Abstract
Background
The preoperative diagnosis of biliary stenosis is associated with low accuracy. As a consequence, probe-based confocal laser endomicroscopy (pCLE), an in-vivo histological imaging technique, was applied in the bile duct. The aim of this study was to establish whether previous inflammation of the bile duct affects confocal interpretation. The findings from pCLE were compared in two groups of patients: those in whom there had been no cholangitis nor stenting and those in whom stents had been used and subsequently retrieved or who had suffered cholangitis.
Patients and methods
pCLE was performed on 54 patients (mean age 66 years; 31 men, 23 women) from September 2008 to July 2011. Patients were divided in two groups: group 1: 39 patients who had not undergone a biliary procedure in the month preceding the pCLE procedure; and group 2: 15 patients who had undergone stent placement or presented with cholangitis in the month preceding the pCLE procedure. Endoscopic and pCLE data were collected prospectively. pCLE results were compared to benchmark histology (surgery, endoultrasonography, percutaneous biopsy). Patients with a benign stricture who did not undergo operation were followed for 1 year. pCLE images of the bile duct were obtained during endoscopic retrograde cholangiopancreatography procedures. pCLE images were interpreted prospectively using the Miami classification in vivo and in real time.
Results
In group 1, sensitivity, specificity, and accuracy were 88, 83, and 87%, respectively. In group 2, sensitivity, specificity, and accuracy were 75, 71, and 73%, respectively. Diagnostic accuracy of pCLE was lower when applied to group 2 (p < 0,001). The investigation is less reliable in bile ducts affected by inflammation from cholangitis or previous stenting.
Conclusions
Inflammatory lesions of the bile duct interfere with interpretation of pCLE. A refined pCLE description of inflammatory lesions should improve accuracy of pCLE in bile duct stenosis.
Keywords: Biliary stenosis, confocal, endoscopic retrograde cholangiopancreatography, probe-based confocal laser endomicroscopy
Introduction
Biliary strictures can be caused by various inflammatory and neoplastic diseases, both benign and malignant. Presently, the preoperative diagnosis of biliary stenosis, and in particular of cholangiocarcinoma, is associated with low accuracy. Cholangiocarcinoma occurs in the hilar region in about 65% of cases, in the distal common bile duct in 20%, and as an intrahepatic lesion in 15%.1,2
Surgical resection of Klatskin tumours comprises extrahepatic suprapancreatic bile duct resection and hepatic resection combined with resection of the portal vein and caudate lobe, and lymph node dissection. In this approach, mortality rates have been reported to range from 7.5 to 18% and morbidity rates from 19 to 85%.3–7 Hence, a reliable preoperative diagnosis is crucial in deciding to proceed to this type of major surgery.
Because of the low accuracy of preoperative histological diagnosis, there is a risk for patients to undergo potentially unnecessary surgery, due to the inability to confirm whether a stricture is benign or malignant. False-positive preoperative diagnoses of malignancy have been reported in 9–15% of published case series.8–11
Consequently, probe-based confocal laser endomicroscopy (pCLE), an in-vivo histological imaging technique, has been applied in the bile duct with promising results.12–16 In these initial series, all stenoses were enrolled. In order to increase the accuracy of pCLE, we wondered if certain conditions might interfere with the interpretation of the confocal images. The aim of the present study was to compare the performance of pCLE for the characterization of biliary strictures (benign or malignant) when applied before stenting or after stent retrieval or cholangitis. In other words, does inflammation of the bile duct affect confocal laser endomicroscopy interpretation?
Patients and methods
Patients
pCLE was performed in 54 patients (mean age 66 years; 31 men, 23 women) from September 2008 to July 2011. All patients presented with a biliary stenosis with or without symptoms. All patients received at least a computed tomography scan. No histology was available at the time of pCLE.
