Abstract
Background
Some esophageal strictures resist endoscopic treatments. There is a need for new treatments, such as specifically designed stents.
Objective
Our study sought to compare the results achieved with a standard, fully covered metallic stent (FCMS) and those achieved using a stent designed specifically for benign strictures (BS-FCMS).
Patients and methods
The study used a prospective, multicenter, controlled design, with patients recruited from tertiary referral centers. Patients with refractory esophageal strictures were included. Standard FCMS were used in group 1 (N = 24), and BS-FCMS were used in group 2 (N = 17). Patients were followed for 24 months after stent removal. The main outcomes measured were stricture resolution rate, 24 months’ recurrence rate and stent-related morbidity.
Results
Early stent migrations occurred in one (4.1%) patient from group 1 and five (29.4%) from group 2 (p < 0.05). During esophageal stenting, complications occurred in six patients (25%) in group 1 and six patients (35.3%) in group 2 (p = 0.47), respectively. Fifty percent of complications were attributed to migration. There was no procedure-related morbidity associated with the extraction of the stent. The stricture resolution rate was, respectively, 95.2% in group 1 and 87.5% in group 2 (the difference between the two groups is not significant). During follow-up, stricture recurrence occurred in 15/19 patients (group 1, 79%) and 7/8 patients (group 2, 87.5%; p = 1.0). The median time to recurrence of esophageal stricture was 1.7 months (group 1, 0.6–12 months) and 1 month (group 2, 0.1–6 months). Study limitations include its nonrandomized design.
Conclusion
The stricture resolution rate was high at the end of the stenting period for both types of stents without any statistical difference between the two groups, but the long-term results were disappointing, with stricture recurring frequently and rapidly in both groups.
Keywords: Benign esophageal stricture, refractory stricture, fully covered self-expandable metallic stent
Introduction
Benign esophageal strictures are a common endoscopic problem. Endoscopic bougienage and balloon dilation are the most commonly used treatments. However, some strictures, which do not respond to dilation or resume despite repeat sessions, are defined as refractory. Various treatments have been proposed for refractory strictures, such as electrocautery therapy, steroid injections and stent placement.
Stenting can be achieved with either expandable plastic or metallic devices. Plastic stents have been evaluated in several trials, with initially interesting results.1,2 However, further studies have been disappointing, with only 6%–30% of patients experiencing long-term improvement of dysphagia.3,4 Additionally, stent-related morbidity is important, mainly because of frequent stent migrations.3,5 Moreover, the procedure carries significant risks such as bleeding, severe chest pain and even death.3
Metallic stents are commonly used for the palliation of dysphagia due to malignant stricture. Partially and fully covered metallic stents (FCMS) have been developed by stent manufacturers to prevent obstruction due to hyperplasic or neoplastic tissue ingrowth. Covered stents can easily be repositioned after implantation, but after a few weeks, stent removal is often difficult or unsuccessful. Published studies included small numbers of patients and most were retrospective.6–8 More recently, new “benign specific” metallic stents, from here referred to as BS-FCMS, have been designed with the intent to overcome the risk of stent incarceration and to facilitate extraction. Both “standard” FCMS and new BS-FCMS require further evaluation.
The aim of this study was to prospectively compare outcomes in patients bearing severe refractory esophageal strictures after implantation of these two types of covered stents.
Patients and methods
Patients
This study was conducted prospectively in 10 French academic hospitals, under of the aegis of the French Society of Digestive Endoscopy. Patients with histologically proven benign esophageal strictures were considered for temporary placement of esophageal FCMS and included after informed, signed consent was obtained. Inclusion criteria were an esophageal stricture of benign nature beginning at least 2 cm below the Killian’s orifice and recurring after more than three endoscopic dilations of more than 15 mm in diameter during the previous 12 months. Benign esophageal stricture was defined as an esophageal stenosis with a history of gastro-oesophageal reflux, esogastric surgery, caustic ingestion, thoracic radiotherapy or eosinophilic esophagitis with biopsies proving its benign nature. A stricture was considered as clinically relevant if it was deemed responsible for the patient’s dysphagia. Severity of dysphagia was assessed by the score defined by Adam et al. (0 = normal, 1 = solid food possible in limited quantity, 2 = liquids only, 3 = difficulty for liquids ingestion, 4 = aphagia).9
Patients who did not consent to FCMS placement were offered esophageal dilation or plastic stent placement as an alternative, after informed consent was obtained. The study received International Review Board approval by the Comité de Protection des Personnes Ile de France 3.
Study endpoints
The primary study outcome measure was the success of the endoscopic treatment, defined clinically by improvement of dysphagia as assessed by a decreased Adam’s score and morphologically by the resolution or the attenuation of the stricture after removal of the FCMS. Conversely, treatment failure was defined by a persistent stricture at the initial stricture site at the time of FCMS removal, with no improvement of the dysphagia score.
