Abstract
Blastocystis sp. is among the few enteric parasites with a prevalence that often exceeds 5% in the general population of industrialized countries and can reach 30–60% in developing countries. This parasite is frequently found in people who are immunocompromised (patients with human immunodeficiency virus/acquired immunodeficiency syndrome or cancer) and a higher risk of Blastocystis sp. infection has been found in people with close animal contact. Such prevalence in the human population and the zoonotic potential naturally raise questions about the impact of these parasites on public health and has increased interest in this area. Recent in vitro and in vivo studies have shed new light on the pathogenic power of this parasite, suggesting that Blastocystis sp. infection is associated with a variety of gastrointestinal disorders, may play a significant role in irritable bowel syndrome, and may be linked with cutaneous lesions (urticaria). Despite recent significant advances in the knowledge of the extensive genetic diversity of this species, the identification of extracellular proteases as virulence factors and the publication of one isolate genome, many aspects of the biology of Blastocystis sp. remain poorly investigated. In this review, we investigate several biological aspects of Blastocystis sp. (diversity and epidemiology, diagnosis tools and pathophysiology). These data pave the way for the following challenges concerning Blastocystis sp. research: deciphering key biological mechanisms and pathways of this parasite and clarification of its clinical impact in humans.
Keywords: Blastocystis, pathogenesis, diagnosis, subtypes, gut
Blastocystis sp. is an anaerobic intestinal parasite of humans and a wide range of animals [Stenzel and Boreham, 1996; Tan, 2004, 2008]. This parasite belongs to the stramenopiles, a complex and heterogeneous evolutionary assemblage of heterotrophic and photosynthetic protozoa [Silberman et al. 1996; Arisue et al. 2002; Riisberg et al. 2009]. Interestingly, Blastocystis sp. is the only stramenopiles known to cause infection in humans. Four major morphological forms of Blastocystis sp. were described in stools or in vitro cultures: vacuolar (Figure 1), granular, amoeboid and cyst forms [Stenzel and Boreham, 1996; Tan, 2008; Suresh et al. 2009]. The two former forms are the most easily recognizable and frequently observed in laboratory culture and stool samples. Although rarely reported, the irregular amoeboid form was postulated to play a role in pathogenesis [Tan et al. 2006, Katsarou-Katsari et al. 2008 but this hypothesis was contradicted [Souppart et al. 2009]. Experimental infectivity studies in animals with the cyst form demonstrated that the water-resistant and environmentally resistant infective cysts represented the transmissible stage of the parasite [Suresh et al. 1993, 2005; Moe et al. 1996; Yoshikawa et al. 2004]. Taking into account these observations and those of in vitro encystations studies [Suresh et al. 1993; Villar et al. 1998; Chen et al. 1999], a life cycle for Blastocystis sp. was proposed with the cyst as the infectious stage [Tan, 2008]. Upon ingestion of cysts, the parasite undergoes excystation in the large intestine and develops into vacuolar forms. These vacuolar forms divide by binary fission and may develop into amoeboid or granular forms. Then, encystation may occur while crossing along the colon before cyst excretion in the faeces [Tan, 2008]. Therefore, Blastocystis sp. lives in oxygen-poor environments and is characterized by the presence of some double-membrane surrounded organelles called mitochondria-like organelles (MLOs) (Figure 1) [Nasirudeen and Tan, 2004]. Sequencing of complete circular DNA in the MLO of these parasites by different authors [Perez-Brocal and Clark, 2008; Stechmann et al. 2008; Wawrzyniak et al. 2008] showed that the cellular compartments have the metabolic properties of both aerobic and anaerobic mitochondria. The nuclear genome of Blastocystis sp. was recently sequenced and revealed a compact nature and an intriguing architecture [Denoeud et al. 2011]. This genome has also provided clues to decipher the genetic diversity and pathogenesis of this parasite.
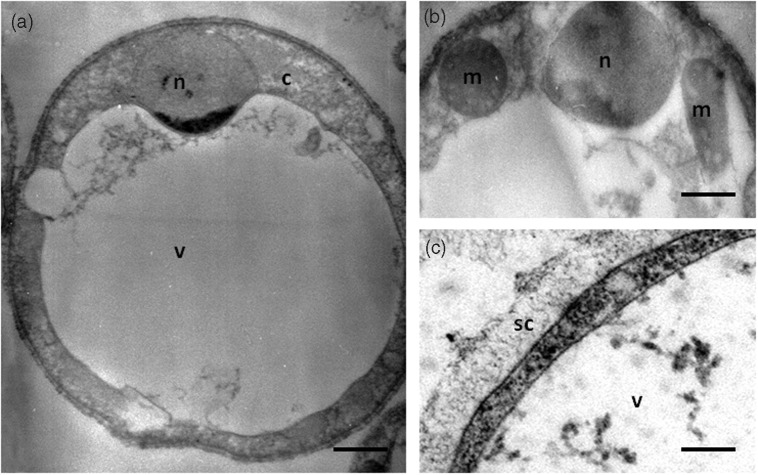
Figure 1.
Vacuolar form of in vitro cultivated Blastocystis sp. viewed under transmission electron microscopy (a). This form is spherical with a large central vacuole (v) and a thin peripheral band of cytoplasm (c) around the vacuole. (b) The cytoplasm contains the nucleus (n) and mitochondrion-like organelles (m). (c) Blastocystis sp. cell is surrounded by a surface coat (sc). Bars, 2 µm for (a) and 500 nm for (b) and (c).
Summary of diagnosis tools
The most common approaches for the detection of Blastocystis sp. [Stenzel and Boreham, 1996; Stensvold et al. 2007a; Tan, 2008] consist of direct smear examination by light microscopic or xenic in vitro culture. However, given the occurrence of different forms of Blastocystis sp. (especially the hardly recognizable cystic form), deterioration caused by environmental conditions or drug treatment and the fact that Blastocystis sp. can be confused with other microorganisms, this method seems to have largely underestimated this parasite in the context of enteric parasite diagnosis. Moreover, culturing this parasite is time consuming and can bias subsequent genotyping due to the different ability of isolates to grow in selective medium [Roberts et al. 2011]. Therefore, to overcome these limitations, several molecular polymerase chain reaction (PCR)-based diagnostic approaches using faeces directly or after culture of faecal specimens have been described [Santin et al. 2011; Abe et al. 2003a; 2003b; Yoshikawa et al. 2004; Scicluna et al. 2006; Stensvold et al. 2006; Roberts et al. 2011]. Studies comparing the relative performances of these various diagnostic methods [Suresh and Smith, 2004; Parkar et al. 2007; Stensvold et al. 2007a] showed that the PCR approach was as sensitive as the culture approach. More recently, Poirier and colleagues reported a highly sensitive real-time quantitative PCR (qPCR) assay developed to detect Blastocystis sp. in stool samples [Poirier et al. 2011]. This assay targets a region of the small subunit rRNA gene (SSU-rDNA) and allowed subtyping of isolates by direct sequencing of qPCR products. Moreover, Stensvold and colleagues developed a qPCR on stool samples using the SSU-rDNA marker, including an internal process control enabling the evaluation of potential PCR inhibitors [Stensvold et al. 2012]. This approach had the advantage of increasing the specificity and avoiding the amplification of false positives. Therefore, currently, SSU-rDNA genotyping is the method of choice for diagnosis [Poirier et al. 2011; Stensvold et al. 2012].
