Abstract
Molecular imaging focuses on the molecular signature of cells rather than morphological changes in the tissue. The need for this novel type of imaging arises from the often difficult detection and characterization especially of small and/or premalignant lesions. Molecular imaging specifically visualizes biological properties of a lesion and might thereby be able to close diagnostic gaps, e.g. when differentiating hyperplastic from neoplastic polyps or detecting the margins of intraepithelial neoplastic spread. Additionally, not only the detection and discrimination of lesions could be improved: based on the molecular features identified using molecular imaging, therapy regimens could be adjusted on the day of diagnosis to allow for personalized medicine and optimized care for each individual patient.
Keywords: Colorectal carcinoma, confocal laser endomicroscopy, endoscopy, gastric cancer, molecular imaging
Introduction
Molecular imaging is a newly emerging discipline in medicine and its combination with gastrointestinal endoscopy offers decisive insights into the molecular blueprint of tissues during an ongoing examination. Especially regarding the development of targeted therapies such as monoclonal antibodies, the so-far purely morphological view on macroscopic features of a lesion can be supported by molecular imaging to be able to initiate appropriate, individualized therapy for each patient. Disease-specific molecular alterations have to be taken into account, and therapies should be started early − at best even before substantial morphological changes occur. It is therefore a self-evident goal to improve diagnostics in order to facilitate the detection of precursor lesions with few macroscopic or histomorphological changes.
Molecular imaging employs a targeted approach to visualize cells based on their molecular signature. Therefore, in contrast to conventional imaging, molecular imaging does not rely on morphological changes for diagnostic assessment. Small molecular alterations taking place early in aetiopathogenesis may suffice for visualization, potentially allowing for much earlier detection of premalignant lesions or inflammation. At the same time, tissues can be analysed for specific molecular features and biomarkers, with potential implications on the choice of targeted therapies.
The systems currently used for molecular imaging mostly rely on fluorescence, as this approach allows for the radiation-free detection and amplification of molecular alterations in the tissue. Exogenous fluorescence is established by administering fluorescent dyes consisting of molecular probes that bind specifically to molecular targets (Figure 1).
Figure 1.
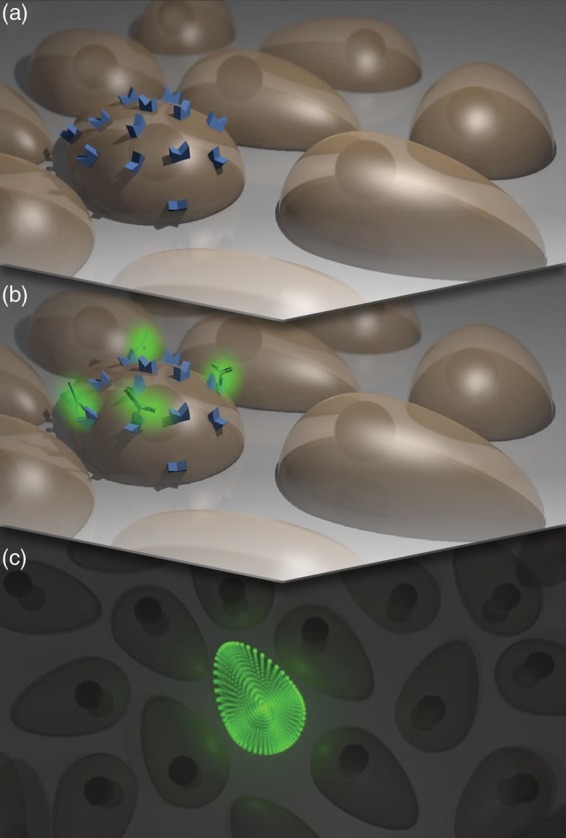
Molecular imaging. A cell shows specific molecular characteristics (a), distinguishing itself from other cells in the tissue. The target structures are located on the cell membrane and thereby easily reachable by extracellular agents. After binding of a fluorescently labelled molecular probe, e.g. an antibody (b), the cell can be fluorescently visualized due to its molecular signature (c).