A complete stricture resolution was defined as a straight esophageal lumen with no visible narrowness on either upper endoscopy or contrast opacification.
An attenuated stricture was defined as the persistence of a visible narrowness on either endoscopy or opacification, but reduced by more than 50% with pre-stenting measurement and with no resistance to the passage of a 10 mm endoscope.
Secondary outcomes were the ability to conveniently insert and remove the FCMS, procedure-related morbidity and stricture recurrence during follow-up. A recurrent stricture was defined as both clinical and endoscopic evidence of stricture after an initial success.
Endoscopic protocol and stent group allocation
All procedures were performed under anesthesia by propofol, usually with airway intubation. Upper endoscopy was conducted with a standard gastroscope. During the endoscopic procedure, an esophageal contrast opacification through the scope was performed under fluoroscopy in order to evaluate the length and shape of the stricture. When the stricture was determined to be clinically significant, an FCMS was placed across the stricture so as to allow it to be largely covered by the FCMS. Stricture dilation with a balloon or bougienage prior to stent placement was left to the investigator’s choice. All investigators had prior expertise in esophageal stenting.
Standard FCMS (Hanarostent NES®, diameter: 18–22 mm, length: 80–170 mm, Life Partners Europe, Bagnolet, France), were implanted in a first group (group 1) and left in place for four weeks. In a second group (group 2), a novel, specifically designed stent (Hanarostent EBN®, diameter: 18–20 mm, length: 80–120 mm, Life Partners Europe) was used (referred to here as benign specific “BS-FCMS”) and left in place for three months before removal. Both FCMS and BS-FCMS are made of a single-wire reinforced nitinol mesh with proximal and distal shoulders to prevent migration and gold radiopaque markers. The main difference between the two types of stents lies in the silicone layer. Whereas the standard FCMS has only an internal silicone layer, the coverage of the BS-FCMS is multilayer, both internal and external. Standard FCMS were left in place for four weeks, as is now commonly recommended to avoid stent impaction due to epithelial hyperplasia, which may compromise stent extraction. As BS-FCMS had a thicker silicone layer to prevent tissue granulation, we considered the likelihood of impaction to be lower, allowing for a longer, three months indwelling period. Figure 1a and 1b show an FCMS and a BS-FCMS, respectively. The protocol did not require the use of clips to secure the proximal wire of the stent to the mucosa. Since BS-FCMS were not commercially available at the beginning of the study, randomization was not possible; group allocation was thus sequential, starting with FCMS (group 1) and following with BS-FCMS (group 2).
Figure 1.
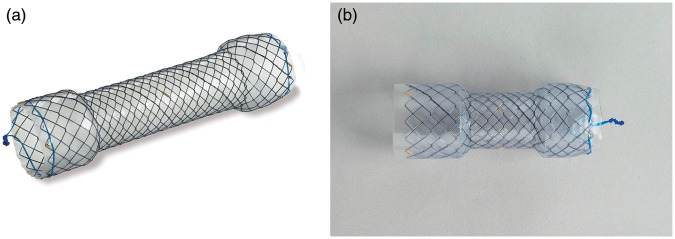
(a) Fully covered metallic stent. (b) Benign specific fully covered metallic stent.
Follow-up–during stenting
Early and late morbidity data were collected prospectively. Early complications were defined as occurring within the first 24 hours after stent placement. Delayed complications were defined as occurring more than 24 hours after stent placement and until the date of stent extraction. The need for stent removal due to chest pain or stent migration was considered as a failure in an intention-to-treat (ITT) perspective. The patients were therefore excluded in these cases. Severe complications were defined as any leading to transfer in an intensive care unit, a surgical operation or the patient’s death. The stenting period corresponded to the period of time that the stent was left in place in the esophagus.
Stents were removed using a rat-tooth forceps, by grasping either the proximal or distal wire of the stent and pulling the endoscope out of the patient after having grasped the stent. No overtube was used for stent removal.
Contrast opacification of the esophagus was performed immediately after FCMS removal: contrast was injected trough the working channel in order to evaluate esophageal patency.
Follow up—after stenting
Patients were followed clinically at 1, 2, 3, 6, 9, 12 and 24 months after stent removal. Dysphagia score, weight, ability to ingest solid food and pain were assessed. One year after stent removal, a consultation, an upper endoscopy and a radiological opacification of the esophagus were performed.
In case of recurrent stricture, patients were offered a new treatment (plastic or a new metallic stent, balloon dilation or surgical treatment), which was left to the investigator’s choice, and follow-up was stopped.
Data collection
Patient characteristics, etiology of the stricture and history of endoscopic procedures before FCMS placement (technique, number and diameter of esophageal dilations, stent placement, steroid injection therapy) were collected before study inclusion. Clinical information (weight, dysphagia score, type of diet, pain) was prospectively collected at the time of upper endoscopies and at 1, 2, 3, 6, 9 and 12 months after stent removal. In the case of stricture recurrence after initial success, the date and type of the treatment undertaken were recorded.
