Abstract
Background
In recent years, three national gastroenterology societies established guidelines for the diagnosis and therapy of pancreatic exocrine insufficiency (PEI). In addition, the Cochrane Collaboration issued a review.
Objective
The purpose of this paper is to present an overview of the recommendations and concordance between the four recent published guidelines and stimulate further discussion.
Methods
A review of the Australian, German and Italian guidelines and the Cochrane review was conducted, and a synthesis was made of common statements.
Results
There is a high degree of agreement on almost all items within these guidelines, both in the diagnosis of PEI and in terms of therapy and approach to management of PEI. In addition, novel emerging developments are highlighted, such as the fecal elastase-1 test, which is widely used but is not suitable for measuring mild-to-moderate PEI despite its ability to positively establish the diagnosis of severe PEI. One of the few novel tests proving to be useful is the 13C mixed-chain triglycerides (MCT) breath test. This test, albeit an excellent quantitative test, is not widely used and is rarely available. The use of this test is making it apparent that there is a difference between treating the symptoms of PEI and treating malnutrition, the broader underlying defect. This may have direct consequences for the dosing of pancreatic enzymes (pancreatin), in that the consensus starting dose of all guidelines may be too low for some patients. Although chronic pancreatitis in adults and cystic fibrosis in children account for the main evidence base used for PEI, other indications are also discussed.
Conclusions
There is good concordance between recommendations provided by international groups. More prospective studies are required in many areas, including the use of pancreatic enzymes in other gastrointestinal disorders, such as celiac disease and irritable bowel syndrome (IBS). We also need to assess the feasibility of the 13C MCT breath test. At the same time, it needs to be confirmed that higher doses of pancreatic enzymes are really necessary to not only relieve the symptoms of PEI but also treat malnutrition appropriately.
Keywords: Pancreatic exocrine insufficiency, pancreatitis, systematic review, Cochrane, enzyme therapy, pancreatic function test
Background and objective
Chronic pancreatitis (CP) in adults and cystic fibrosis (CF) in children are the major indications for the use of pancreatic enzyme replacement therapy. These conditions both lead to impairment in both the endocrine and the exocrine pancreas. The treatment of diabetes mellitus is well standardized in diagnostic methods and therapeutic pathways,1 but the diagnosis and treatment of pancreatic exocrine insufficiency is a continuous challenge. Recently, several national societies defined guidelines, among them Australia,2 Italy3 and Germany.4 In addition, a Cochrane Review5 exists (Table 1).
Table 1.
Synopsis of the guidelines on diagnosis and treatment of pancreatic exocrine insufficiency
This paper provides a summary of the evidence and recommendations as a guideline for clinical pancreatologists. The levels of evidence and the grades of recommendation used in the published guidelines follow the established terms and definitions of the Center for Evidence Based Medicine (www.cebm.com). This article is not meant to provide a comprehensive review on CP but rather to highlight the consensus of the three guidelines and the Cochrane Review. For more detailed information, a study of the published guidelines is recommended.2–5
Pancreatic exocrine insufficiency (PEI) is defined as inadequate pancreatic enzyme activity, due to insufficient enzyme production, insufficient enzyme activation or early enzyme degradation.6 Further, PEI can be classified as primary or secondary. Primary PEI is due to a lack of exocrine pancreatic tissue or disturbances in the innervation. In secondary PEI, pancreatic enzymes are released but cannot work appropriately due to anatomical changes after surgery, or due to inappropriate activation or inactivation. Before treatment, a diagnosed pancreatic disease should be established as the cause of PEI. Pancreatic function tests may be used to diagnose CP (Evidence 1c/recommendation B).7 Symptoms of PEI can be anticipated after 10 years of disease duration (Evidence 1b; recommendation/good clinical practice [GCP]). In hereditary pancreatitis, the cumulative risk (95% confidence interval [CI]) at 50 years of age for exocrine failure was found to be 37.2% (28.5%, 45.8%) and 47.6% (37.1%, 58.1%) for endocrine failure.8 In cystic fibrosis, PEI is present in 85% of infants at birth.9 A typical symptom of advanced PEI is steatorrhea. Steatorrhea, however, may not be present or may have another cause. Finally, no symptom definitively proves or excludes PEI (Evidence 1b). Regular meetings should occur with patients every 6 to 12 months in order to detect complications (deterioration of PEI, pain, diabetes, cancer) (Evidence 5; recommendation D). Patients with diabetes mellitus, for example, have an increased risk of developing PEI (Evidence 2B) because of atrophy of the exocrine acinar tissue.10