In a clinical setting, a two-step approach to molecular imaging would be ideal. Macroscopic molecular imaging modalities such as fluorescence imaging using wide-field endoscopes could serve as red flag techniques to rapidly screen large parts of the mucosal wall for suspicious areas. Microscopic techniques could then follow for further characterization and visualization of molecular probes bound to their targets on a subcellular level.
Techniques
For macroscopic molecular imaging, fluorescence wide-field endoscopes can be used. At this time, most groups employ customized or experimental endoscopes.1–6 In the future, wide-field endoscopes with detection and excitation filters for different wavelengths (comparable to narrow-band imaging devices) could be used to combine or overlay fluorescence endoscopy with high-resolution white-light endoscopy images. This could serve as a red flag technique for the detection of suspicious lesions.
For microscopic molecular imaging, confocal laser endomicroscopy (CLE) has been studied. It enables the endoscopist to examine the tissue under high magnification during an ongoing endoscopic examination, with a high spatial resolution sufficient to visualize subcellular details. Two CLE systems are currently in clinical use.7 One system consists of a built-in device in the tip of an endoscope (eCLE) (Pentax, Tokyo, Japan; Optiscan, Notting Hill, Australia). It offers high spatial resolution and variable imaging plane depth from surface to 250 µm within the tissue. However, this comes at the price of a lower temporal resolution of approximately 1 frame per second. A second system currently in use is probe based and fits through the working channel of conventional white-light endoscopes (pCLE) (Mauna Kea Technologies, Paris, France). It provides faster image acquisition with a rate of 12 frames per second and allows for ad-hoc CLE. However, the spatial resolution is lower and the field of view is smaller. Besides, probes must be swapped entirely to move the depth of the imaging plane.8 Both systems use blue laser light for excitation and detect emission at wavelengths >505 nm. In conventional CLE, unspecific dyes such as fluorescein are used to visualize the histomorphological features of the tissue, comparable to haematoxylin and eosin staining in pathology. By employing molecularly targeted fluorescent agents, molecular CLE becomes feasible and cells can be visualized based on their molecular signature, comparable to immunohistochemistry.
Probes
In principle, a variety of wavelengths could be used for molecular imaging. Most studies using molecular imaging for macroscopic detection of lesions have favoured (near-infra)red wavelengths. However, most microscopic devices are currently restricted to excitation and emission wavelengths around 500 nm, limiting fluorophore options. Infrared or near-infrared wavelengths would allow for increased imaging plane depths due to less scattering in the tissue and reduced absorption by physiological substances such as haemoglobin. This was already tested for an excitation wavelength of 780 nm using a prototype near-infrared eCLE device (Optiscan) in a preclinical study.9 For pCLE, a laser scanning unit with an excitation wavelength of 660 nm is available (Mauna Kea Technologies). Multicolour imaging could in perspective allow for the simultaneous visualization of several molecular dyes or molecular imaging on a background of morphological data.
With the availability of fluorescence labelling, fluorophores such as the fluorescein-derived fluorescein isothiocyanate (FITC) or AlexaFluor dyes can be conjugated with a variety of different agents to be used as probes for molecular CLE. Antibodies, affinity peptides, activatable probes, physiological substances, and nanoparticles can be employed for molecular imaging (Table 1).10
Table 1.