Statistical analysis
It was assumed that standard stents could achieve a 30% stricture resolution rate in the “long term” and we hypothesized that BS-FCMS could achieve a two-fold improvement, with a 60% sustained resolution rate. Under this hypothesis, with a unilateral test, an α-risk of 0.05 and a power of 0.80, the number of patients to include in each group was estimated at 24. Continuous variables were reported as median/mean and range/interquartile range (IQR) and categorical variables as count and percentage. Differences between groups were tested using the Wilcoxon rank-sum or unpaired t-tests for continuous variables, and Fisher’s exact or Chi-square tests for categorical variables, as appropriate. Kaplan-Meier’s curves were plotted to display the survival curves of each group, considering the occurrence of stricture recurrence as the endpoint. Differences in survival between groups were compared by means of the log-rank test. Logistic regression was then performed to assess (1) unadjusted odds ratios (ORs) for migration or recurrence (univariate analysis) and (2) independent risk factors for migration or recurrence (multivariate analysis) using Firth’s bias correction to take into account the small sample size. Predictors associated with migration or recurrence with a p value <0.2 in univariate analysis were entered in the model. Statistical analyses were two-tailed and p < 0.05 was considered to have statistical significance. Analyses were performed using Stata 11.0 (StataCorp LP, TX, USA).
Results
Forty-one patients (27 men, 14 women), aged 62 ± 17, (median 62, range 21–85) were included, with 24 patients belonging to group 1 using standard FCMS, and 17 patients to group 2 using BS-FCMS.
Patients’ demographic and disease characteristics are summarized in Table 1. Overall, median stricture length was 2 (0.2–12) cm, the median stent diameter was 20 (18–22) mm, and the median stent length was 90 mm (80–170). All patients were deemed refractory to standard endoscopic treatment. Table 2 summarizes all endoscopic interventions undertaken in patients included.
Table 1.
Patient characteristics
| Group 1 N = 24 | Group 2 N = 17 | p | |
|---|---|---|---|
| Females N (%) | 7 (29.2%) | 7 (41.2%) | 0.42 |
| Age (years) median (IQR); | 62 (50–75); | 61 (60–74); | 0.65 |
| Mean age (range) | 59.7 (21–83) | 65 (40–85) | |
| Etiology of esophageal stricture | N (%) | N (%) | 0.89 |
| Peptic | 10 (41.7%) | 6 (35.3%) | |
| Post-surgical | 6 (25%) | 6 (35.3%) | |
| Post-radiation | 5 (20.8%) | 3 (17.6%) | |
| Caustic ingestion | 2 (8.3%) | 1 (5.9%) | |
| Ischemic | 0 (0%) | 1 (5.9%) | |
| Eosinophilic esophagitis | 1 (4.2%) | 0 (0%) | |
| Stricture length: median (range) in cm | 2 (0.5–6) | 2 (0.2–12) | 0.65 |
IQR: interquartile range.
Table 2.
Endoscopic treatments
| Endoscopic treatments prior to inclusion | Group 1 N = 24 | Group 2 N = 17 | p |
|---|---|---|---|
| No. dilation sessions: median (range) | 4 (3–17) | 4 (3–8) | 0.26 |
| Max. diameter achieved by dilation: median (range) | 15 (15–18) | 15 (15–20) | 0.69 |
| Dilation technique | 0.03 | ||
| Balloon dilation N (%) | 15 (62.5%) | 11 (64.7%) | |
| Bougienage N (%) | 7 (29.2%) | 2 (11.8%) | |
| Balloon + bougienage n (%) | 2 (8.3%) | 0 (0%) | |
| Bougienage + stent | 0 (0%) | 4 (23.5%) | |
| Endoscopic treatment after the inclusion | |||
| Dilation before stent placement N (%) | 10 (41.7%) | 11 (64.7%) | 0.15 |
| Stent length: median (range) | 90 (80–170) | 80 (80–120) | 0.02 |
| Stent diameter: median (range) | 18 (18–22) | 20 (18–20) | 0.97 |
Complications that occurred after FCMS placement are shown in Table 3. Early complications (within 24 h) occurred in 1 (4.1%) and 6 (35.3%) patients in group 1 and group 2, respectively (p = 0.01), consisting mostly of stent migrations (group 1, N = 1 [4.1%], group 2, N = 5 [29.4%]; p = 0.07). Early migrations were observed in peptic strictures: four of six patients (66.7%), as against one patient each for anastomotic and caustic strictures. Pre-stenting dilations were performed in five of six patients with early stent migration (83.3%), as against 17 of 35 of those with no migration (48.6%), but this difference did not reach statistical significance (p = 0.19). One patient from group 2 had vomiting for three days following BS-FCMS placement. Symptoms resolved with medical therapy.