Diagnosis of pancreatic exocrine insufficiency
PEI can be diagnosed clinically, if steatorrhea is present. However, steatorrhea may not be present or may have other causes, including mucosal disease or postcibal asynchrony, which can occur in post-gastrectomy patients, and finally, no symptom definitively proves or excludes PEI (Evidence 1b). At the time of clinical suspicion, a pancreatic function test should be performed (Evidence 1b; recommendation B) because even mild-to-moderate PEI may have a clinical implication. The secretin-pancreozymin test, although it is the “gold standard” (Evidence 1b, recommendation A), has practically disappeared from clinical practice. Instead, non-invasive tests should be used (Evidence 5, recommendation B). The most widely used test is the fecal elastase-1 (FE-1) test, which is easy to use; however, it is only reliable in moderate-to-severe PEI.11 Measuring FE-1 in diarrhea, for example, in patients with irritable bowel syndrome (IBS), can yield false results.12 The 13C breath test with mixed-chain triglycerides (MCT) is an alternative (Evidence 5, recommendation B) which may become more widely used in the future. It is the only test that can monitor and measure the success of PERT (pancreatic enzyme replacement therapy—see below).13
Pancreatic enzyme preparations
The US Food and Drug Administration (FDA) recently decided to end all over-the-counter (OTC) preparations of pancreatin because they vary dramatically in activity, and consequently, all pancreatin preparations for the US market had to undergo a new drug application (NDA). Indeed, the few studies addressing the subject have demonstrated huge differences in the activity and release kinetics of the enzymes.14 The choice of the particular pancreatin is in part also dependent on the situation of the patient (see below).
Medical therapy for pancreatic exocrine insufficiency
Medical therapy must address the pain as well as the functional and nutritional deficits. Furthermore, there are general measures to be taken, for example, a change in the patient’s lifestyle.
General measurements
With regard to the known most prominent factors in CP, alcohol should be avoided (Evidence 2b; recommendation A), and this can reduce the pain in CP (Evidence 2b; recommendation B). There is no evidence and hence no recommendation for smoking cessation in CP and PEI despite some reports,5,15 even in acute pancreatitis.2–5 Smoking cessation is recommended (Evidence 3b, recommendation A). In chronic pancreatitis, however, smoking cessation will reduce the pain relapses during acute attacks (Evidence 4; recommendation C).
Therapy for pancreatic pain
Patients with CP suffer from severe visceral pain. Therefore, adequate pain therapy is mandatory (Evidence 2b, recommendation A). The pain therapy is following the established World Health Organization (WHO) scheme with a non-steroidal anti-inflammatory drug (NSAID) as an initial therapy, stepping up all the way to opioids. Avoiding morphine, even during an acute attack, is obsolete (Evidence 2b). Continuous pain should be considered as an indication for interventional or surgical therapy (Evidence 2b/2b).
Pancreatic enzyme replacement therapy
Before treating a patient with PEI, the diagnosis should be established, for example, via FE-1 or a breath test (Evidence 2b; recommendation B). The secretin-pancreozymin test is still considered the gold standard (Evidence 1, recommendation A); however, it is no longer in routine clinical use. There is also a recommendation to test endocrine function before starting the therapy, for example, via fasting blood glucose (Evidence 4; recommendation C), as many patients with CP may already have an impaired glucose tolerance, chemical or clinically non-overt diabetes mellitus, called type 3c.16 Patient compliance regarding diet and intake of medication should be assured (recommendation A). It is generally recommended that a dietician should be involved (GCP/recommendation D).
There is no doubt that pancreatic enzyme replacement therapy (PERT) is indicated in patients with CP and PEI (Evidence 1a; recommendation A). Besides clinical improvement, there is also some evidence that PERT improves the quality of life (Evidence 4; recommendation D).