Endoscopic and pCLE data were collected prospectively. Precise inclusion criteria were based on the criteria for Endomicroscopy intra-ductal (EMID) (Table 1).12 For each pCLE procedure, a diagnosis of benign or malignant stenosis was reported. The aim was to determine if there was a significant difference of accuracy for malignant stenosis according to whether patients had had a stent (or a cholangitis) before pCLE. Patients were divided in two groups: group 1: 39 patients who did not undergo biliary procedure and had not suffered cholangitis in the month preceding the pCLE procedure; and group 2: 15 patients who previously underwent stent placement14 or who presented with cholangitis1 in the month preceding the pCLE procedure (pre-stenting procedure) and with unclear diagnosis. The patient with cholangitis had no fever and no pain at the time of pCLE procedure. These patients had a history of previous endobiliary injections (previous stent) or recent cholangitis and were considered at risk for suffering from inflammation or infection of the bile duct. No primary sclerosing cholangitis stricture was included.
Table 1.
Inclusion and exclusion criteria for EMID study
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patients older than 18 years | No indication of ERCP |
| Patients with indication of ERCP: unclear biliary stenosis | Allergic disease, in particular allergy to fluorescein |
| Patients able to sign informed consent | Pregnant women or potentially pregnant women (no contraception) |
| Patients with health insurance | Kidney or cardiac failure |
| Cirrhosis | |
| Emergency management | |
| Coagulation disorders | |
| Patients unable to sign informed consent |
pCLE results were compared to benchmark histology obtained by surgery, fine needle aspiration, endoultrasonography, and percutaneous biopsy, and the combined pCLE / benchmark histology results (sensibility, specificity, and accuracy) were then compared between groups 1 and 2.
All enrolled patients who had a benign stricture received a 1-year follow up, unless the definitive diagnosis was obtained by surgery. The study was approved by the institutional review board of Marseille University. Signed informed consent was obtained from all patients before inclusion.
pCLE procedure
Systemic antibiotic prophylaxis was administered to all patients (amoxicillin-clavulanic acid 1 g). pCLE images of bile duct were obtained during endoscopic retrograde cholangiopancreatography (ERCP) procedures using an 0.96 mm diameter probe (cholangioFlex probe; Mauna Kea Technologies) inserted in the bile duct through a double lumen catheter (8.5F; Cook-Endoscopy, Winston Salem, NC, USA) after sphincterotomy. The probe was then advanced until the tip was visible under fluoroscopy. Images were obtained by placing the tip of the probe in contact with the mucosa after injection of 2.5 ml of 10% fluorescein.