Table 3.
Complications after FCMS placement
| Group 1 N = 24 (%) | Group 2 N = 17 (%) | Management | p | |
|---|---|---|---|---|
| Early complications (<24 h)* | 1 (4.1%) | 6 (35.3%) | 0.01 | |
| Stent migration | 1 (4.1%) | 5 (29.4%) | Stent exchanged N = 2 | 0.07 |
| Stent repositioned N = 1 | ||||
| Balloon dilation N = 1 | ||||
| Observation N = 2 | ||||
| Vomiting | 0 (0%) | 1 (5.9%) | ConservativeN = 1 | 0.41 |
| Delayed complications | 6 (25%) | 6 (35.3%) | 0.51 | |
| Stent migration | 2 (8.2%) | 4 (23.5%) | Stent repositioned N = 1 | 0.21 |
| Bougienage N = 1 | ||||
| Balloon dilation N = 1 | ||||
| Stent exchanged N = 1 | ||||
| Observation N = 2 | ||||
| Pain | 4 (16.7%) | 1 (5.9%) | Stent extraction N = 2 | 0.38 |
| Stent repositioned N = 1 | ||||
| Stent exchanged N = 1 | ||||
| Conservative N = 1 | ||||
| Pneumonia | 0 (0%) | 1 (5.9%) | Conservative N = 1 | 0.41 |
p value <0.05.
Delayed complications occurred in six (25%) and six (35.3%) patients in group 1 and group 2, respectively (p = 0.47), with mainly stent migrations in both groups (group 1, N = 2 [8.2%], group 2, N = 4 [23.5%]; p = 0.21). Stricture length in patients with delayed migrations was short, at 0.75 cm (0.2–3), and etiology was evenly distributed (peptic N = 2 [33.3%]; anastomotic N = 2 [33.3%]; caustic, post-radiation and due to an eosinophilic esophagitis in one case each [16.7%]). Three of six patients (50%) whose stents migrated, as against 19 of 35 patients (54.3%) whose stents did not migrate, had undergone pre-stenting dilation (p = 1.0). Considering the overall stent migration rate (early and delayed), there were more stent migrations in group 2 than in group 1 (N = 9 [52.9%] vs. N = 3 [12.5%]; p = 0.005), but no statistical difference in stricture length, stent length or diameter between patients with or without stent migration. Chest pain during stenting was the second-most frequent complication (group 1 N = 4 [16.7%], group 2 N = 1 [5.9%]; p = 0.38). One patient (5.9%) in group 2 developed pneumonia following the endoscopic procedure.
The median duration of stenting was 27 (20–80) and 90 (80–140) days in group 1 and group 2, respectively. Three (12.5%) and nine (52.9%) patients were excluded from, respectively, group 1 and group 2, during stenting because of the need for stent removal (due to chest pain, N = 3, or stent migration, N = 6), the late development of a malignant stricture (N = 1) or loss to follow-up (N = 2).
Extraction of the stent was possible in 100% of patients within less than 10 minutes, with no procedure-related morbidity. None of the removed stents presented structural damage such as metal mesh fracture or covering decay.
Table 4 summarizes the outcome and management of esophageal strictures after stenting. During the extraction procedure, upper endoscopy showed a persistent stricture in one (4.7%) patient in group 1 and one (12.5%) patient in group 2 (p = 0.48). The stricture was treated immediately by bougienage (N = 1) or stent exchange (N = 1). One (12.5%) patient presented an attenuated stricture, and 26 patients (group 1, N = 20 [95.2%], group 2, N = 6 [75%]) had complete stricture resolution. Twenty (83.3%) and seven (41.1%) patients, respectively, in group 1 and group 2, had initial success with the endoscopic procedure from an ITT perspective (p = 0.005).
Table 4.
Outcome at the end of stenting perioda
| Upper endoscopy findings | Group 1 N (%) | Group 2 N (%) | p | Management |
|---|---|---|---|---|
| Persistent stricture | 1/21 (4.7%) | 1/8 (12.5%) | 0.48 | Bougienage N = 1 |
| Stent exchanged N = 1 | ||||
| Attenuated stricture | 0/21 (0%) | 1/8 (12.5%) | 0.28 | Observation |
| No stricture | 20/21 (95.2%) | 6/8 (75%) | 0.18 | Observation |
| Initial success | 20/21 (95.2%) | 7/8 (87.5%) | 0.48 | |
| Initial success from an ITT perspective | 20/24 (83.3%) | 7/17 (41.1%) | 0.005 |
Follow-up was stopped in case of stent extraction or late diagnostic of malignant stricture.
ITT = intention to treat.