Agents for molecular CLE
| Class | Pros | Cons | Use |
|---|---|---|---|
| Antibodies1,11,27,28,30–33 | Highly specific | Slow distribution | Animals and humans |
| Target known | Risk of allergic reactions | ||
| Drug visualization | |||
| Affinity peptides5,6,12 | Fast distribution | Variable affinity | Animals and humans |
| Low immunogenicity | Target unclear | ||
| Activatable probes2,15,25 | Specific activation | Difficult delivery, internalization required | Animals |
| Visualization of proteasome | Safety | ||
| Physiological substances17,19,23,26 | (Partially) physiologically occurring | (Partially) limited use for molecular characterization | Humans |
| Good safety profile | |||
| Nanoparticles29 | Signal amplification | Slow distribution | Cell culture and animals |
| Possible radiosensitizing effects of certain types | Unclear kinetics | ||
| Certain types useable for MRI | Potential toxicity |
Antibodies (approxiamately 150 kDa) are among the first agents that were used because commercially available antibodies (e.g. for fluorescence-activated cell sorting) could also be employed for molecular imaging.1 An advantage of antibodies as molecular probes is their highly specific binding to their epitope. Additionally, the epitope is usually known, therefore permitting the direct correlation of fluorescence with antigen distribution. In recent studies, therapeutic antibodies were fluorescently labelled. This opens a completely new perspective for molecular imaging: by visualizing targeted therapeutics binding to their molecular targets, drugs can be assessed in action, potentially allowing a transition from solely diagnostic imaging to the prediction of therapeutic success with targeted drugs.11
So-called affinity peptides consist of few (usually 6–8) amino acids and are mostly developed using phage display, which allows the selection of peptides specifically binding to certain, e.g. dysplastic, tissues.12 The advantages of affinity peptides result from their small size (<1 kDa), leading to lower immunogenicity and faster distribution throughout the tissue, which is of high importance especially when the molecular dye is administered topically. However, specific binding can be an issue when developing these agents, and the molecular binding sites are not known. This predestines this class of molecular imaging agents for diagnostic purposes, when aiming for detection but not necessarily molecular characterization of a lesion. Antibody fragments could serve as an alternative, combining the advantages of smaller peptides with those of antibodies.
Nanoparticles (approximately 2–100 nm without ligands) are among the larger molecular probes currently used, and consist of a metalloid nanocrystal with a bioavailable shell that can be loaded with multiple ligands for specific binding. One advantage of nanoparticles lies in the possibility of signal amplification: fluorescence of core components (espcially quantum dots) is usually stronger compared to other classes of fluorophores, so that lowest expression of the target antigen suffices to evoke clearly detectable signals. Additionally, several antigens can be targeted at once using a multitarget nanoparticle. However, their often large size leads to slower distribution throughout the tissue and concerns about potential toxicity have been raised.13 Apart from gastrointestinal imaging, nanoparticles (especially superparamagnetic iron oxide particles) might be of high interest for the development of molecular contrast agents for MRI, and certain types might have radiosensitizing capabilities.14
‘Smart’, activatable probes (approximately 40 kDa) are another promising type of molecular beacon. The fluorescence of these probes is suppressed in their native state. Dequenching occurs and fluorescence intensity increases only after specific activation. This activation can take place due to pH shifts or changes in electrolyte concentration, or after the probe has been processed by tumour-specific proteases, optimizing the signal-to-noise ratio.15 Activatable probes combine molecular dyes with functional imaging and may in perspective allow insights into metabolic homeostasis and protein activity of cells. Safety profiles are, however, not yet well established.
A different approach to molecular imaging employs metabolic characteristics, for example those of neoplastic cells. Physiological substrates like folate can be linked to fluorophores, or fluorescent endogenous substances such as porphyrins are induced and accumulate in the tumorous tissue. Lectins are carbohydrate-binding proteins that can be used to visualize changes in glycan expression. As these agents often occur physiologically in the human body or are part of normal human diet, concerns of toxicity are not as high as with other types of molecular probes, lowering safety concerns and thereby facilitating the employment in humans.
For all these probes, the mode of delivery can differ: a topical administration might be preferable for diagnostic endoscopy and might be performed by spraying the molecular dye directly onto the surface of the tissue through a catheter (molecular chromoendoscopy). However, when characterizing a given lesion in a known cancer patient or monitoring therapy, a small testing dose of a fluorescently labelled targeted therapeutic could be administered intravenously.
Topical administration probably results in lower systemic concentrations of a fluorescent agent, potentially reducing immunogenicity and the occurrence of adverse events. However, in a clinical setting, large volumes of a fluorescent dye would be needed for the examination of larger parts of the mucosa, as in conventional chromoendoscopy. Intravenous injection could lead to a more regular distribution of the molecular agent throughout the tissue, comparable to computed tomography and MRI contrast agents or tracers for scintigraphy and positron emission tomography. This could make it easier to standardize protocols and compare studies, but comes at the cost of a potentially higher risk for unwanted side effects and reactions.