This probe has a magnification of × 400 and a depth of penetration into the tissue of 40 µm. The lateral resolution is 3.5 µm and the total field of view on a picture is 325 × 325 µm. Intraductal images were recorded in the hard disk of the computer connected to the probe.
pCLE interpretation criteria
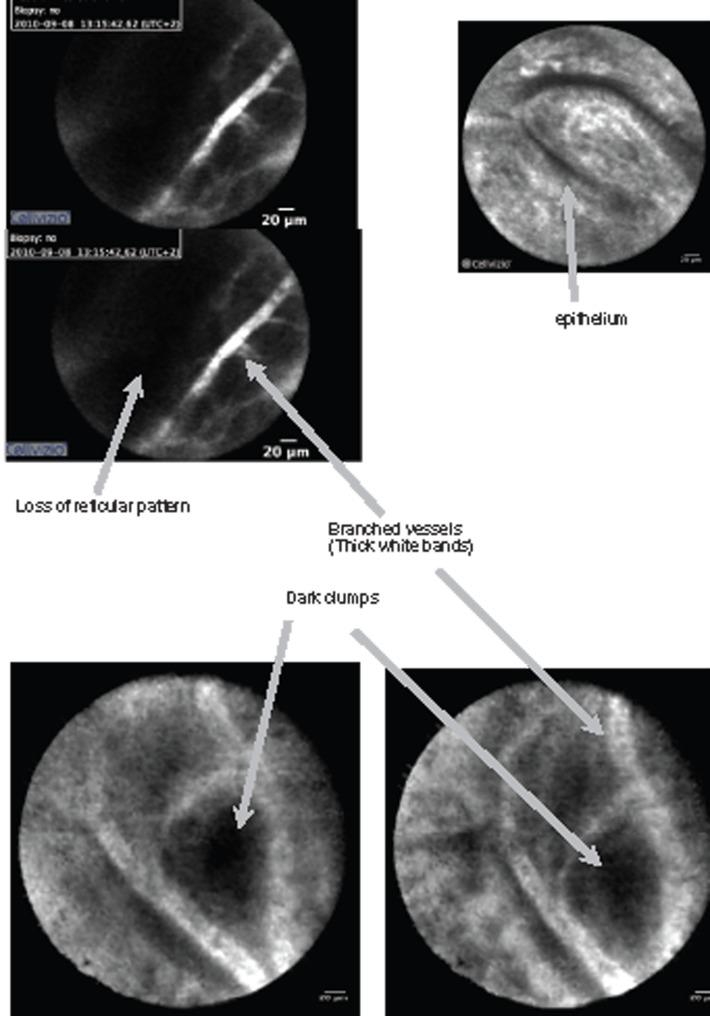
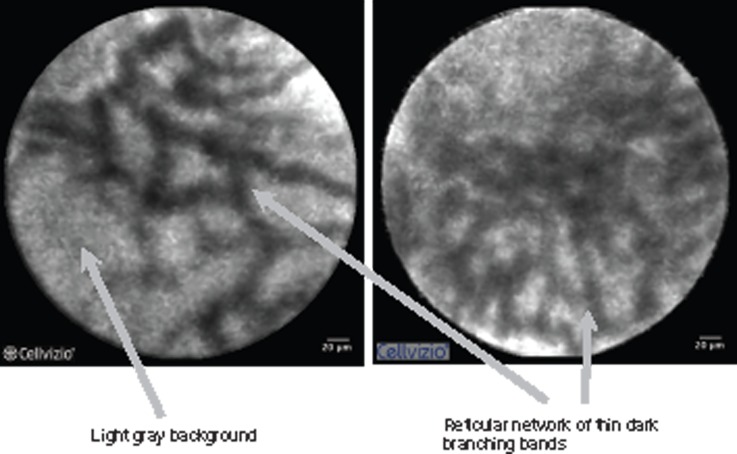
pCLE images were interpreted prospectively using the Miami classification, in vivo and in real time.17 The criteria characteristic of malignancy are listed in Table 2 and are illustrated in Figure 1.18 A normal bile duct is illustrated in Figure 2.
Table 2.
Miami criteria
| Tumoural bile duct | Normal bile duct |
|---|---|
| Thick white bands (>20 µm) | Vessels <20 µm |
| Thick dark bands (>40 µm) | Reticular network of thin dark branching bands (<20 µm) |
| Dark clumps | Light grey background |
| Epithelium |
Figure 1.
Miami criteria.
Figure 2.
Normal bile duct.
Data analysis
Statistical analysis was done using the chi-squared test. p-values < 0.05 were considered statistically significant.
Results
In group 1, benchmark histology showed pancreatic adenocarcinoma in 16 cases, cholangiocarcinoma in six cases, ampulloma in four cases, metastatic lesions (lung, breast, colon) in three cases, lymphoma in two cases, gallbladder carcinoma in one case, hepatocarcinoma in one case and benign lesions in six cases. Histology was obtained by endoultrasonography in 20 cases, by percutaneous biopsy in two cases, by surgery in 12 cases, by endobiliary biopsy in two cases and by a follow up of 1 year in the case of three of the six benign lesions. There were four localizations in the hilum (13%), three localizations in the ampulla of Vater (7%), and 32 in the main bile duct (82%).
In group 2, histology showed cholangiocarcinoma in five cases, ampullary tumour in two cases, pancreatic adenocarcinoma in one case and benign lesions in seven cases. Histology was obtained by endoultrasonography in four cases, by percutaneous biopsy in one case, by surgery in six cases, endobiliary biopsy in one case. Four patients out of seven with benign lesions received a 1-year follow up. There were two localizations in the ampulla of Vater (13%), two in the hilum (13%), and 11 in the main bile duct (73%).