Blastocystis sp., a highly prevalent and divergent parasite
Numerous epidemiological surveys carried out in different countries identify Blastocystis sp. as the most common eukaryotic parasite reported in human faecal samples [Tan, 2008]. Overall the prevalence of Blastocystis sp. is higher than those of other intestinal protozoan parasites such as Giardia, Entamoeba and Cryptosporidium, as observed in France [The ANOFEL Cryptosporidium National Network, 2010] and the United States [Boorom et al. 2008]. An increasing trend in identification of Blastocystis sp. suggests that it is an emerging parasite with a worldwide distribution [WHO, 2008]. Prevalence varies widely from country to country and within communities of the same country [Tan, 2008; Souppart et al. 2009; Alfellani et al. 2013]. Developing countries have a higher prevalence of the parasite than industrialized countries. This difference can be explained by poor hygiene practices, close animal contact and consumption of contaminated food or water [Li et al. 2007; Leelayoova et al. 2008; Eroglu and Koltas, 2010; Baldursson and Karanis, 2011; Ithoi et al. 2011; Lee et al. 2012; Nagel et al. 2012] since the faecal–oral route is considered to be the main mode of transmission of this parasite [Yoshikawa et al. 2004]. Prevalence is low in developed countries such as Japan (0.5–1%) [Hirata et al. 2007] and Singapore (3.3%) [Wong et al. 2008] and high in developing nations including Brazil (40.9%) [Aguiar et al. 2007], Egypt (33.3%) [Rayan et al. 2007] and Indonesia (60%) [Pegelow et al. 1997]. In some countries, the prevalence can be rather variable and ranges from 1.9% to 32.6% in China [Li et al. 2007] and from 0.9% to 45.2% in Thailand [Saksirisampant et al. 2003, 2006], depending on the subpopulation studied. Such variations within the same country could reflect true differences between communities or the use of different diagnostic approaches [Stensvold, 2013].
Blastocystis sp. isolates from humans and other animals have been reported to be morphologically indistinguishable. However, extensive genetic variation among numerous Blastocystis sp. isolates from both humans and animals was mainly observed by PCR restriction fragment length polymorphism [Hoevers et al. 2000; Kaneda et al. 2001; Abe et al. 2003b; Rivera and Tan, 2005] and PCR using sequenced-tagged site primers [Yoshikawa et al. 2004; Yan et al. 2006; Li et al. 2007; Yoshikawa et al. 2009]. This considerable genetic divergence among isolates was subsequently confirmed by molecular phylogenies, mainly inferred from SSU-rDNA sequences [Arisue et al. 2003; Abe, 2004; Noël et al. 2005; Scicluna et al. 2006; Jones et al. 2008; Ozyurt et al. 2008; Souppart et al. 2009, 2010; Stensvold et al. 2009; Parkar et al. 2010; Whipps et al. 2010]. From these molecular analyses, a consensus on Blastocystis sp. terminology was proposed in an international collaborative project [Stensvold et al. 2007b]. In this new classification, all isolates should be designated Blastocystis sp. and assigned to one of the described subtypes (STs). Each of the STs exhibiting sufficient genetic diversity should be classified as separate species. Thereafter, a new ST was identified from primates and artiodactyls and designated as Blastocystis sp. ST10 [Stensvold et al. 2009]. More recently, seven additional STs (ST11–17) were identified from zoo animals [Parkar et al. 2010; Fayer et al. 2012; Alfellani et al. 2013; Roberts et al. 2013].
Most of the samples included in published epidemiological surveys represented simple infections. Mixed infections (i.e. infections by at least two different STs) probably result from multiple sources of infection. The prevalence of these infections is similar in different countries and roughly comprised between 2.6% and 14.3% [Yan et al. 2006; Li et al. 2007; Dogruman-Al et al. 2008; Souppart et al. 2009; Meloni et al. 2012]. However, the true distribution of mixed infections remains difficult to ascertain in a particular individual and is likely underestimated as this depends on the method employed for subtyping [Meloni et al. 2012]. In almost all the studies reported so far, including those in Europe [Souppart et al. 2009; Meloni et al. 2011; Forsell et al. 2012; Alfellani et al. 2013], Africa [Souppart et al. 2010; Alfellani et al. 2013], Oceania [Roberts et al. 2013], Asia [Jantermtor et al. 2013] and the Middle East [Moosavi et al. 2012], a large majority of human infections with Blastocystis sp. were attributable to ST3 isolates. Only a few exceptions showed a higher prevalence of ST4 in Spain [Dominguez-Marquez et al. 2009], Denmark [Stensvold et al. 2011] and in a region of France [Poirier et al. 2011], and of ST1 in Thailand [Thathaisong et al. 2013]. Collectively, these studies suggest that the dominant ST3 was the only ST of human origin as was first proposed by Noël and colleagues [Noël et al. 2005], even if it can also be found in some animals. Consequently, the predominance of this ST might be mainly explained by large-scale human to human transmission [Yoshikawa et al. 2000]. Proportions of ST1 to ST4 differ between locations. ST6 and ST7 are common in Asia but rarely observed in European countries. ST5, ST8 and ST9 are found episodically in humans [Yan et al. 2007; Tan, 2008; Stensvold et al. 2009; Tan et al. 2010; Moosavi et al. 2012].
Comparison of SSU-rDNA gene sequences, cross-transmission experiments and respective prevalence of different STs in the human population indicate that almost all of the known STs of supposed animal origin are likely zoonotic and able to infect human (Figure 2) [Noël et al. 2005; Parkar et al. 2007, 2010; Yan et al. 2007; Yoshikawa et al. 2009]. Therefore, a higher risk of Blastocystis sp. infection was found in people with close animal contact, including zoo keepers [Parkar et al. 2010] and abattoir workers [Rajah Salim et al. 1999; Parkar et al. 2010], indicating that animals may represent a significant zoonotic source of this parasite for humans.
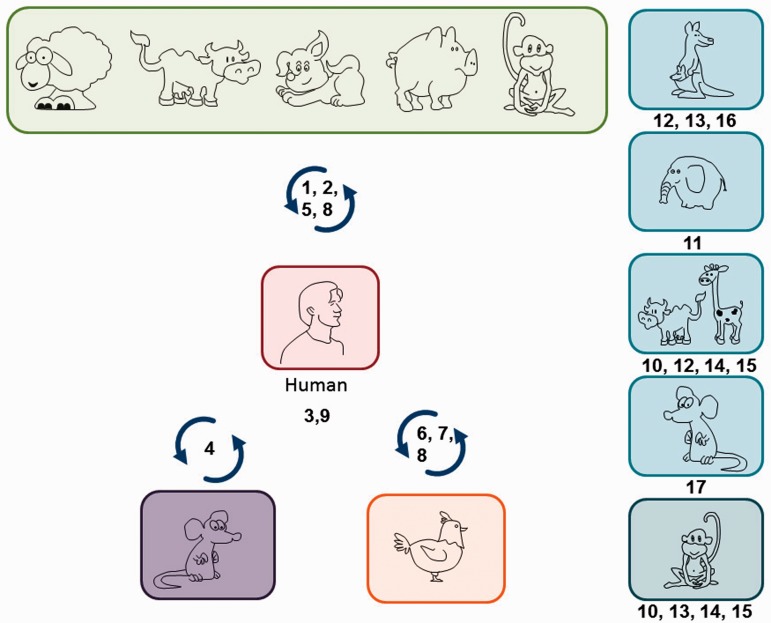
Figure 2.