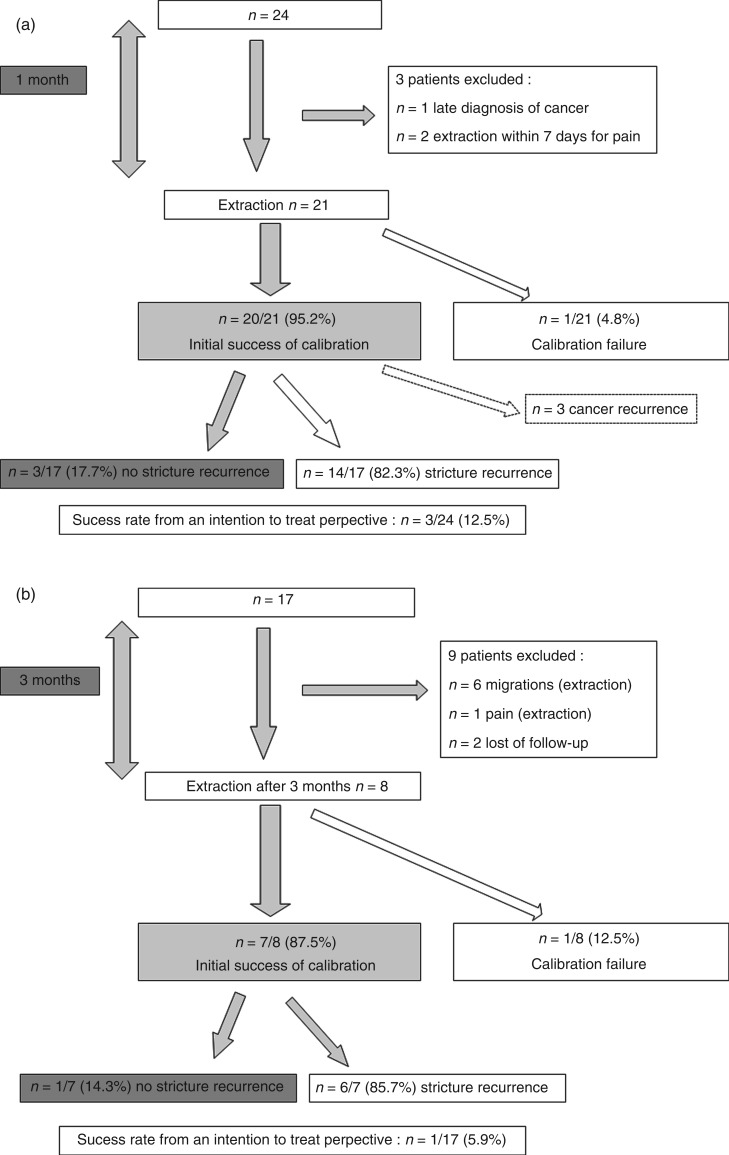
Follow-up data are displayed as flow charts in Figure 2a (group 1) and 2b (group 2). During follow-up, benign stricture recurrence occurred in 14 (group 1, 82.3%) and 6 (group 2, 85.7%) of the remaining patients. The median time to recurrence of esophageal stricture was 1.6 (0.6–12) and 1.6 (0.1–4.5) months in group 1 and group 2, respectively. Success rates from an ITT perspective were therefore 3/24 (12.5%) in group 1 and 1/17 (5.9%) in group 2 (p = 0.63).
Figure 2.
(a) Flow chart representing follow-up data for group 1. (b) Flow chart representing follow-up data for group 2.
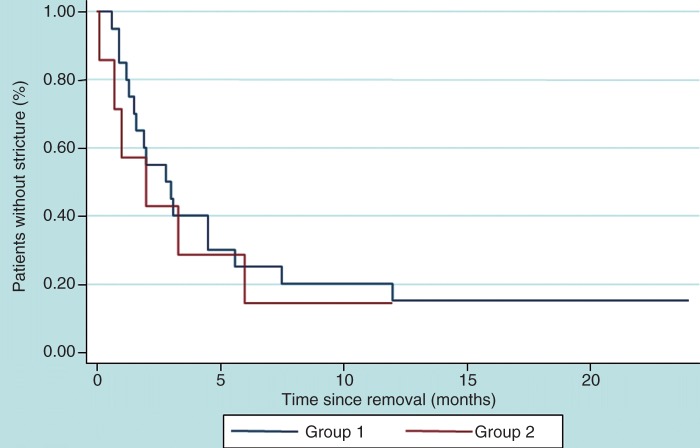
Figure 3 displays Kaplan-Meier’s plots for survival without stricture recurrence in the two groups. No significant association was found between recurrence-free survival and the group, sex or age, whereas recurrence-free survival was found to be decreased in anastomotic-related strictures versus other causes of esophageal stricture (p = 0.006).
Figure 3.
Kaplan-Meier plots for survival without stricture recurrence in the two study groups.