Preclinical and clinical studies
A wide array of probes has been studied for different diseases. Which type of probe is suited best for molecular imaging depends strongly on both the investigated disease entity and the examiner’s intention: for diagnostic purposes alone, sensitivity is of highest importance. It is not necessarily required to know the probe’s exact binding site. However, specific binding to a known target structure is crucial for molecular characterization. To be able to predict response to therapy, the target structure of the probe and the drug to be used should be the same, or the drug itself should be employed for imaging.
Several studies in gastrointestinal endoscopy and endoscopy in related fields examined the use of molecular imaging for diagnostic purposes. For photodynamic diagnosis of bladder cancer in urology, hexaminolevulinate is employed as an indirect optical imaging agent. On a molecular level, aminolevulinic acid is a compound of the porphyrin synthesis pathway and leads to the generation of protoporphyrins which then accumulate in neoplastic tissue and whose fluorescence can be visualized. Especially the detection of otherwise easily overlooked sites of carcinoma in situ could be improved using this method.16 A similar technique has been used to improve surgery of malignant gliomas: fluorescence-guided surgery using 5-aminolevulinic acid (5-ALA) lead to an improvement in tumour resection and progression-free survival.17 Additionally, protoporphyrins serve as endogenous photosensitizing agents which can be used for photodynamic therapy.18 These imaging techniques were shown to be transferable to upper gastrointestinal imaging: using orally administered 5-aminolevulinic acid, it was possible to detect dysplastic areas in Barrett’s oesophagus.19 This could be of high use when taking targeted biopsies or might serve as macroscopic red-flag technique for subsequent microscopic in-vivo imaging. Studies were also performed examining the detection of colonic dysplasia and cancer using 5-ALA as the fluorescent agent.20 However, remaining stool and inflammation proved to lower the specificity of this method and led to false-positive results.21 Another study focused on the detection of lymph node metastases: in a mouse model, metastatic lymph nodes could be visualized 3 hours after intraperitoneal administration of 5-ALA, but not after oral administration.22 Sensitivity and specificity rates for this new application are still unclear.
Molecular imaging has also been used for the detection of peritoneal carcinomatosis: in patients with advanced-stage ovarian cancer, FITC-labelled folate was administered before operation. Tumour deposits showed fluorescence during surgery which allowed the detection and excision of lesions <1 mm in diameter.23 This implementation of molecular imaging in a clinical setting could impact strongly on protocols for cytoreductive surgery, as both intraoperative navigation/staging and resection could be improved.
Affinity peptides were used for molecular diagnostics in gastrointestinal imaging using both confocal laser endomicroscopy and macroscopic fluorescence endoscopy: after topical administration of a fluorescently labelled heptapeptide on 18 neoplastic lesions during ongoing colonoscopy, strong binding to dysplastic cells could be observed via pCLE with sensitivity and specificity of 81 and 82%, respectively.12 A different peptide, also targeting colonic dysplasia, was used successfully for wide-field detection of dysplastic polyps in an animal model, using different fluorophores.4,5 In another study using a mouse model, multiple affinity peptides were combined to a peptide multimer conjugated with a near-infrared dye, resulting in an increased binding affinity to colonic adenomas, comparable to the affinity of an antibody but with the advantages of a potentially lower risk of side effects and faster distribution throughout the tissue.6 After topical administration, the dye was allowed to incubate for 5 minutes before imaging took place − an interval compatible with the use during colonoscopy. Toxicity profiles seem to make this approach transferable to the use in humans, and follow-up studies are awaited. Other studies examined the use of peptides with preferential binding to dysplastic areas in Barrett’s oesophagus: 12 oesophagus specimens were examined at 1-mm intervals and a heptapeptide, selected using phage display, bound specifically to dysplastic areas.3 Another peptide was then also evaluated in vivo.24 These studies exemplify the use of molecular imaging for the detection of lesions in high-risk patients and for the discerning of limited mucosal areas that are at risk to develop intraepithelial neoplasia.