Blastocystis sp. subtypes (STs 1–9) with various host specificities. Humans can be infected by nine STs, some being mainly found in humans (ST3 and ST9). ST1, 2, 5 and 8 are found both in human and mammalian isolates (primate, pig, human, cattle and pig), while ST4 is also present among rodent isolates, and ST6, 7 and 8 among avian isolates. Some STs are exclusively found in animals (ST10–17). ST10 and 15 are present among Artiodactyla and nonhuman primates, ST11 among Proboscidea, ST12 among Artiodactyla and marsupials, ST13 among nonhuman primates and marsupials, ST14 among Artiodactyla, ST16 among marsupials and ST17 among rodents.
Insights into Blastocystis sp. pathogenesis
The pathogenic status of Blastocystis sp. was widely debated in the literature to determine whether this microorganism was a truly pathogenic or commensal organism [Stenzel and Boreham, 1996; Boorom et al. 2008; Tan, 2008; Tan et al. 2010], although an increasingly number of recent studies cited Blastocystis sp. as an emerging pathogen [Tan, 2008, Tan et al. 2010; Poirier et al. 2012; Scanlan, 2012]. This is mainly due to the fact that Blastocystis sp. can be found in both symptomatic and asymptomatic patients [Dogruman-Al et al. 2008; Eroglu et al. 2009; Souppart et al. 2009]. However, recent in vitro and in vivo studies show that Blastocystis sp. infection is associated with a variety of gastrointestinal disorders (called blastocystosis), especially in irritable bowel syndrome (IBS) and cutaneous lesions.
Blastocystis sp. is associated with various clinical symptoms in humans
In humans, blastocystosis is mainly characterized by nonspecific gastrointestinal symptoms, like diarrhoea, abdominal pain, flatulence, nausea, vomiting, constipation, weight loss or fatigue [Stenzel and Boreham, 1996; Boorom et al. 2008; Tan, 2008; Tan et al. 2010]. The severity of these diseases is variable and ranges from acute to chronic infections [Tan, 2008]. A hypothesis to explain differences in the disease caused by Blastocystis sp. is its genetic diversity [Hussein et al. 2008; Tan, 2008; Tan et al. 2010; Scanlan, 2012], although no association was detected between symptoms and Blastocystis subtypes in several studies [Ozyurt et al. 2008; Dogruman-Al et al. 2009; Souppart et al. 2009; Jantermtor et al. 2013]. However, ST4 isolates are more common in symptomatic patients in Sweden, Denmark and Spain [Dominguez-Marquez et al. 2009; Stensvold et al. 2011; Forsell et al. 2012], arguing for an important role of this subtype that needs to be investigated further.
In addition to aspecific gastrointestinal symptoms, studies associated the parasite with cutaneous disorders and chronic or acute urticaria [Vogelberg et al. 2010; Hameed et al. 2011; Zuel-Fakkar et al. 2011; Verma and Delfanian, 2013]. These diseases were correlated with the presence of Blastocystis sp. belonging to the ST2 [Vogelberg et al. 2010] or ST3 [Zuel-Fakkar et al. 2011] in the patient stools. An association was also found between urticaria and amoeboid forms of a ST3 isolate [Katsarou-Katsari et al. 2008]. It was suggested that the amoeboid form adheres efficiently to the intestinal epithelium, affecting gut immune homeostasis and causing an inflammatory response against the parasite that led to urticaria [Valsecchi et al. 2004].
Blastocystis sp. is also suspected to be involved in IBS [Tan et al. 2010; Poirier et al. 2012; Scanlan, 2012]. Indeed several studies reported a higher incidence of the parasite in patients with IBS compared with healthy populations [Poirier et al. 2012]. IBS is a common gastrointestinal disorder characterized by abdominal pain and discomfort associated with changes in bowel habits [Longstreth et al. 2006]. Studies on the IBS population showed a higher prevalence of ST1 and ST3 isolates of Blastocystis sp. [Yakoob et al. 2010; Fouad et al. 2011; Jimenez-Gonzalez et al. 2012]. However, these studies did not conclude that Blastocystis sp. was the sole etiologic agent. Moreover, a recent study demonstrated that even though Blastocystis sp. was more frequent in symptomatic patients with IBS, the differences compared with controls were not significant [Cekin et al. 2012]. The presence of Blastocystis sp. in symptomatic patients can also indicate that this parasite could be involved with other factors in this disease pathophysiology [Poirier et al. 2012]. It is possible that the alteration of the intestinal environment, provoked by pathogens (bacteria), genetic or environmental factors promotes its development. Furthermore, studies showing the concomitant eradication of Blastocystis sp. with the disappearance of symptoms in patients with IBS are needed to clarify the role of this parasite.
High-risk populations
As in many parasitic infections, some populations are more susceptible to Blastocystis sp. infection. Therefore, this parasite is frequently found in immunocompromised individuals such as those with human immunodeficiency virus/acquired immunodeficiency syndrome or cancer [Kurniawan et al. 2009; Tan et al. 2010]. Children from developing countries and those who are immunocompromised are also more susceptible [Calik et al. 2011; Canete et al. 2012; Daryani et al. 2012]. The socioeconomic status, the quality of drinking water, the consumption of contaminated food and the personal hygiene habits are the major risks explaining contamination in children [Abdulsalam et al. 2012; Canete et al. 2012]. A higher risk of Blastocystis sp. infection was also found in people who are in close contact with animals, which increases exposure to the parasite [Yoshikawa et al. 2009; Parkar et al. 2010], reinforcing the zoonotic nature of Blastocystis sp.
Pathophysiology of blastocystosis
One of the major obstacles to the study of the pathogenesis of Blastocystis sp. is the lack of animal models to test Koch's postulate. However, a variety of experimental infections involving different animals have been described, including rats, mice, guinea pigs or chickens [Tan, 2008; Tan et al. 2010]. From these, it was deduced that laboratory mice are not suitable as animal models. Infections were generally self limiting, although some mice showed weight loss and lethargy. However, histological examination of the cecum and colon revealed intense inflammatory cell infiltration, oedematous lamina propria and mucosal sloughing [Moe et al. 1997]. These authors also showed that there was an age-related susceptibility to Blastocystis sp. in mice. Indeed, juvenile BALB/c mice were more susceptible than adult mice and 8-week-old adult BALB/c mice were totally resistant to Blastocystis sp.
Studies on rat models suggest that this species is more suitable for developing an animal model [Tan, 2008]. Ten to a hundred cysts were able to establish an infection via intracecal or oral inoculations in 3-week-old Wistar rats and all contents from the cecum and large intestine were positive for Blastocystis sp. infection [Yoshikawa et al. 2004]. Hussein and colleagues tested the infectivity of human ST1–4 isolates obtained from both asymptomatic and symptomatic patients in 4-week-old male Wister rats orally inoculated with 4-day-old cultures of Blastocystis sp. negative for Cryptosoridium, Cyclospora, Isospora, Microsporidium and common bacterial pathogens [Hussein et al. 2008]. Interestingly, the moderate and severe degrees of pathological changes observed 6 weeks post infection were found only with symptomatic isolates while mild changes were found only with asymptomatic isolates. Interestingly, ST1 symptomatic isolates induced 25% mortality in rats, indicating its pathogenesis. In parallel, the authors described intense inflammatory reaction and sloughing mucosa, oedema and precancerous polyps in cecum and proximal colon tissues in symptomatic infected rats. It was suggested that the inflammation induced by Blastocystis sp. had the ability to alter tight junctions between the intestinal epithelial cells and the intestinal content, which led to disturbances of the barrier function and permeability [Hussein et al. 2008]. However, the possibility that these pathologies could be of bacterial or viral origin was not excluded.