In group 1, sensitivity, specificity, and accuracy of pCLE for the diagnosis of cancer were 88, 83, and 87%, respectively. In group 2, sensitivity, specificity, and accuracy of pCLE were 75, 71, and 73%, respectively. The difference in sensitivity and specificity was not statistically significant. However, the diagnostic accuracy of pCLE was significantly lower when applied to group 2 (p < 0.001). As a result, there is significantly lower accuracy for pCLE in a bile duct with inflammation (due to stent placement or cholangitis before pCLE) (Table 3).
Table 3.
Sensitivity, specificity, and accuracy of probe-based confocal laser endomicroscopy
| Sensitivity | Specificity | Accuracy | |
|---|---|---|---|
| Group 1 | 88 (29/33) | 83 (5/6) | 87 (34/39) |
| Group 2 | 75 (6/8) | 71 (5/7) | 73 (11/15) |
| p-value | 0.71 | 1 | <0.001 |
Values are % (n/total).
Group 1, patients without pre-stenting procedure; group 2, patients with pre-stenting procedure.
In group 1, the sensitivity and specificity were, respectively, for brush cytology 24 and 66% and for endobiliary biopsy 50 and 60% (Table 4). In group 2, sensitivity and specificity were, respectively, for brush cytology 40 and 66%, and for endobiliary biopsy 50 and 83% (Table 5).
Table 4.
Results of sensitivity and specificity for brush cytology, endobiliary biopsy, and pCLE in patients without previous stent placement (group 1) to detect malignant bile duct stricture
| Sensitivity | Specificity | |
|---|---|---|
| Brush cytology | 24 (4/17) | 66 (2/3) |
| Endobiliary biopsy | 50 (7/14) | 60 (3/5) |
| pCLE | 88 | 83 |
Values are % (n/total) or %.
Table 5.
Results of sensitivity and specificity for brush cytology, endobiliary biopsy, and pCLE in patients with pre-stenting procedure (group 2)
| Sensitivity | Specificity | |
|---|---|---|
| Brush cytology | 40 (2/5) | 66 (2/3) |
| Endobiliary biopsy | 50 (4/8) | 83 (5/6) |
| pCLE | 75 | 71 |
Values are % (n/total) or %.
To clarify the role of the stent rather than the different histologies in the lower accuracy of group 2 (with stent), we compared the two groups by subgroup: benign lesions, cholangiocarcinoma and malignant lesions. In groups 1 and 2, respectively, we had six and seven benign lesions, six and five cholangiocarcinoma, and 33 and eight malignant lesions. Accuracy for the different subgroups in groups 1 and 2 were 83 and 71%, 100 and 80%, and 91 and 75%, respectively. In all three subgroups, the accuracy is less high in group 2, although not to a degree that is statistically significant.
Discussion
Reliable preoperative diagnosis is crucial for the management of a potentially malignant biliary stenosis. Magnetic resonance imaging (MRI) can reach a sensitivity and a specificity of 90% in diagnosing biliary obstruction.19–21 Combining improved diagnostic methods, such as thin-section spiral computed tomography, MRI/magnetic resonance cholangiopancreatography, and positron emission tomography, may increase diagnostic accuracy, but still remains insufficiently reliable to differentiate between malignant and benign lesions.22–24 The use of imaging modalities (computed tomography, MRI/magnetic resonance cholangiopancreatography, or endoscopic ultrasound) often fails to distinguish benign conditions causing pancreaticobiliary strictures (such as ischaemia after gall bladder resection or inflammation associated with primary sclerosing cholangitis) from malignant strictures due to cholangiocarcinoma, pancreatic cancer, or metastatic disease.25
Histological confirmation provides only limited diagnostic accuracy, with about 50% sensitivity. The best outcomes are obtained when several sampling techniques are combined, but these remain suboptimal. Sensitivity rates of combining brush cytology with forceps biopsy yielded a sensitivity of 54–71%.26–28 Triple tissue sampling of malignant biliary strictures by ERCP (brush, fine needle aspiration, and biopsy) has been evaluated and has demonstrated a sensitivity of 62% and a specificity of 90% for high grade atypia and cancer.29 In one review of biliary sampling methods, sensitivity of cytology brushing was 30–57%, sensitivity of biopsy 43–81%, and fine needle aspiration sensitivity 26–62%.30,31 In a study comparing the triple tissue sampling method by ERCP, when atypia was excluded, the correct diagnosis of cholangiocarcinoma could only be made in 23% of cases with cytology brushing. When cytology brushing was combined with biopsy and fine needle aspiration, this positive diagnostic yield increased to (only) 47%.29