Tables 5 and 6 display logistic regression analysis assessing unadjusted ORs for migration and recurrence (univariate analysis). Patients in group 2 with a BS-FCMS had higher risk of stent migration compared to group 1 (OR = 6.87; confidence interval [CI] 95[1.59–29.57]). In multivariate analysis, the BS-FCMS remained significantly associated with migration (p = 0.023) after adjusting for the number of dilation sessions and the maximum diameter achieved by dilation prior to study inclusion. No significant risk factor for recurrence was clearly identified, although a trend was observed concerning stent length and diameter: the longer and larger the stent was, the less promptly the stricture had recurred.
Table 5.
Logistic regression assessing unadjusted ORs for migration (univariate analysis)
| Migration | |||||
|---|---|---|---|---|---|
| Unadjusted OR | p value | CI 95% | |||
| Group | Group 1 | 1 (Ref) | |||
| Group 2 | 6.87 | 0.010 | 1.59 | 29.57 | |
| Gender | Men | 1 (Ref) | |||
| Women | 0.98 | 0.981 | 0.25 | 3.86 | |
| Aetiology of oesophageal stricture | Peptic | 1 (Ref) | |||
| Anastomotic | 0.60 | 0.517 | 0.12 | 2.86 | |
| Others | 0.54 | 0.435 | 0.11 | 2.55 | |
| Dilation before stent placement | No | 1 (Ref) | |||
| Yes | 1.89 | 0.358 | 0.49 | 7.32 | |
| No. dilation sessions | 0.67 | 0.134 | 0.40 | 1.13 | |
| Age | 1.00 | 0.965 | 0.96 | 1.04 | |
| Stricture length | 0.95 | 0.713 | 0.74 | 1.23 | |
| Stent length | 1.00 | 0.884 | 0.97 | 1.03 | |
| Stent diameter | 0.92 | 0.720 | 0.60 | 1.42 | |
| Max. diameter achieved by dilation | 0.64 | 0.171 | 0.34 | 1.21 | |
OR = odds ratio. CI = confidence interval.
Table 6.
Logistic regression assessing unadjusted ORs for recurrence from an ITT perspective (univariate analysis)
| Recurrence | |||||
|---|---|---|---|---|---|
| Unadjusted OR | p value | CI 95% | |||
| Group | Group 1 | 1 (ref) | |||
| Group 2 | 1.79 | 0.571 | 0.24 | 13.46 | |
| Gender | Men | ||||
| Women | 0.49 | 0.457 | 0.07 | 3.21 | |
| Aetiology of esophageal stricture | Peptic | ||||
| Anastomotic | 9.00 | 0.155 | 0.44 | 185.36 | |
| Others | 9.72 | 0.140 | 0.47 | 199.44 | |
| Dilation before stent placement | No | ||||
| Yes | 1.24 | 0.820 | 0.19 | 8.05 | |
| No. dilation sessions | 0.92 | 0.477 | 0.72 | 1.17 | |
| Age | 1.01 | 0.630 | 0.96 | 1.07 | |
| Stricture length | 0.85 | 0.318 | 0.63 | 1.16 | |
| Stent length | 0.97 | 0.065 | 0.93 | 1.00 | |
| Stent diameter | 0.48 | 0.074 | 0.21 | 1.07 | |
| Max diameter achieved by dilation | 2.26 | 0.323 | 0.45 | 11.34 | |
OR = odds ratio. ITT = intention to treat. CI = confidence interval.
Discussion
Approximately 30% of esophageal benign strictures can be deemed refractory, because they exhibit a very high propensity to recur after any kind of mechanical treatment.10 This is usually due to a long-standing process of deep and thick fibrosis, which is generally irreversible, whatever the trigger to initial inflammation—peptic, post-surgical, caustic or otherwise. Corticosteroids have shown limited efficacy in one randomized trial,11 but mechanical methods are more widely used. Once repeat dilations have failed, stenting is an attractive alternative, provided that stents can be safely removed. Plastic stents have been evaluated in several trials, with disappointing results in terms of clinical success, with only 6%–30% long-term symptom improvement.3,4 Complications were frequent and consisted mainly of migrations (22%–64.2%), chest pain (11%), bleeding (8%) and perforations (5.5%).3 One death from massive bleeding was reported by Dua et al.3
Data concerning metal stents are sparse, with published studies including only small numbers of patients6–8 or patients with particular lesions, such as benign hypopharyngeal strictures6 or achalasia.7 Song et al. included 25 patients with common benign esophageal strictures such as caustic ingestion, reflux esophagitis and radiation fibrosis.8 The use of a “Z stent”, a precursor of current metal stents, was encouraging, with a stricture recurrence in only one of two patients, but dilation up to 10 mm was not always performed before stent placement, suggesting that the strictures were not as severe as in other studies. In a meta-analysis of eight studies on the use of expansive plastic and metallic stents, Thomas et al. reported a 46% improvement rate in dysphagia.12 As was recognized by the authors, the pooled outcome of all the included studies can be biased to the heterogeneity of the cohort population, in terms not only of stricture etiology but also stricture length and location, definition of dysphagia improvement, the retrospective nature of some studies, time to stent removal and the duration of follow-up. Our study, as a multicenter, prospective one, may overcome some of these shortcomings, although it is clearly limited by its nonrandomized design and its small sample size. A summary of published data is shown in Table 7.5,13–15
Table 7.