Some in vitro studies were conducted to investigate the mechanisms of physiopathology by studying the cytopathic effects of Blastocystis sp. on mammalian cell cultures. A first study [Long et al. 2001] showed that 24 h incubation with Blastocystis sp. ST1 cells or culture filtrates induced the production of proinflammatory cytokines interleukin (IL)-8 and granulocyte–macrophage colony-stimulating factor, suggesting that the parasite was able to modulate the host immune response (Figure 3). The induction of IL-8 production from human colonic epithelial cells (HT84) was demonstrated to be activated by cysteine proteases from Blastocystis sp. ST4 in a nuclear factor κB dependent manner [Puthia et al. 2008]. The same authors also showed that coincubation of intestinal epithelial cells of rat interstitial IEC6 with either Blastocystis sp. ST4 isolates or parasite lysates induced the apoptosis of host cells [Puthia et al. 2006] in a contact-independent manner. A decrease in transepithelial resistance and an increase in epithelial permeability were also observed and could be explained by the rearrangement of actin filaments [Tan et al. 2010; Mirza et al. 2012]. These data suggest that Blastocystis sp. is able to disturb host gut homeostasis (Figure 3). As observed in other parasitic protozoa [Sajid and McKerrow, 2002], cysteine proteases of Blastocystis sp. should be involved in parasite survival in vivo and represent virulence factors [Tan et al. 2010; Scanlan, 2012]. Variations in cysteine protease activity were observed between ST4 and ST7 isolates, which may be attributable to differences in virulence [Mirza and Tan, 2009]. Protease activities were identified in Blastocystis sp. secretory products and were able to cleave human secretory immunoglobulin A, the prevalent immunoglobulin defence at the mucosal surface [Puthia et al. 2005]. Another study revealed that cysteine proteases increased permeability of human epithelium by reorganization of the tight junction complex and modulation of the rho associated kinase/phosphorylation of myosin light chain pathway [Mirza et al. 2012]. The sequence of the Blastocystis sp. ST7 complete genome provides molecular candidates that could be involved in pathogenesis [Denoeud et al. 2011]. Twenty-two proteases were predicted to be secreted [Denoeud et al. 2011] and could therefore play a role at the host–parasite interface. Among them, 20 cysteine proteases, 1 serine protease and 1 aspartic protease were characterized and 2 cysteine proteases were experimentally identified and characterized in the secretory products by mass spectrometry analysis [Wawrzyniak et al. 2012]. These secreted proteases are serious candidates to explain gut function disruption observed in intestinal pathologies [Poirier et al. 2012]. Apart from proteases, hydrolases and protease inhibitors were predicted to be secreted and could participate in the blastocystosis physiopathology. Moreover, genes coding a polyketide synthase and nonribosomal peptide synthases were identified [Denoeud et al. 2011]. These enzymes may produce molecules with interesting pharmacological activities like antibiotics and toxins and may be implicated in dysbiosis (Figure 3). The next challenge is to understand the role of Blastocystis sp. in gut dysfunctions [Poirier et al. 2012].
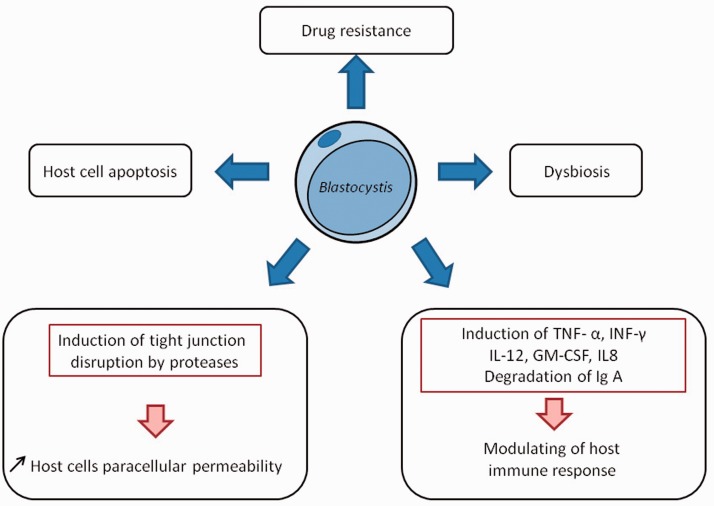
Figure 3.
Mechanisms of blastocystosis physiopathology. Blastocystis sp. may release cysteine protease that may participate in the attack of intestinal epithelium with other hydrolases and may cause the increase in paracellular permeability that is observed in several digestive pathologies such as irritable bowel syndrome (IBS). Blastocystis sp. is able to induce physiological disturbances linked to IBS: host cell apoptosis, the modulation of host immune response and a microinflammation. Some as yet uncharacterized secondary metabolites produced by the polyketide synthase or nonribosomal peptide synthases could participate in host intestinal symptoms by inducing changes in the host microbiota, another feature of IBS. Finally, drug-resistant isolates of the parasite could be explained by the presence of multidrug resistance proteins that could eject active drugs. GM-CSF, granulocyte–macrophage colony-stimulating factor; Ig, immunoglobulin; IL, interleukin; INF, interferon; TNF, tumour necrosis factor.
Treatment of blastocystosis
This treatment is usually considered if diarrhoea is persistent and no other pathogen apart from Blastocystis sp. is identified in faecal specimens [Coyle et al. 2012]. In this case, metronidazole is considered as the first-line therapy for Blastocystis sp. infection [Nigro et al. 2003; Cassano et al. 2005; Moghaddam et al. 2005; Stensvold et al. 2010]. In a first evaluation of this drug efficacy, Nigro and colleagues showed that immunocompetent individuals with Blastocystis sp. infection as the only evident cause of diarrhoea responded to metronidazole treatment and consequently they suggested that the parasite induced intestinal disease [Nigro et al. 2003]. However, there were accumulating reports of treatment failure, particularly in patients with severe Blastocystis sp. infections [Haresh et al. 1999; Moghaddam et al. 2005; Stensvold et al. 2008, 2010], suggesting the existence of extensive variations in drug susceptibility [Mirza et al. 2011]. Accordingly, some genes coding for multidrug resistance pump proteins (ATP-binding cassette transporters) were identified in the Blastocystis sp. ST7 genome (Figure 3) [Denoeud et al. 2011]. Several standard antimicrobials (cotrimoxazole, ornidazole, nitazoxanide, paromomycin, chloroquine, trimethoprim-sulfamethoxazole, iodoquinol, tinidazole, emetine, pentamidine, iodochlorhydroxyquin and furazolidone) may be considered as second-choice drugs [Ok et al. 1999; Cimerman et al. 2003; Diaz et al. 2003; Rossignol et al. 2005; Stensvold et al. 2008; Mirza et al. 2011; Coyle et al. 2012]. Even if some of these drugs were shown to be globally effective against Blastocystis sp., treatment failures were also largely reported [Haresh et al. 1999; Nigro et al. 2003; Mirza et al. 2011].
Conclusion
Blastocystis sp. was included in the Water Sanitation and Health programmes of the World Health Organization [WHO, 2008]. Increasing interest of the scientific and medical community in Blastocystis sp. was coupled with new data about epidemiology, pathogeny and more recently the first whole genome of Blastocystis ST7. Accumulating in vivo, in vitro and in silico data assessed the importance of Blastocystis sp. in human health, with probably the association with major host and environmental factors to be determined. To answer most of the crucial questions regarding Blastocystis sp., we need to develop and standardize axenization of new STs and diagnosis tools, transfection and animal models, microarrays and genotyping markers. Sequencing of genomes from different STs and comparative genomic projects are in progress and will be useful to identify specific virulence factors. Multicentre studies are also required to further our comprehension of the clinical implications of Blastocystis sp. in IBS and other pathologies. To conclude, the interaction of Blastocystis sp. with gut microbiota needs to be studied because of the increasing interest in microbiota disturbances in the genesis of various gastrointestinal dysfunctions.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The authors declare no conflicts of interest in preparing this article.