Fluorescence in-situ hybridization (FISH) uses fluorescently-labelled DNA probes to detect chromosomal abnormalities that may be indicative of neoplasia. Recent evidence has shown that FISH can detect chromosomal abnormalities in the setting of biliary malignancy.32,33 In one prospective study of 66 patients, of whom 39 had cholangiocarcinoma, the sensitivity of cytology was 15% and that of FISH was 34% (p < 0.01). The specificity of FISH was 91% while that of cytology was 98% (p = 0.06).34
Digital image analysis (DIA) uses Feulgen dye and spectrophotometric principles to quantify abnormalities in nuclear DNA and determine the amount of ploidy in the cell. A prospective study comparing DIA and cytology brushing in 110 consecutive patients who underwent ERCP for suspicious biliary strictures demonstrated significantly higher sensitivity of DIA (39.3%) as compared to 17.9% sensitivity for cytology (p = 0,014). However, the specificity profiles were converse, with cytology specificity (97.7%) being superior to that obtained for DIA (77.3%) (p = 0,003).35 FISH, DIA, and composite DIA/FISH are able to predict a malignant diagnosis in 62, 14, and 67%, respectively.36
We report in this study a sensitivity and a specificity of 24 and 66% for brush cytology compared to values of 50 and 66% for endobiliary biopsy (all patients included). Atypia and dysplasia were considered as tumoural. The specificity was not 100% because atypia and dysplasia were considered as tumoural. However, we accepted, following the literature, endobiliary biopsy with carcinoma as having a specificity of 100%.30,31
Cholangiocarcinomas are particularly difficult to diagnose because most grow along the bile duct wall rather than radially to form a mass.37,38 This is probably one of the reasons why pCLE has the potential to provide an efficacious tool for preoperative diagnosis. In our study, we included different proportions of the various aetiologies of bile duct stenosis in two patient groups. This could be considered a major limitation. We did this, however, assuming that the effects of pre-stenting of bile duct stenoses do not depend much on the nature of the stenosis because the main change after pre-stenting affects the normal bile duct Moreover, our subgroup results may mitigate the limitation mentioned above, namely that the proportion of etiologies in the two groups is different.
In the future, the aim of pCLE should be to diagnose biliary stenosis without mass; in other words, a preoperative diagnosis of the sort which is difficult to obtain with only endobiliary histology and MRI (typically Klatskin tumours). We also suggest that the diagnosis of benign and malignant pathology in all cases of biliary stenoses is the first step in the learning curve of this technique. Because pCLE was used in situ during the ERCP examination, it is possible that investigators may have been influenced by the ERCP information as well as by the patient history. However, histopathologists have access to the same clinical data and ERCP findings. Therefore, the results of tissue sampling were obtained in an unblinded manner. Another potential limitation is the obtaining of benchmark histology. We could not exclusively include patients who had undergone surgical resection, and in some cases histology was not obtained at the same time as ERCP but after a mass appeared.
Our results are similar to other studies focusing on pCLE in the bile duct12–14 (Table 6). In the present study, the aim was not to compare our results to those of other teams but to understand how to increase our diagnostic accuracy. Nevertheless, the similarity of our results further support the use of the technique. Moreover, we know that imaging quality is very important to assure a good diagnosis.39 Imaging quality will increase with progress in the technology of the probe.40 The accuracy of pCLE therefore is expected to increase with progress of technologies.
Table 6.