There is some evidence and therefore a recommendation that small meals are often better tolerated (recommendation D). If PERT is conducted properly, no fat restrictions are necessary (recommendation D). Patients should take pancreatin along with a normal diet instead of changing their diet. If deficits in vitamins and trace elements are recorded,17 these should be substituted accordingly (Evidence 1c; recommendation A/B). Antioxidants can be helpful in pain treatment in CP (Evidence 1b; recommendation C).18
Pancreatin for PERT should contain sufficient lipase units (recommendation A), with 20,000–40,000 units as a starting dose for a meal and 10,000–20,000 lipase units for a snack (Evidence 1b; recommendation B). In contrast to the consensus of all these guidelines, recent evidence suggest that this may not be sufficient: according to results of the 13C MCT breath test, this dose may not be sufficient to normalize nutrition.13 Therefore, we might consider higher doses when initiating PERT.19 The only side effect of PERT is constipation; nevertheless, one of the guidelines presents an upper limit of dosing, 80,000/day (Evidence 2b)—however, without a recommendation. Pancreatin should be given for every meal (recommendation A) and enzymes should be administered during the meal (Evidence 1b; recommendation A). PERT dosing in children uses either body weight or is dosed according to fat intake. Patients are commenced on the minimum dose and then up titrated based on weight gain and bowel signs to ascertain the lowest effective dose.9,20 A maximum dose recommendation of 10,000 units of lipase per kilogram per day is used because of the possible risk of fibrosing colonopathy.2
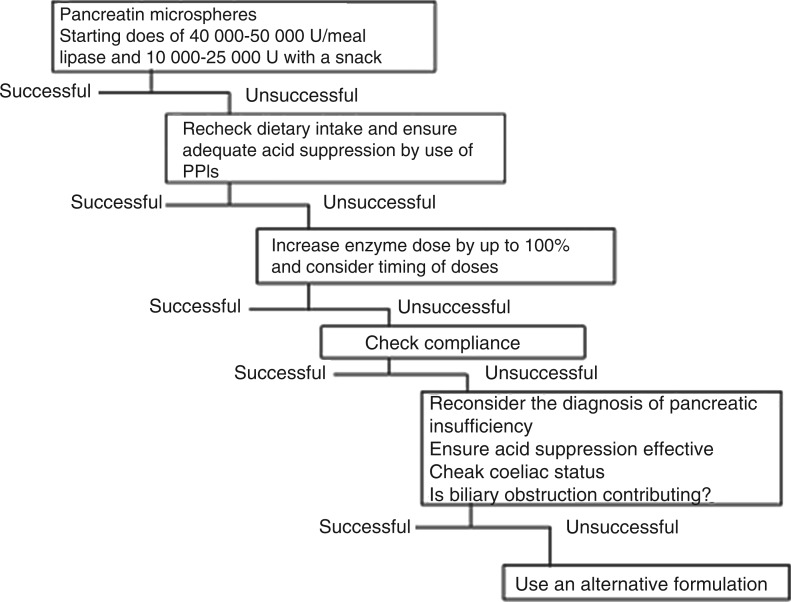
In case of insufficient response to PERT, the pancreatin dose should be doubled or tripled (Evidence 5; recommendation B) and/or a proton pump inhibitor should be administered (Evidence 1b; recommendation B) (Figure 1). Depending on the situation of the individual patient (e.g. [partial] gastrectomy, anatomic divergence from normal [e.g. Roux-en-Y]), pancreatin as a granulate can be used—however, it is not available in all countries.
Figure 1.
Algorithm summarizing the rationale for conducting pancreatic enzyme replacement therapy in patients with pancreatic exocrine insufficiency. (Adapted from Imrie et al.19)
Besides the typical patient with chronic pancreatitis and PEI, there is emerging evidence for treating other patient groups with PERT. These include patients with unresectable pancreatic cancer (Evidence 5; GCP), patients with celiac disease,21 diagnosed with PEI (Evidence 2b; GCP) and especially infants (Evidence 1b; GCP). About one-third of patients with AIDS may suffer from PEI and should be treated (Evidence 3b).
The effect of PERT in IBS has long been considered to be a placebo effect; recent studies, however, demonstrate that there is a diarrhea-predominant subgroup of IBS patients (D-IBS according to the Rome-II criteria) with PEI.22 These should be treated (Evidence 2b/3b; GCP). On the other hand, there is no indication whatsoever to use PERT for treatment of pain in CP (Evidence 1a; recommendation A).5
Patients with acute pancreatitis may have transient PEI due to several different proposed mechanisms; they should not, however, be treated with enzymes during the acute pancreatitis (Evidence 1a; recommendation A). As an episode of acute pancreatitis sometimes represents the first manifestation of previously undiagnosed chronic pancreatitis, patients should be reassessed and followed up between 6 and 18 months so as not to overlook developing or preexisting PEI (Evidence 2b).