References
- Abdulsalam A., Ithoi I., Al-Mekhlafi H., Ahmed A., Surin J., Mak J. (2012) Drinking water is a significant predictor of Blastocystis infection among rural Malaysian primary schoolchildren. Parasitology 139: 1014–1020 [DOI] [PubMed] [Google Scholar]
- Abe N. (2004) Molecular and phylogenetic analysis of Blastocystis isolates from various hosts. Vet Parasitol 120: 235–242 [DOI] [PubMed] [Google Scholar]
- Abe N., Wu Z., Yoshikawa H. (2003a) Molecular characterization of Blastocystis isolates from birds by PCR with diagnostic primers and restriction fragment length polymorphism analysis of the small subunit ribosomal RNA gene. Parasitol Res 89: 393–396 [DOI] [PubMed] [Google Scholar]
- Abe N., Wu Z., Yoshikawa H. (2003b) Zoonotic genotypes of Blastocystis hominis detected in cattle and pigs by PCR with diagnostic primers and restriction fragment length polymorphism analysis of the small subunit ribosomal RNA gene. Parasitol Res 90: 124–128 [DOI] [PubMed] [Google Scholar]
- Aguiar J., Goncalves A., Sodre F., Pereira Sdos R., Boia M., De Lemos E., et al. (2007) Intestinal protozoa and helminths among Terena Indians in the State of Mato Grosso do Sul: high prevalence of Blastocystis hominis. Rev Soc Bras Med Trop 40: 631–634 [DOI] [PubMed] [Google Scholar]
- Alfellani M., Stensvold C., Vidal-Lapiedra A., Onuoha E., Fagbenro-Beyioku A., Clark C. (2013) Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop 126: 11–18 [DOI] [PubMed] [Google Scholar]
- Alfellani M., Taner-Mulla D., Jacob A., Imeede C., Yoshikawa H., Stensvold C., et al. (2013) Genetic diversity of Blastocystis in livestock and zoo animals. Acta Trop 126: 11–18 [DOI] [PubMed] [Google Scholar]
- Arisue N., Hashimoto T., Yoshikawa H. (2003) Sequence heterogeneity of the small subunit ribosomal RNA genes among Blastocystis isolates. Protist 164: 497–509 [DOI] [PubMed] [Google Scholar]
- Arisue N., Hashimoto T., Yoshikawa H., Nakamura Y., Nakamura G., Nakamura F., et al. (2002) Phylogenetic position of Blastocystis hominis and of stramenopiles inferred from multiple molecular sequence data. J Eukaryot Microbiol 49: 42–53 [DOI] [PubMed] [Google Scholar]
- Baldursson S., Karanis P. (2011) Waterborne transmission of protozoan parasites: review of worldwide outbreaks – an update 2004–2010. Water Res 45: 6603–6614 [DOI] [PubMed] [Google Scholar]
- Boorom K., Smith H., Nimri L., Viscogliosi E., Spanakos G., Parkar U., et al. (2008) Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection. Parasit Vectors 1: 40–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calik S., Karaman U., Colak C. (2011) Prevalence of microsporidium and other intestinal parasites in children from Malatya, Turkey. Indian J Microbiol 51: 345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canete R., Diaz M., Avalos Garcia R., Laud Martinez P., Manuel Ponce F. (2012) Intestinal parasites in children from a day care centre in Matanzas City, Cuba. PLoS One 7: e51394–e51394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano N., Scoppio B., Loviglio M., Vena G. (2005) Remission of delayed pressure urticaria after eradication of Blastocystis hominis. Acta Derm Venereol 85: 357–358 [DOI] [PubMed] [Google Scholar]
- Cekin A., Cekin Y., Adakan Y., Tasdemir E., Koclar F., Yolcular B. (2012) Blastocystosis in patients with gastrointestinal symptoms: a case-control study. BMC Gastroenterol 12: 122–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Singh M., Howe J., Ho L., Tan S., Yap E. (1999) In vitro encystation and excystation of Blastocystis ratti. Parasitology 118: 151–160 [DOI] [PubMed] [Google Scholar]
- Cimerman S., Ladeira M., Iuliano W. (2003) Blastocystosis: nitazoxanide as a new therapeutic option. Rev Soc Bras Med Trop 36: 415–417 [DOI] [PubMed] [Google Scholar]
- Coyle C., Varughese J., Weiss L., Tanowitz H. (2012) Blastocystis: to treat or not to treat. Clin Infect Dis 54: 105–110 [DOI] [PubMed] [Google Scholar]
- Daryani A., Sharif M., Nasrolahei M., Khalilian A., Mohammadi A., Barzegar G. (2012) Epidemiological survey of the prevalence of intestinal parasites among schoolchildren in Sari, Northern Iran. Trans R Soc Trop Med Hyg 106: 455–459 [DOI] [PubMed] [Google Scholar]
- Denoeud F., Roussel M., Noel B., Wawrzyniak I., Da Silva C., Diogon M., et al. (2011) Genome sequence of the stramenopile Blastocystis, a human anaerobic parasite. Genome Biol 12: R29–R29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E., Mondragon J., Ramirez E., Bernal R. (2003) Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am J Trop Med Hyg 68: 384–385 [PubMed] [Google Scholar]
- Dogruman-Al F., Dagci H., Yoshikawa H., Kurt O., Demirel M. (2008) A possible link between subtype 2 and asymptomatic infections of Blastocystis hominis. Parasitol Res 103: 685–689 [DOI] [PubMed] [Google Scholar]
- Dogruman-Al F., Yoshikawa H., Kustimur S., Balaban N. (2009) PCR-based subtyping of Blastocystis isolates from symptomatic and asymptomatic individuals in a major hospital in Ankara, Turkey. Parasitol Res 106: 263–268 [DOI] [PubMed] [Google Scholar]
- Dominguez-Marquez M., Guna R., Munoz C., Gomez-Munoz M., Borras R. (2009) High prevalence of subtype 4 among isolates of Blastocystis hominis from symptomatic patients of a health district of Valencia (Spain). Parasitol Res 105: 949–955 [DOI] [PubMed] [Google Scholar]
- Eroglu F., Genc A., Elgun G., Koltas I. (2009) Identification of Blastocystis hominis isolates from asymptomatic and symptomatic patients by PCR. Parasitol Res 105: 1589–1592 [DOI] [PubMed] [Google Scholar]
- Eroglu F., Koltas I. (2010) Evaluation of the transmission mode of B. hominis by using PCR method. Parasitol Res 107: 841–845 [DOI] [PubMed] [Google Scholar]
- Fayer R., Santin M., Macarisin D. (2012) Detection of concurrent infection of dairy cattle with Blastocystis, Cryptosporidium, Giardia, and Enterocytozoon by molecular and microscopic methods. Parasitol Res 111: 1349–1355 [DOI] [PubMed] [Google Scholar]
- Forsell J., Granlund M., Stensvold C., Clark C., Evengard B. (2012) Subtype analysis of Blastocystis isolates in Swedish patients. Eur J Clin Microbiol Infect Dis 31: 1689–1696 [DOI] [PubMed] [Google Scholar]
- Fouad S., Basyoni M., Fahmy R., Kobaisi M. (2011) The pathogenic role of different Blastocystis hominis genotypes isolated from patients with irritable bowel syndrome. Arab J Gastroenterol 12: 194–200 [DOI] [PubMed] [Google Scholar]
- Hameed D., Hassanin O., Zuel-Fakkar N. (2011) Association of Blastocystis hominis genetic subtypes with urticaria. Parasitol Res 108: 553–560 [DOI] [PubMed] [Google Scholar]
- Haresh K., Suresh K., Khairul, Anus A., Saminathan S. (1999) Isolate resistance of Blastocystis hominis to metronidazole. Trop Med Int Health 4: 274–277 [DOI] [PubMed] [Google Scholar]