Summary of published results of stenting with covered metallic stents for refractory esophageal benign strictures
| Author | Publication year | Stent placed, N | Mean age, years | Follow-up, weeks | Long term success, % of patients | Migration, N (%) |
|---|---|---|---|---|---|---|
| Kim et al.13 | 2009 | 55 | 49 | 152 (mean) | 31 | 14 (25.4) |
| Oh et al.5 | 2010 | 13 | 66 | NA | 23 | 4 (30.8) |
| Eloubeidi et al.14 | 2011 | 19 | 61 | 23+/−15 (mean) | 21% | 34% |
| Hirdes et al.15 | 2012 | 15 | 61 | 13 (median) | 0% | 60% (migration and intolerance) |
| Chaput et al. (present study) | Total = 41 | Total = 61.9 | 104 (median) | 18.5 | 12 (29.2) | |
| Group 1 = 24 | Group 1 = 59.7 | 21 | 3 (12.5) | |||
| Group 2 = 17 | Group 2 = 65 | 12.5 | 9 (52.9) |
NA = data not available.
The definition of a refractory stricture is not uniform in previously published studies and remains controversial. In the present work, we defined such cases as strictures recurring after more than three endoscopic dilations of more than 15 mm during the previous 12 months, because it was consensual among the study investigators and seemed to fit with the actual practice of many gastroenterologists, who consider switching from dilations to an alternative therapy above this threshold.
Nevertheless, our results are at least equally disappointing, with a failure to achieve a sustainable symptomatic improvement and with stricture recurrence rates exceeding 80% after stent removal, within a median time of 1.6 months from stenting. Although the sample size target was not reached in one group, the relative lack of power was unlikely to affect the final conclusions.
The major complication observed was stent migration. Migration is an important factor in treatment failure. We did not undertake a multivariate analysis of predictive factors for success or failure because of an insufficient number of events. It is noteworthy that none of the factors analyzed in the meta-analysis of 199 patients by Thomas et al. (sex, patient age, corrosive etiology, stricture location, stricture length, time of removal and duration of follow-up) had any significant influence on outcomes.12 We also observed that the length of stricture did not seem to play a role, as it varied from 0.2 to 8 cm (median 2 cm) in patients experiencing migration. However, we noted that early migrations occurred more often in cases of peptic strictures of the lower esophagus.
Performing dilation before stenting could facilitate early stent migration, with a trend toward a higher migration rate in patients with pre-stenting dilation (83.3% vs. 48.6%), but the difference was not statistically significant (p = 0.19). In the study by Holm et al., a similarly nonsignificant trend was observed, with a migration rate of 69.7% in patients with dilation before stenting as against 53.3% when no dilation was required.4 The higher migration rate in group 2 is probably due in part to the presence of a thicker silicone cover, not only internal but also external, which efficiently prevents the stent from sticking or incarcerating in the esophageal wall but also makes it more slippery. The diameter and length of the stent did not seem to influence the likelihood of migrations, since these occurred also with the largest stents, with an inner diameter of 22 mm, as well as the longest stents. We did not use clips to stitch the stents to the mucosa, because published evidence for this technique is limited.16 No other internal (i.e. embedded in the stent design) or external “anti-migration system” has been proven to date.
The time from implantation to removal is another potentially important factor of outcome. In the previously quoted meta-analysis, time to removal was not statistically significant, but a trend was observable in favor of a longer stenting period.12 A longer remodeling period might indeed improve the initial success of the endoscopic treatment, but optimal duration depends probably on the cause, morphology and biological nature of the stricture. In particular, the content in collagen and the presence of an ongoing inflammatory process can weigh heavily on the refractory pattern. Since it can be unsafe to leave stents in place more than six to eight weeks, due to the likelihood of hyperproliferation with stent impaction,17 a sequential stent placement, such as for biliary strictures, could also be considered in some cases.
Conclusion
Despite a positive outcome at the end of the stenting period, both types of stents in this study were equally disappointing in refractory benign esophageal strictures. The safety of insertion and removal of both standard FCMS and specific BS stents is confirmed, but none of these stents appears to be a satisfactory treatment of this condition. However, it is noteworthy that no severe complication was observed with BS stents, which remained in place for several months. The BS stent could possibly help improve esophageal remodeling provided that a modified design substantially reduces the rate of stent migration. The fact that no tissue overgrowth is observed,15 makes it possible to leave the stent in place for several months and exchange it easily, contrary to other stents. Stents remain an interesting alternative to surgery, particularly for fragile patients, but improvements in stent design and anti-inflammatory properties are needed to recommend an extension of their use.