For molecular characterization of lesions however, molecular probes with known target structures are preferred. In an experimental setting using an animal model, activatable probes were used for molecular imaging of xenograft-bearing mice. The probe was activated only after being processed by lysine–lysine-cleaving proteases such as cathepsin B, a cysteine protease frequently overexpressed in several gastrointestinal tumours. Fluorescence was detected on near-infrared wavelengths and tumours could be visualized with a high tumour-to-background ratio of 8.86 in the treatment group vs. 1.56 in controls, resulting from the strong difference in expression of proteases between neoplastic and healthy tissue.2 In another study illustrating the feasibility of visualizing enzyme activity, a rodent model of gastric cancer was visualized using cathepsin-activatable and matrix metalloproteinase-activatable probes.25 Alterations in glycan expression during oncogenesis were used for molecular imaging in another study, employing a specific lectin as molecular probe. Changes in lectin binding patterns allowed the visualization of high-grade dysplastic areas not detected by conventional endoscopy in human specimens of Barrett’s oesophagus.26
Several studies used antibodies targeting different tumour antigens to molecularly characterize lesions. Using a fluorescein-labelled monoclonal antibody against carcinoembryogenic antigen, it was possible to visualize binding in 27 patients in one study. The antibody was administered topically; endoscopy with an experimental fluorescence endoscope was performed after an incubation period of 10 minutes. Bleeding and ulceration of tumorous tissue was shown to be interfering with fluorescence detection, but targeting of carcinoembryogenic antigen visualized 19/25 carcinomas and 3/8 adenomas in this setting.1 In an animal study, a cocktail of antibodies was administered to differentiate two types of tumour xenografts simultaneously using macroscopic in-vivo biofluorescence imaging. Antibodies were labelled with different fluorophores and targeted different tumour antigens (EGFR1 and HER2), and two blinded investigators were able to discriminate the tumour types visualized with an accuracy of 100% (40/40).27 EGFR1 also was the target structure of a study using CLE for visualization of colon cancer xenografts and human tissue samples with a diagnostic antibody: after intravenous injection in the xenograft model, epidermal growth factor receptor (EGFR) expression levels of tumours of different cell lines could be discriminated. Topical administration, i.e. the incubation of human colon cancer specimens with the antibody, allowed discerning neoplastic tissue from healthy mucosa.28 In a study on oral squamous cell carcinoma, near-infrared quantum dots were conjugated with antibodies targeting EGFR. After administration of these nanoparticles, cancer xenografts could be visualized macroscopically in vivo with good tissue penetration of the signal.29 Another study targeted the vascular endothelial growth factor (VEGF) for CLE imaging: in rodent and xenografted models of colon cancer, as well as in human specimens, the distribution of VEGF in the malignantly transformed tissue could be displayed clearly.30 A study on gastric cancer used both diagnostic antibodies targeting EGFR and cetuximab as molecular probes for the visualization of xenografts. Fluorescence was quantified and showed a significant increase of fluorescence in treated animals versus isotype controls, both in the group receiving the diagnostic antibody and the group receiving cetuximab. Due to the high spatial resolution of CLE, the distribution of EGFR could be visualized on a subcellular level and different staining patterns could be discriminated.11 Another study recently illustrated the feasibility of discerning EGFR-expressing colon cancer cells in humans.31