Studies of sensitivity, specificity, and accuracy of probe-based confocal laser endomicroscopy in bile duct
| n | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|
| Meining et al.13 | 14 | 83 | 86 | 86 |
| Giovannini et al.12 | 37 | 83 | 75 | 86 |
| Meining et al.14 | 89 | 98 | 67 | 81 |
| Our study (without/with stent) | 54 | 88/75 | 83/71 | 87/73 |
Values are %.
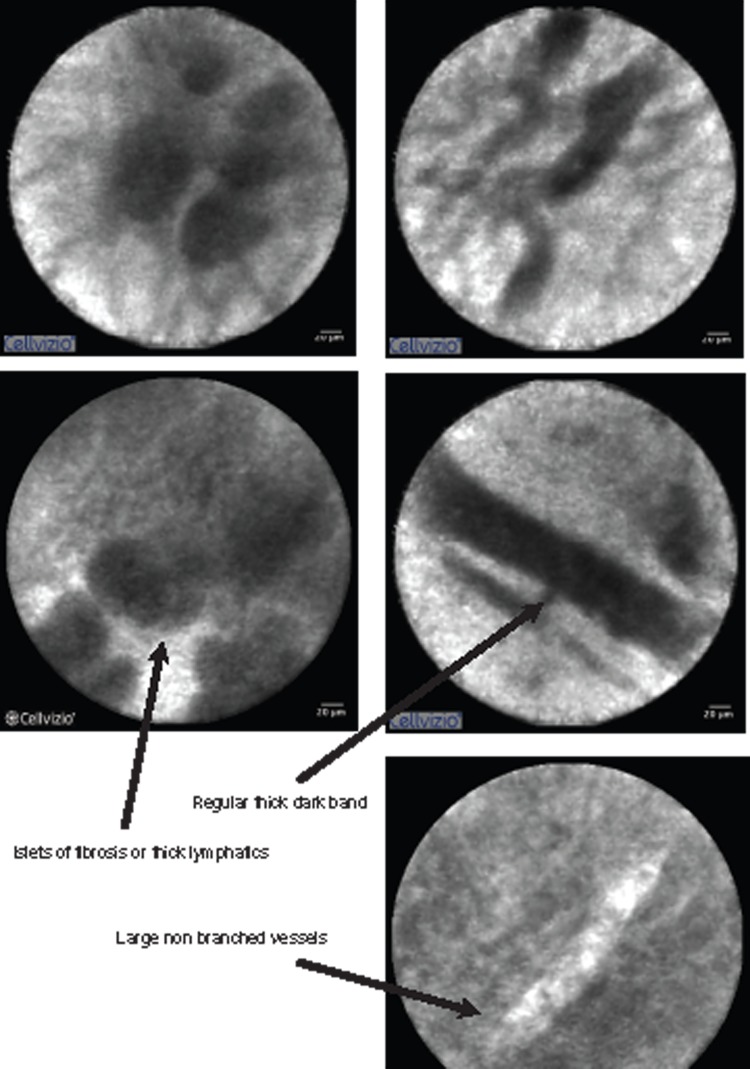
The question arising from this work is: how can we, with the same probe and the same endoscopists, obtain a statistically significant difference in the accuracy of our two groups? Reviewing our pCLE videos, we saw malignant criteria in benign stenosis in group 2. The main malignant criteria visualized was epithelioma. We never saw all malignant criteria in the same group-2 patient with benign stenosis. Nor did we see typical tumoural vessels in benign stenosis of group 2. We have to remember that group 2 should be considered as a group of inflammatory stenoses. We suggest that inflammatory lesions of the bile duct might induce changes in the structure of the bile duct wall and that these changes might be misclassified by pCLE. It is necessary to define the cut off for the number of Miami criteria to establish a clear diagnosis. Refined image interpretation criteria which defines inflammatory criteria needs to be established for this category of stenoses (Figure 3).
Figure 3.
Inflammatory stenosis.
In conclusion, we have confirmed good sensitivity and specificity of pCLE in the bile duct. Pre-stenting procedures interfere with pCLE diagnosis. Accepting this pre-stenting procedure as an equivalent of inflammation of the bile duct, we have found that inflammation interferes with pCLE. We suggest that the pCLE criteria defining inflammation have to be refined to increase the accuracy of the technique.
