (DAAs) demonstrated to work in HIV/HCV coinfection: so how are treatment paradigms in HIV/HCV coinfection changing now? In Summary from CROI 2012 for Hepatitis Co-infection, http://www.natap.org/2012/CROI/croi_89.htm.
- Schaefer M., Mauss S. (2012) Management of adverse drug reactions. In Mauss, Berg, Rockstroh, Sarrazin, Wedemeyer Hepatology 2012, third edition. Berlin: Flying Publisher [Google Scholar]
- Schwarze-Zander C., Rockstroh J.K. (2012) HIV protease inhibitors in combination with boceprevir: are drug-drug interactions the same for all patients? AIDS. 26: 1845–1846 [DOI] [PubMed] [Google Scholar]
- Soriano V., Vispo E., Poveda E., Labarga P., Martin-Carbonero L., Fernandez-Montero J., et al. (2011) Directly acting antivirals against hepatitis C virus. J Antimicrob Chemother. 66: 1673–1686 [DOI] [PubMed] [Google Scholar]
- Sulkowski M. (2013) HCV therapy in HIV-infected patients. Liver Int. 33(Suppl. S1): 63–67 [DOI] [PubMed] [Google Scholar]
- Sulkowski M., Sherman K., Soriano V., Rockstroh J., Dieterich D., Girard P., et al. (2012) Telaprevir in combination with peginterferon alfa 2a / ribavirin in HCV/HIV co-infected patients: SVR24 final study results. Hepatology. 56(4 Suppl.): 219A–219A22334397 [Google Scholar]
- Wilby K., Greanya E., Ford J., Yoshida E., Partovi N. (2012) A review of drug interactions with boceprevir and telaprevir: implications for HIV and transplants patients. Ann Hepatol. 11: 179–185 [PubMed] [Google Scholar]