Patients with autoimmune pancreatitis can develop both an endocrine and an exocrine pancreatic insufficiency.23–25 Their PEI should be treated according to the established guidelines (Evidence 2b, recommendation A). It should be noted that some patients may live to see a restitutio at integrum, whereas a good one-third of these patients develop atrophy of the pancreatic gland requiring continuous PERT.26
Due to pancreatic acinar atrophy,10 patients with diabetes mellitus have an increased risk of developing PEI (Evidence 2B), as evident from reduced FE-1 in both type 1 and type 2 diabetes mellitus.27 These patients also ought to be treated (GCP).
Monitoring treatment success
The success of PERT and/or compliance can be monitored in several ways. In the normal clinical setting, the clinical improvement of the patient is a sufficient enough criterion to evaluate response (Evidence 2a; recommendation B). There is good evidence that both the fecal fat excretion (coefficient of fat absorption [CFA]) and the 13C MCT breath test are reliable instruments to measure success and compliance (Evidence 1b/2b). The 13C MCT breath test is even recommended in non-responders (Evidence 2b; recommendation B).
Conclusions
The three published guidelines together with the Cochrane Review provide a sound basis for diagnosing and treating PEI. Nevertheless, diagnosing PEI remains a challenge in clinical practice due to the lack of a reliable test measuring mild-to-moderate PEI. The FE-1 test, although well established, may not measure mild-to-moderate PEI reliably. Accordingly, evidence is sparse and recommendations are few. All scientific work, even guidelines which have to undergo a certain default process prior to being published, constitutes merely a snapshot. A new pancreatic function test is emerging: the 13C MCT breath test—however, experience is yet limited to a few centers. Secretin-stimulated magnetic resonance cholangiopancreatography might represent another modality to measure pancreatic function. There is good consensus among all guidelines on how to treat patients with PEI, as demonstrated here, especially on the starting dose and how to proceed in case of insufficient response to therapy (Figure 1). Recent evidence suggests that the starting dose may be too low if only clinical symptoms are taken as a surrogate. The emerging concept points to treatment of malnutrition, that is, not only correcting symptoms of PEI. These new developments warrant further clinical studies, both diagnostic and therapeutic, building the basis for later updates of guidelines to come.
Acknowledgments
This comprehensive review is the based on a plenary lecture delivered during the 44th European Pancreatic Club, held June 20–23, 2012, in Prague, Czech Republic. It is dedicated to Paul G Lankisch, Professor Emeritus of Gastroenterology, on the occasion of his 75th birthday.
Funding
The ongoing work is funded by a Link Award of the United European Gastroenterology (UEG) to JML via the Swedish Gastroenterology Society (SGF).
Conflict of interest
The authors have received honoraria from Abbott Pharmaceuticals and Axcan for lecturing.
References
- 1.American Diabetes Association. Introduction: The American Diabetes Association's (ADA) evidence-based practice guidelines, standards, and related recommendations and documents for diabetes care. Diabetes Care 2012; 35(Suppl 1): S1–S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toouli J, Biankin AV, Oliver MR, et al. Management of pancreatic exocrine insufficiency: Australasian Pancreatic Club recommendations. Med J Aust 2010; 193461–467 [DOI] [PubMed] [Google Scholar]
- 3.Frulloni L, Falconi M, Gabbrielli A, et al. Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis 2010; 42(Suppl 6): S381–S406 [DOI] [PubMed] [Google Scholar]
- 4.Hoffmeister A, Mayerle J, Beglinger C, et al. S3-Leitlinie Chronische Pankreatitis: Definition, Ätiologie, Diagnostik, konservative, interventionell endoskopische und operative Therapie der chronischen Pankreatitis. Leitlinie der Deutschen Gesellschaft für Verdauungs- und Stoffwechselkrankheiten (DGVS). Z Gastroenterol 2012; 501176–1224 [DOI] [PubMed] [Google Scholar]
- 5.Shafiq N, Rana S, Bhasin D, et al. Pancreatic enzymes for chronic pancreatitis. Cochrane Database Syst Rev 2009. CD006302 [DOI] [PubMed] [Google Scholar]