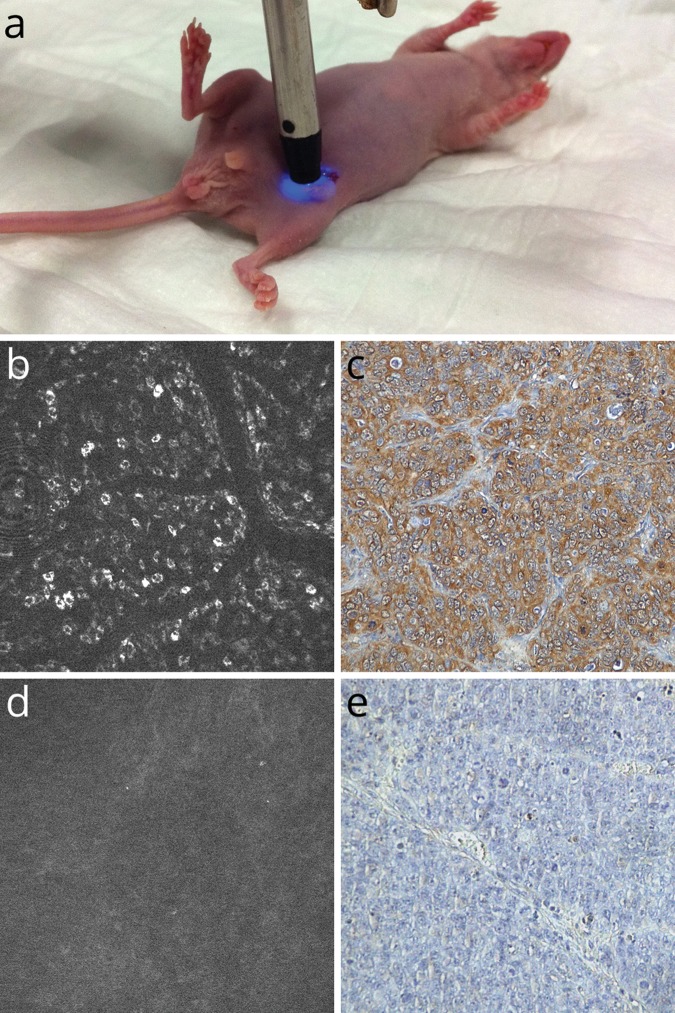
To address the potential use of molecular imaging for the prediction of tumour response to therapy, nude mice bearing human colon cancer xenografts with either high or low expression of EGFR were imaged using molecular CLE with cetuximab before and after therapy with this drug (Figure 2): high fluorescence before therapy went hand in hand with significantly slower tumour growth, better physical condition, and better overall survival up to day 30 of therapy.32 A different study correlated molecular CLE imaging using FITC-labelled adalimumab with response to anti-tumour necrosis factor therapy in 15 patients with Crohn’s disease. A high visualization of membranous tumour necrosis factor coincided with higher response rates.33 Although the last two studies are only available as abstract at the time of publication, this was the first time that endoscopic visualization of a target for molecular therapy was correlated to response of therapy. It also points to using endoscopy for therapeutic stratification of patients based on their molecular disease pattern.
Figure 2.
Molecular confocal laser endomicroscopy of human colon cancer xenografts in nude mice (a). Whereas tumour cells in xenografts overexpressing EGFR show strong fluorescence in molecular CLE with cetuximab (b), low-expressing xenografts stay dark (d). These in-vivo findings could be verified ex vivo using immunohistochemistry against in-vivo bound human IgG (cetuximab) (c, e).
Perspective
Molecular imaging is a novel approach to the visualization of diseases. It is already feasible in animal models and patients and can be used for macroscopic and microscopic specific visualization. In gastrointestinal endoscopy, especially the diagnosis of dysplastic and neoplastic lesions could be improved using this method, as molecular alterations occurring during oncogenesis can be visualized even before morphological changes take place. This offers a window for early and improved detection of lesions and visualization of metastases in gastrointestinal endoscopy (Figure 3). The wide range of molecular agents suited for molecular imaging opens a broad perspective of future applications: diagnosis can be combined with molecular characterization, potentially impacting on therapy protocols and optimizing the implementation of individualized treatment. By using therapeutic molecular agents as probes, drugs can be visualized in vivo in direct interaction with their target structures, potentially allowing for the prediction of tumour response to targeted therapies.
Figure 3.
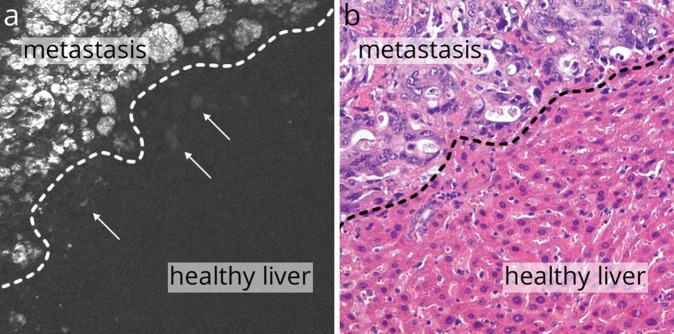
Molecular confocal laser endomicroscopy against EGFR using cetuximab identified metastases in the liver of xenografted nude mice (a). Individual tumour cells could be visualized (arrows), surrounded by healthy liver tissue. These findings could be verified ex vivo (b).