- Hirata T., Nakamura H., Kinjo N., Hokama A., Kinjo F., Yamane N., et al. (2007) Prevalence of Blastocystis hominis and Strongyloides stercoralis infection in Okinawa, Japan. Parasitol Res 101: 1717–1719 [DOI] [PubMed] [Google Scholar]
- Hoevers J., Holman P., Logan K., Hommel M., Ashford R., Snowden K. (2000) Restriction-fragment-length polymorphism analysis of small-subunit rRNA genes of Blastocystis hominis isolates from geographically diverse human hosts. Parasitol Res 86: 57–61 [DOI] [PubMed] [Google Scholar]
- Hussein E., Hussein A., Eida M., Atwa M. (2008) Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol Res 102: 853–860 [DOI] [PubMed] [Google Scholar]
- Ithoi I., Jali A., Mak J., Wan, Sulaiman W., Mahmud R. (2011) Occurrence of Blastocystis in water of two rivers from recreational areas in Malaysia. J Parasitol Res 2011: 123916–123916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantermtor S., Pinlaor P., Sawadpanich K., Pinlaor S., Sangka A., Wilailuckana C., et al. (2013) Subtype identification of Blastocystis spp. isolated from patients in a major hospital in Northeastern Thailand. Parasitol Res 112: 1781–1786 [DOI] [PubMed] [Google Scholar]
- Jimenez-Gonzalez D., Martinez-Flores W., Reyes-Gordillo J., Ramirez-Miranda M., Arroyo-Escalante S., Romero-Valdovinos M., et al. (2012) Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol Res 110: 1269–1275 [DOI] [PubMed] [Google Scholar]
- Jones M., 2nd, Ganac R., Hiser G., Hudson N., Le A., Whipps C. (2008) Detection of Blastocystis from stool samples using real-time PCR. Parasitol Res 103: 551–557 [DOI] [PubMed] [Google Scholar]
- Kaneda Y., Horiki N., Cheng X., Fujita Y., Maruyama M., Tachibana H. (2001) Ribodemes of Blastocystis hominis isolated in Japan. Am J Trop Med Hyg 65: 393–396 [DOI] [PubMed] [Google Scholar]
- Katsarou-Katsari A., Vassalos C., Tzanetou K., Spanakos G., Papadopoulou C., Vakalis N. (2008) Acute urticaria associated with amoeboid forms of Blastocystis sp. subtype 3. Acta Derm Venereol 88: 80–81 [DOI] [PubMed] [Google Scholar]
- Kurniawan A., Karyadi T., Dwintasari S., Sari I., Yunihastuti E., Djauzi S., et al. (2009) Intestinal parasitic infections in HIV/AIDS patients presenting with diarrhoea in Jakarta, Indonesia. Trans R Soc Trop Med Hyg 103: 892–898 [DOI] [PubMed] [Google Scholar]
- Lee L., Chye T., Karmacharya B., Govind S. (2012) Blastocystis sp.: waterborne zoonotic organism, a possibility? Parasit Vectors 5: 130–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelayoova S., Siripattanapipong S., Thathaisong U., Naaglor T., Taamasri P., Piyaraj P., et al. (2008) Drinking water: a possible source of Blastocystis spp. subtype 1 infection in schoolchildren of a rural community in central Thailand. Am J Trop Med Hyg 79: 401–406 [PubMed] [Google Scholar]
- Li L., Zhang X., Lv S., Zhang L., Yoshikawa H., Wu Z., et al. (2007) Cross-sectional surveys and subtype classification of human Blastocystis isolates from four epidemiological settings in China. Parasitol Res 102: 83–90 [DOI] [PubMed] [Google Scholar]
- Li L., Zhou X., Du Z., Wang X., Wang L., Jiang J., et al. (2007) Molecular epidemiology of human Blastocystis in a village in Yunnan Province, China. Parasitol Int 56: 281–286 [DOI] [PubMed] [Google Scholar]
- Long H., Handschack A., Konig W., Ambrosch A. (2001) Blastocystis hominis modulates immune responses and cytokine release in colonic epithelial cells. Parasitol Res 87: 1029–1030 [DOI] [PubMed] [Google Scholar]
- Longstreth G., Thompson W., Chey W., Houghton L., Mearin F., Spiller R. (2006) Functional bowel disorders. Gastroenterology 130: 1480–1491 [DOI] [PubMed] [Google Scholar]
- Meloni D., Poirier P., Mantini C., Noel C., Gantois N., Wawrzyniak I., et al. (2012) Mixed human intra- and inter-subtype infections with the parasite Blastocystis sp. Parasitol Int 61: 719–722 [DOI] [PubMed] [Google Scholar]
- Meloni D., Sanciu G., Poirier P., El, Alaoui H., Chabe M., Delhaes L., et al. (2011) Molecular subtyping of Blastocystis sp. isolates from symptomatic patients in Italy. Parasitol Res 109: 613–619 [DOI] [PubMed] [Google Scholar]
- Mirza H., Tan K. (2009) Blastocystis exhibits inter- and intra-subtype variation in cysteine protease activity. Parasitol Res 104: 355–361 [DOI] [PubMed] [Google Scholar]
- Mirza H., Teo J., Upcroft J., Tan K. (2011) A rapid, high-throughput viability assay for Blastocystis spp. reveals metronidazole resistance and extensive subtype-dependent variations in drug susceptibilities. Antimicrob Agents Chemother 55: 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza H., Wu Z., Teo J., Tan K. (2012) Statin pleiotropy prevents rho kinase-mediated intestinal epithelial barrier compromise induced by Blastocystis cysteine proteases. Cell Microbiol 14: 1474–1484 [DOI] [PubMed] [Google Scholar]
- Moe K., Singh M., Howe J., Ho L., Tan S., Chen X., et al. (1997) Experimental Blastocystis hominis infection in laboratory mice. Parasitol Res 83: 319–325 [DOI] [PubMed] [Google Scholar]
- Moe K., Singh M., Howe J., Ho L., Tan S., Ng G., et al. (1996) Observations on the ultrastructure and viability of the cystic stage of Blastocystis hominis from human feces. Parasitol Res 82: 439–444 [DOI] [PubMed] [Google Scholar]
- Moghaddam D., Ghadirian E., Azami M. (2005) Blastocystis hominis and the evaluation of efficacy of metronidazole and trimethoprim/sulfamethoxazole. Parasitol Res 96: 273–275 [DOI] [PubMed] [Google Scholar]
- Moosavi A., Haghighi A., Mojarad E., Zayeri F., Alebouyeh M., Khazan H., et al. (2012) Genetic variability of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Iran. Parasitol Res 111: 2311–2315 [DOI] [PubMed] [Google Scholar]
- Nagel R., Cuttell L., Stensvold C., Mills P., Bielefeldt-Ohmann H., Traub R. (2012) Blastocystis subtypes in symptomatic and asymptomatic family members and pets and response to therapy. Intern Med J 42: 1187–1195 [DOI] [PubMed] [Google Scholar]
- Nasirudeen A., Tan K. (2004) Isolation and characterization of the mitochondrion-like organelle from Blastocystis hominis. J Microbiol Methods 58: 101–109 [DOI] [PubMed] [Google Scholar]
- Nigro L., Larocca L., Massarelli L., Patamia I., Minniti S., Palermo F., et al. (2003) A placebo-controlled treatment trial of Blastocystis hominis infection with metronidazole. J Travel Med 10: 128–130 [DOI] [PubMed] [Google Scholar]
- Noël C., Dufernez F., Gerbod D., Edgcomb V., Delgado-Viscogliosi P., Ho L., et al. (2005) Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J Clin Microbiol 43: 348–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ok U., Girginkardesler N., Balcioglu C., Ertan P., Pirildar T., Kilimcioglu A. (1999) Effect of trimethoprim-sulfamethaxazole in Blastocystis hominis infection. Am J Gastroenterol 94: 3245–3247 [DOI] [PubMed] [Google Scholar]