Acknowledgements
The authors thank Professor Rolfe Birch, Isa Birch, and Nick Birch for their help with the translation.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest
References
- 1.Anderson CD, Pinson CW, Berlin J, et al. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004; 9: 43–57 [DOI] [PubMed] [Google Scholar]
- 2.Fevery J, Verslype C, Lai G, et al. Incidence, diagnosis, and therapy of cholangiocarcinoma in patients with primary sclerosing cholangitis. Dig Dis Sci 2007; 52: 3123–3135 [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki S, Imamura H, Kobayashi A. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg 2003; 238: 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pichlmayr R, Weimann A, Klempnauer J. Surgical treatment in proximal bile duct cancer. A single centre experience. Ann Surg 1996; 224: 628–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuhaus P, Jonas S, Bechstein WO. Extended resections for hilar cholangiocarcinoma. Ann Surg 1999; 230: 808–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Fong Y, DeMatteo RP. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001; 234: 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witzigmann H, Ringel U, Caca K, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma. Ann Surg 2006; 244: 230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verbeek PCM, Van Leeuwen DJ, De Wit LT, et al. Benign fibrosing disease at the hepatic confluence mimicking Klatskin tumors. Surgery 1992; 112: 866–871 [PubMed] [Google Scholar]
- 9.Gerhards MF, Vos P, Van Gulik TM, et al. Incidence of benign lesion in patients resected for suspicious hilar obstruction. Br J Surg 2001; 88: 48–51 [DOI] [PubMed] [Google Scholar]
- 10.Knoefel WT, Prenzel KL, Peiper M, et al. Klatskin mimicking lesions of the biliary tree. EJSO 2003; 29: 658–661 [DOI] [PubMed] [Google Scholar]
- 11.Uhlmann D, Wiedmann M, Schmidt F, et al. management and outcome in patient with Klatskin-mimicking lesions of the biliary tree. J Gastrointest Surg 2006; 10: 1144–1150 [DOI] [PubMed] [Google Scholar]
- 12.Giovannini M, Bories E, Monges G, et al. Results of a phase I-II study on intraductal confocal microscopy (IDCM) in patients with common bile duct (CBD) stenosis. Surg Endosc 2011; 25: 2247–2253 [DOI] [PubMed] [Google Scholar]
- 13.Meining A, Frimberger E, Becker V, et al. Detection of cholangiocarcinoma in vivo using miniprobe-based confocal fluorescence microscopy. Clin Gastroenterol Hepatol 2008; 6: 1057–1060 [DOI] [PubMed] [Google Scholar]
- 14.Meining A, Chen YK, Plewkow, et al. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointest Endosc 2011; 5: 961–968 [DOI] [PubMed] [Google Scholar]
- 15.Lim LG, von Delius S, Meining A. Cholangioscopy and probe-based confocal laser endomicroscopy in the diagnosis of an usual liver cyst. Gastroenterology 2011; 141: e5–e6 [DOI] [PubMed] [Google Scholar]
- 16.Chennat J, Konda V, Madrigal-Hoyos E, et al. Biliary confocal laser endomicroscopy real-time detection of cholangiocarcinoma. Dig Dis Sci 2011; 56: 3701–3706 [DOI] [PubMed] [Google Scholar]
- 17.Chen YK, Pleskow DK, Chuttani R, et al. Miami classification (MC) of probe-based confocal laser endomicroscopy (pCLE) findings in the pancreaticobiliary (PB) system for evaluation of indeterminate strictures: interim results from an international multicentre registry. Gastrointest Endosc 2010; 71: AB134–AB134 [Google Scholar]
- 18.Loeser CS, Robert ME, Mennone A, et al. Confocal endomicroscopic examination of malignant biliary strictures and histologic correlation with lymphatics. J Clin Gastroenterol 2011; 45(3): 246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee WJ, Lim HK, Jang KM, et al. Radiologics spectrum of cholangiocarcinoma: emphasis on unusual manifestations and differential diagnoses. Radiographics 2001; 21: S97–S116 [DOI] [PubMed] [Google Scholar]