- 6.Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut 2005; 54(Suppl 6): 1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegmund E, Löhr JM, Schuff-Werner P. The diagnostic validity of non-invasive pancreatic function tests—a meta-analysis. Z Gastroenterol 2004; 421117–1128 [DOI] [PubMed] [Google Scholar]
- 8.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol 2004; 2252–261 [DOI] [PubMed] [Google Scholar]
- 9.Van de Vijver E, Desager K, Mulberg AE, et al. Treatment of infants and toddlers with cystic fibrosis-related pancreatic insufficiency and fat malabsorption with pancrelipase MT. J Pediatr Gastroenterol Nutr 2011; 5361–64 [DOI] [PubMed] [Google Scholar]
- 10.Löhr M, Klöppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia 1987; 30757–762 [DOI] [PubMed] [Google Scholar]
- 11.Lankisch PG, Schmidt I, Konig H, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mild to moderate exocrine pancreatic insufficiency. Gut 1998; 42551–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer B, Hoh S, Wehler M, et al. Faecal elastase-1: lyophilization of stool samples prevents false low results in diarrhoea. Scand J Gastroenterol 2001; 36771–774 [DOI] [PubMed] [Google Scholar]
- 13.Dominguez Munoz JE, Iglesias-Garcia J, Vilarino-Insua M, et al. 13C-mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2007; 5484–488 [DOI] [PubMed] [Google Scholar]
- 14.Löhr J-M, Hummel FM, Pirilis KT, et al. Properties of different pancreatin preparations used in pancreatic exocrine insufficiency. Eur J Gastroenterol Hepatol 2009; 211024–1031 [DOI] [PubMed] [Google Scholar]
- 15.Talamini G, Bassi C, Falconi M, et al. Smoking cessation at the clinical onset of chronic pancreatitis and risk of pancreatic calcifications. Pancreas 2007; 35320–326 [DOI] [PubMed] [Google Scholar]
- 16.Ewald N, Kaufmann C, Raspe A, et al. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev 2012; 28338–342 [DOI] [PubMed] [Google Scholar]
- 17.Lindkvist B, Domínguez-Muñoz JE, Luaces-Regueira M, et al. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology 2012; 12305–310 [DOI] [PubMed] [Google Scholar]
- 18.Singh N, Bhardwaj P, Pandey RM, et al. Oxidative stress and antioxidant capacity in patients with chronic pancreatitis with and without diabetes mellitus. Indian J Gastroenterol 2012; 31226–231 [DOI] [PubMed] [Google Scholar]
- 19.Imrie CW, Connett G, Hall RI, et al. Review article: enzyme supplementation in cystic fibrosis, chronic pancreatitis, pancreatic and periampullary cancer. Aliment Pharmacol Ther 2010; 321–25 [DOI] [PubMed] [Google Scholar]
- 20.Graff GR, McNamara J, Royall J, et al. Safety and tolerability of a new formulation of pancrelipase delayed-release capsules (CREON) in children under seven years of age with exocrine pancreatic insufficiency due to cystic fibrosis: an open-label, multicentre, single-treatment-arm study. Clin Drug Investig 2010; 30351–364 [DOI] [PubMed] [Google Scholar]
- 21.Leeds JS, Hopper AD, Hurlstone DP, et al. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Aliment Pharmacol Ther 2006; 25265–271 [DOI] [PubMed] [Google Scholar]
- 22.Leeds JS, Hopper AD, Sidhu R, et al. Some Patients With Irritable Bowel Syndrome May Have Exocrine Pancreatic Insufficiency. Clin Gastroenterol Hepatol 2010; 8433–438 [DOI] [PubMed] [Google Scholar]
- 23.Detlefsen S, Löhr JM, Drewes AM, et al. Current concepts in the diagnosis and treatment of type 1 and type 2 autoimmune pancreatitis. Recent Pat Inflamm Allergy Drug Discov 2011; 5136–149 [DOI] [PubMed] [Google Scholar]
- 24.Kamisawa T, Chari ST, Giday SA, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas 2011; 40809–814 [DOI] [PubMed] [Google Scholar]
- 25.Frulloni L, Scattolini C, Katsotourchi AM, et al. Exocrine and endocrine pancreatic function in 21 patients suffering from autoimmune pancreatitis before and after steroid treatment. Pancreatology 2010; 10129–133 [DOI] [PubMed] [Google Scholar]
- 26.Maire FEDER, Le Baleur Y, Rebours V, et al. Outcome of Patients With Type 1 or 2 Autoimmune Pancreatitis. Am J Gastroenterol 2010; 106151–156 [DOI] [PubMed] [Google Scholar]
- 27.Hardt PD, Hauenschild A, Nalop J, et al. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1,021 diabetic patients. Pancreatology 2003; 3395–402 [DOI] [PubMed] [Google Scholar]