- Ozyurt M., Kurt O., Molbak K., Nielsen H., Haznedaroglu T., Stensvold C. (2008) Molecular epidemiology of Blastocystis infections in Turkey. Parasitol Int 57: 300–306 [DOI] [PubMed] [Google Scholar]
- Parkar U., Traub R., Kumar S., Mungthin M., Vitali S., Leelayoova S., et al. (2007) Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology 134: 359–367 [DOI] [PubMed] [Google Scholar]
- Parkar U., Traub R., Vitali S., Elliot A., Levecke B., Robertson I., et al. (2010) Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Vet Parasitol 169: 8–17 [DOI] [PubMed] [Google Scholar]
- Pegelow K., Gross R., Pietrzik K., Lukito W., Richards A., Fryauff D. (1997) Parasitological and nutritional situation of school children in the Sukaraja district, West Java, Indonesia. Southeast Asian J Trop Med Public Health 28: 173–190 [PubMed] [Google Scholar]
- Perez-Brocal V., Clark C. (2008) Analysis of two genomes from the mitochondrion-like organelle of the intestinal parasite Blastocystis: complete sequences, gene content, and genome organization. Mol Biol Evol 25: 2475–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P., Wawrzyniak I., Albert A., El, Alaoui H., Delbac F., Livrelli V. (2011) Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: prospective study of patients with hematological malignancies. J Clin Microbiol 49: 975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P., Wawrzyniak I., Vivares C., Delbac F., El Alaoui H. (2012) New insights into Blastocystis spp.: a potential link with irritable bowel syndrome. PLoS Pathog 8: e1002545–e1002545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthia M., Lu J., Tan K. (2008) Blastocystis ratti contains cysteine proteases that mediate interleukin-8 response from human intestinal epithelial cells in an NF-kappaB-dependent manner. Eukaryot Cell 7: 435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthia M., Sio S., Lu J., Tan K. (2006) Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells. Infect Immun 74: 4114–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthia M., Vaithilingam A., Lu J., Tan K. (2005) Degradation of human secretory immunoglobulin a by Blastocystis. Parasitol Res 97: 386–389 [DOI] [PubMed] [Google Scholar]
- Rajah, Salim H., Suresh, Kumar G., Vellayan S., Mak J., Khairul, Anuar A., Init I., et al. (1999) Blastocystis in animal handlers. Parasitol Res 85: 1032–1033 [DOI] [PubMed] [Google Scholar]
- Rayan H., Ismail O., El, Gayar E. (2007) Prevalence and clinical features of Dientamoeba fragilis infections in patients suspected to have intestinal parasitic infection. J Egypt Soc Parasitol 37: 599–608 [PubMed] [Google Scholar]
- Riisberg I., Orr R., Kluge R., Shalchian-Tabrizi K., Bowers H., Patil V., et al. (2009) Seven gene phylogeny of heterokonts. Protist 160: 191–204 [DOI] [PubMed] [Google Scholar]
- Rivera W., Tan M. (2005) Molecular characterization of Blastocystis isolates in the Philippines by riboprinting. Parasitol Res 96: 253–257 [DOI] [PubMed] [Google Scholar]
- Roberts T., Barratt J., Harkness J., Ellis J., Stark D. (2011) Comparison of microscopy, culture, and conventional polymerase chain reaction for detection of Blastocystis sp. in clinical stool samples. Am J Trop Med Hyg 84: 308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T., Stark D., Harkness J., Ellis J. (2013) Subtype distribution of Blastocystis isolates from a variety of animals from New South Wales, Australia. Vet Parasitol 196: 85–89 [DOI] [PubMed] [Google Scholar]
- Rossignol J., Kabil S., Said M., Samir H., Younis A. (2005) Effect of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis. Clin Gastroenterol Hepatol 3: 987–991 [DOI] [PubMed] [Google Scholar]
- Sajid M., McKerrow J. (2002) Cysteine proteases of parasitic organisms. Mol Biochem Parasitol 120: 1–21 [DOI] [PubMed] [Google Scholar]
- Saksirisampant W., Nuchprayoon S., Wiwanitkit V., Yenthakam S., Ampavasiri A. (2003) Intestinal parasitic infestations among children in an orphanage in Pathum Thani Province. J Med Assoc Thai 86(Suppl. 2): S263–S270 [PubMed] [Google Scholar]
- Saksirisampant W., Prownebon J., Kulkumthorn M., Yenthakam S., Janpla S., Nuchprayoon S. (2006) Prevalence of intestinal parasitic infections among school children in the central region of Thailand. J Med Assoc Thai 89: 1928–1933 [PubMed] [Google Scholar]
- Santin M., Gomez-Munoz M., Solano-Aguilar G., Fayer R. (2011) Development of a new PCR protocol to detect and subtype Blastocystis spp. from humans and animals. Parasitol Res 109: 205–212 [DOI] [PubMed] [Google Scholar]
- Scanlan P.D. (2012) Blastocystis: past pitfalls and future perspectives. Trends Parasitol 28: 327–334 [DOI] [PubMed] [Google Scholar]
- Scicluna S., Tawari B., Clark C. (2006) DNA barcoding of Blastocystis. Protist 157: 77–85 [DOI] [PubMed] [Google Scholar]
- Silberman J., Sogin M., Leipe D., Clark C. (1996) Human parasite finds taxonomic home. Nature 380: 398–398 [DOI] [PubMed] [Google Scholar]
- Souppart L., Moussa H., Cian A., Sanciu G., Poirier P., El, Alaoui H., et al. (2010) Subtype analysis of Blastocystis isolates from symptomatic patients in Egypt. Parasitol Res 106: 505–511 [DOI] [PubMed] [Google Scholar]
- Souppart L., Sanciu G., Cian A., Wawrzyniak I., Delbac F., Capron M., et al. (2009) Molecular epidemiology of human Blastocystis isolates in France. Parasitol Res 105: 413–421 [DOI] [PubMed] [Google Scholar]
- Stechmann A., Hamblin K., Perez-Brocal V., Gaston D., Richmond G., Van Der, Giezen M., et al. (2008) Organelles in Blastocystis that blur the distinction between mitochondria and hydrogenosomes. Curr Biol 18: 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold C. (2013) Comparison of sequencing (barcode region) and sequence-tagged-site PCR for Blastocystis subtyping. J Clin Microbiol 51: 190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold C., Ahmed U., Andersen L., Nielsen H. (2012) Development and evaluation of a genus-specific, probe-based, internal-process-controlled real-time PCR assay for sensitive and specific detection of Blastocystis spp. J Clin Microbiol 50: 1847–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold C., Alfellani M., Norskov-Lauritsen S., Prip K., Victory E., Maddox C., et al. (2009) Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int J Parasitol 39: 473–479 [DOI] [PubMed] [Google Scholar]
- Stensvold C., Arendrup M., Jespersgaard C., Molbak K., Nielsen H. (2007a) Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagn Microbiol Infect Dis 59: 303–307 [DOI] [PubMed] [Google Scholar]