- 20.Rosch T, Meining A, Fruhmorgen S, et al. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endosc 2002; 55: 870–876 [DOI] [PubMed] [Google Scholar]
- 21.Materne R, Van Beers BE, Gigot JF, et al. Extrahepatic biliary obstruction: magnetic resonance imaging compared with endoscopic ultrasonography. Endoscopy 2000; 32: 3–9 [DOI] [PubMed] [Google Scholar]
- 22.Feldy A, Vilgrain V, Benys A, et al. Helical CT assessment in hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Am J Roentgenol 1999; 172: 73–77 [DOI] [PubMed] [Google Scholar]
- 23.Park MS, Kim TK, Kim KW, et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign strictures: findings at MRCP versus ERCP. Radiology 2004; 233: 234–240 [DOI] [PubMed] [Google Scholar]
- 24.Yeh BM, Liu PS, Soto JA, et al. MR imaging and CT of the biliary tract. Radiographics 2009; 29: 1669–1688 [DOI] [PubMed] [Google Scholar]
- 25.Bennet JJ, Green RH. Malignant masquerade: dilemmas in diagnosing biliary obstruction. Surg Oncol Clin N Am 2009; 18: 207–214 [DOI] [PubMed] [Google Scholar]
- 26.Rösh T, Hofrichter K, Frimberger E, et al. ERCP or EUS for tissue diagnosis of biliary stricture? A prospective comparative study. Gastrointest Endosc 2004; 60: 390–396 [DOI] [PubMed] [Google Scholar]
- 27.Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc 1995; 42: 565–572 [DOI] [PubMed] [Google Scholar]
- 28.Schöfl R, Haefner M, Wrba F, et al. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand J Gastroenterol 1997; 32: 363–368 [DOI] [PubMed] [Google Scholar]
- 29.Jailwala J, Fogel EL, Sherman S, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc 2000; 51: 383–390 [DOI] [PubMed] [Google Scholar]
- 30.De Bellis M, Sherman S, Fogel EL, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (part 1). Gastrointest Endosc 2002; 56: 552–561 [DOI] [PubMed] [Google Scholar]
- 31.De Bellis M, Sherman S, Fogel EL, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (part 2). Gastrointest Endosc 2002; 56: 720–730 [DOI] [PubMed] [Google Scholar]
- 32.Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancretobiliary strictures. Gastroenterology 2006; 131: 1064–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harewood GC. Endoscopic tissue diagnosis of cholangiocarcinoma. Curr Opin Gastroenterol 2008; 24: 627–630 [DOI] [PubMed] [Google Scholar]
- 34.Kipp BR, Stadheim LM, Halling SA, et al. A comparaison of routine cytology and fluorescence in situ hybridation for the detection of malignant strictures. Am J Gastroenterol 2004; 99: 1675–1681 [DOI] [PubMed] [Google Scholar]
- 35.Baron TH, Harewood GC, Rumalla A, et al. A prospective comparaison of digital image analysis and routine cytology for the identification of malignancy in biliary strictures. Clin Gastroenterol Hepatol 2004; 2: 214–219 [DOI] [PubMed] [Google Scholar]
- 36.Levy MJ, Baron TH, Clayton AC, et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol 2008; 103: 1263–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology 2005; 128: 1655–1667 [DOI] [PubMed] [Google Scholar]
- 38.Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet 2005; 366: 1303–1314 [DOI] [PubMed] [Google Scholar]
- 39.Caillol F, Filoche B, Gaidhane M, Kahaleh M. Refined probe based confocal laser endomicroscopy classification for biliary strictures: The Paris classification. Dig Dis Sci. Epub ahead of print, 12 January 2013DOI: 10.1007/s10620-012-2533-5 [DOI] [PubMed] [Google Scholar]
- 40.Shieh FK, Drumm H, Nathanson MH, et al. High-definition confocal endomicroscopy of the common bile duct. J Clin Gastroenterol 2012; 46: 401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]