Study support
This study was done under the aegis of the French Society of Digestive Endoscopy.
References
- 1.Evrard S, Le Moine O, Lazaraki G, et al. Self-expanding plastic stents for benign esophageal lesions. Gastrointest Endosc 2004; 60(6): 894–900 [DOI] [PubMed] [Google Scholar]
- 2.Repici A, Conio M, De Angelis C, et al. Temporary placement of an expandable polyester silicone-covered stent for treatment of refractory benign esophageal strictures. Gastrointest Endosc 2004; 60(4): 513–519 [DOI] [PubMed] [Google Scholar]
- 3.Dua KS, Vleggaar FP, Santharam R, et al. Removable self-expanding plastic esophageal stent as a continuous, non-permanent dilator in treating refractory benign esophageal strictures: a prospective two-center study. Am J Gastroenterol 2008; 103(12): 2988–2994 [DOI] [PubMed] [Google Scholar]
- 4.Holm AN, de la Mora Levy JG, Gostout CJ, et al. Self-expanding plastic stents in treatment of benign esophageal conditions. Gastrointest Endosc 2008; 67(1): 20–25 [DOI] [PubMed] [Google Scholar]
- 5.Oh YS, Kochman ML, Ahmad NA, et al. Clinical outcomes after self-expanding plastic stent placement for refractory benign esophageal strictures. Dig Dis Sci 2010; 55(5): 1344–1348 [DOI] [PubMed] [Google Scholar]
- 6.Conio M, Blanchi S, Filiberti R, et al. A modified self-expanding Niti-S stent for the management of benign hypopharyngeal strictures. Gastrointest Endosc 2007; 65(4): 714–720 [DOI] [PubMed] [Google Scholar]
- 7.De Palma GD, lovino P, Masone S, et al. Self-expanding metal stents for endoscopic treatment of esophageal achalasia unresponsive to conventional treatments. Long-term results in eight patients. Endoscopy 2001; 33(12): 1027–1030 [DOI] [PubMed] [Google Scholar]
- 8.Song HY, Jung HY, Park SI, et al. Covered retrievable expandable nitinol stents in patients with benign esophageal strictures: initial experience. Radiology 2000; 217(2): 551–557 [DOI] [PubMed] [Google Scholar]
- 9.Adam A, Ellul J, Watkinson AF, et al. Palliation of inoperable esophageal carcinoma: a prospective randomized trial of laser therapy and stent placement. Radiology 1997; 202(2): 344–348 [DOI] [PubMed] [Google Scholar]
- 10.Pereira-Lima JC, Ramires RP, Zamin I, Jr, et al. Endoscopic dilation of benign esophageal strictures: report on 1043 procedures. Am J Gastroenterol 1999; 94(6): 1497–1501 [DOI] [PubMed] [Google Scholar]
- 11.Ramage JI, Jr, Rumalla A, Baron TH, et al. A prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection therapy for recalcitrant esophageal peptic strictures. Am J Gastroenterol 2005; 100(11): 2419–2425 [DOI] [PubMed] [Google Scholar]
- 12.Thomas T, Abrams KR, Subramanian V, et al. Esophageal stents for benign refractory strictures: a meta-analysis. Endoscopy 2011; 43(5): 386–393 [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Song HY, Choi EK, et al. Temporary metallic stent placement in the treatment of refractory benign esophageal strictures: results and factors associated with outcome in 55 patients. Eur Radiol 2009; 19: 384–390 [DOI] [PubMed] [Google Scholar]
- 14.Eloubeidi MA, Talreja JP, Lopes TL, et al. Success and complications associated with placement of fully covered removable self-expandable metal stents for benign esophageal diseases (with videos). Gastrointest Endosc 2011; 73(4): 673–681 [DOI] [PubMed] [Google Scholar]
- 15.Hirdes MM, Siersema PD, Vleggaar FP. A new fully covered metal stent for the treatment of benign and malignant dysphagia: a prospective follow-up study. Gastrointest Endosc 2012; 75(4): 712–718 [DOI] [PubMed] [Google Scholar]
- 16.Sriram PV, Das G, Rao GV, et al. Another novel use of endoscopic clipping: to anchor an esophageal endoprosthesis. Endoscopy 2001; 33(8): 724–726 [DOI] [PubMed] [Google Scholar]
- 17.Siersema PD, de Wijkerslooth LR. Dilation of refractory benign esophageal strictures. Gastrointest Endosc 2009; 70(5): 1000–1012 [DOI] [PubMed] [Google Scholar]