- Stensvold C., Arendrup M., Nielsen H., Bada A., Thorsen S. (2008) Symptomatic infection with Blastocystis sp. subtype 8 successfully treated with trimethoprim-sulfamethoxazole. Ann Trop Med Parasitol 102: 271–274 [DOI] [PubMed] [Google Scholar]
- Stensvold C., Christiansen D., Olsen K., Nielsen H. (2011) Blastocystis sp. subtype 4 is common in Danish Blastocystis-positive patients presenting with acute diarrhea. Am J Trop Med Hyg 84: 883–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold C., Smith H., Nagel R., Olsen K., Traub R. (2010) Eradication of Blastocystis carriage with antimicrobials: reality or delusion? J Clin Gastroenterol 44: 85–90 [DOI] [PubMed] [Google Scholar]
- Stensvold C., Suresh G., Tan K., Thompson R., Traub R., Viscogliosi E., et al. (2007b) Terminology for Blastocystis subtypes – a consensus. Trends Parasitol 23: 93–96 [DOI] [PubMed] [Google Scholar]
- Stensvold R., Brillowska-Dabrowska A., Nielsen H., Arendrup M. (2006) Detection of Blastocystis hominis in unpreserved stool specimens by using polymerase chain reaction. J Parasitol 92: 1081–1087 [DOI] [PubMed] [Google Scholar]
- Stenzel D., Boreham P. (1996) Blastocystis hominis revisited. Clin Microbiol Rev 9: 563–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh K., Ng G., Ramachandran N., Ho L., Yap E., Singh M. (1993) In vitro encystment and experimental infections of Blastocystis hominis. Parasitol Res 79: 456–460 [DOI] [PubMed] [Google Scholar]
- Suresh K., Smith H. (2004) Comparison of methods for detecting Blastocystis hominis. Eur J Clin Microbiol Infect Dis 23: 509–511 [DOI] [PubMed] [Google Scholar]
- Suresh K., Smith H., Tan T. (2005) Viable Blastocystis cysts in Scottish and Malaysian sewage samples. Appl Environ Microbiol 71: 5619–5620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh K., Venilla G., Tan T., Rohela M. (2009) In vivo encystation of Blastocystis hominis. Parasitol Res 104: 1373–1380 [DOI] [PubMed] [Google Scholar]
- Tan K. (2004) Blastocystis in humans and animals: new insights using modern methodologies. Vet Parasitol 126: 121–144 [DOI] [PubMed] [Google Scholar]
- Tan K. (2008) New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev 21: 639–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K., Mirza H., Teo J., Wu B., Macary P. (2010) Current views on the clinical relevance of Blastocystis spp. Curr Infect Dis Rep 12: 28–35 [DOI] [PubMed] [Google Scholar]
- Tan T., Suresh K. (2006) Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients. Parasitol Res 98: 189–193 [DOI] [PubMed] [Google Scholar]
- Thathaisong U., Siripattanapipong S., Mungthin M., Pipatsatitpong D., Tan-Ariya P., Naaglor T., et al. (2013) Identification of Blastocystis subtype 1 variants in the home for girls, Bangkok, Thailand. Am J Trop Med Hyg 88: 352–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ANOFEL Cryptosporidium National Network (2010) Laboratory-based surveillance for Cryptosporidium in France, 2006–2009. EuroSurveill 15: pii=19642–pii=19642 [PubMed] [Google Scholar]
- Valsecchi R., Leghissa P., Greco V. (2004) Cutaneous lesions in Blastocystis hominis infection. Acta Derm Venereol 84: 322–323 [DOI] [PubMed] [Google Scholar]
- Verma R., Delfanian K. (2013) Blastocystis hominis associated acute urticaria. Am J Med Sci 346: 80–81 [DOI] [PubMed] [Google Scholar]
- Villar J., Carbajal J., Lanuza M., Munoz C., Borras R. (1998) In vitro encystation of Blastocystis hominis: a kinetics and cytochemistry study. Parasitol Res 84: 54–58 [DOI] [PubMed] [Google Scholar]
- Vogelberg C., Stensvold C., Monecke S., Ditzen A., Stopsack K., Heinrich-Grafe U., et al. (2010) Blastocystis sp. subtype 2 detection during recurrence of gastrointestinal and urticarial symptoms. Parasitol Int 59: 469–471 [DOI] [PubMed] [Google Scholar]
- Wawrzyniak I., Roussel M., Diogon M., Couloux A., Texier C., Tan K., et al. (2008) Complete circular DNA in the mitochondria-like organelles of Blastocystis hominis. Int J Parasitol 38: 1377–1382 [DOI] [PubMed] [Google Scholar]
- Wawrzyniak I., Texier C., Poirier P., Viscogliosi E., Tan K., Delbac F., et al. (2012) Characterization of two cysteine proteases secreted by Blastocystis St7, a human intestinal parasite. Parasitol Int 61: 437–442 [DOI] [PubMed] [Google Scholar]
- Whipps C., Boorom K., Bermudez L., Kent M. (2010) Molecular characterization of Blastocystis species in Oregon identifies multiple subtypes. Parasitol Res 106: 827–832 [DOI] [PubMed] [Google Scholar]
- WHO (2008) Guidelines for Drinking-Water Quality (3rd edition, incorporating first and second addenda), Geneva: World Health Organization; [PubMed] [Google Scholar]
- Wong K., Ng G., Lin R., Yoshikawa H., Taylor M., Tan K. (2008) Predominance of subtype 3 among Blastocystis isolates from a major hospital in Singapore. Parasitol Res 102: 663–670 [DOI] [PubMed] [Google Scholar]
- Yakoob J., Jafri W., Beg M., Abbas Z., Naz S., Islam M., et al. (2010) Blastocystis hominis and Dientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitol Res 107: 679–684 [DOI] [PubMed] [Google Scholar]
- Yan Y., Su S., Lai R., Liao H., Ye J., Li X., et al. (2006) Genetic variability of Blastocystis hominis isolates in China. Parasitol Res 99: 597–601 [DOI] [PubMed] [Google Scholar]
- Yan Y., Su S., Ye J., Lai X., Lai R., Liao H., et al. (2007) Blastocystis sp. subtype 5: a possibly zoonotic genotype. Parasitol Res 101: 1527–1532 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Abe N., Iwasawa M., Kitano S., Nagano I., Wu Z., et al. (2000) Genomic analysis of Blastocystis hominis strains isolated from two long-term health care facilities. J Clin Microbiol 38: 1324–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Morimoto K., Wu Z., Singh M., Hashimoto T. (2004) Problems in speciation in the genus Blastocystis. Trends Parasitol 20: 251–255 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Wu Z., Kimata I., Iseki M., Ali I., Hossain M., et al. (2004) Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol Res 92: 22–29 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Wu Z., Pandey K., Pandey B., Sherchand J., Yanagi T., et al. (2009) Molecular characterization of Blastocystis isolates from children and rhesus monkeys in Kathmandu, Nepal. Vet Parasitol 160: 295–300 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Yoshida K., Nakajima A., Yamanari K., Iwatani S., Kimata I. (2004) Fecal–oral transmission of the cyst form of Blastocystis Hominis in rats. Parasitol Res 94: 391–396 [DOI] [PubMed] [Google Scholar]
- Zuel-Fakkar N., Abdel, Hameed D., Hassanin O. (2011) Study of Blastocystis hominis isolates in urticaria: a case-control study. Clin Exp Dermatol 36: 908–910 [DOI] [PubMed] [Google Scholar]