There are several obstacles to overcome before molecular imaging can be integrated into clinical routine, and many promising studies have so far only been conducted in vitro or in animal models. This is in part due to the fact that strict testing for toxicity is mandatory before a molecular agent can be used for imaging in humans, including drugs that are already approved for clinical use but are now fluorescently labelled. Still, the first in-human studies have already been published and several more are underway, demonstrating that the transition from bench to bedside is already taking place. It is, however, not yet fully answered whether the promising results of animal studies can be easily transferred. There are inherent methodological differences to take into account, such as the lack of intra-tumour heterogeneity in xenograft models. Imaging depth is another concern that needs to be addressed; however, near-infrared dyes with less scattering and absorption in the tissue are a promising approach to overcome this problem. The option to choose from several wavelengths for imaging might also allow for multicolour imaging in the future: several structures could be targeted simultaneously using a cocktail of molecular probes, or microscopic molecular imaging could be combined with the visualization of the tissue’s histoarchitecture using unspecific dyes such as fluorescein.
Up to now, most studies focus on either microscopic or macroscopic imaging − or use several techniques independently of each other to demonstrate the feasibility of both imaging modes. In a clinical setting, the two-step approach to molecular imaging should be possible without switching devices, and the technical requirements to achieve this are already established. In principle, a high-definition wide-field endoscope could be combined with fluorescence imaging as described above, and microscopic imaging could be achieved through probe-based or integrated confocal laser endomicroscopes.
The integration of molecular imaging into clinical routine could strongly influence disease management in gastroenterology. With the early identification of precursor lesions, molecular imaging could be used to identify patients and lesions at risk and help to lower the miss rate of conventional endoscopic techniques. The precise molecular characterization of a lesion could be used to tailor therapies in vivo, helping to personalize medicine and preventing time loss due to the sequential testing of therapies. Additionally, repeated molecular imaging of therapeutic molecular probes could be used to monitor therapy regimens and improve disease surveillance. The employment of targeted drugs as probes also holds promise for the prediction of response to therapy, further optimizing and facilitating personalized health care.
Regarding its unique potential and the ongoing transition into clinical practice, it is highly probable that molecular imaging will greatly impact on gastrointestinal endoscopy and personalized health care in the near future.
Acknowledgements
Aspects of this article are part of the MD thesis of MSH.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Keller R, Winde G, Terpe HJ, et al. Fluorescence endoscopy using a fluorescein-labeled monoclonal antibody against carcinoembryonic antigen in patients with colorectal carcinoma and adenoma. Endoscopy 2002; 34: 801–807 [DOI] [PubMed] [Google Scholar]
- 2.Alencar H, Funovics MA, Figueiredo J, et al. Colonic adenocarcinomas: near-infrared microcatheter imaging of smart probes for early detection – study in mice. Endoscopy 2007; 244: 232–238 [DOI] [PubMed] [Google Scholar]
- 3.Li M, Anastassiades CP, Joshi B, et al. Affinity peptide for targeted detection of dysplasia in Barrett’s esophagus. Gastroenterology 2010; 139: 1472–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller SJ, Joshi BP, Feng Y, et al. In vivo fluorescence-based endoscopic detection of colon dysplasia in the mouse using a novel peptide probe. PLoS ONE 2011; 6: e17384–e17384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Miller SJ, Joshi BP, et al. In vivo targeting of colonic dysplasia on fluorescence endoscopy with near-infrared octapeptide. Gut 2012; 62: 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi BP, Liu Z, Elahi SF, et al. Near-infrared-labeled peptide multimer functions as phage mimic for high affinity, specific targeting of colonic adenomas in vivo (with videos). Gastrointest Endosc 2012; 76: 1197–1206e1–e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goetz M, Watson A, Kiesslich R. Confocal laser endomicroscopy in gastrointestinal diseases. J Biophotonics 2011; 4: 498–508 [DOI] [PubMed] [Google Scholar]
- 8.Wallace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology 2009; 136: 1509–1513 [DOI] [PubMed] [Google Scholar]
- 9.Goetz M, Deris I, Vieth M, et al. Near-infrared confocal imaging during mini-laparoscopy: a novel rigid endomicroscope with increased imaging plane depth. J Hepatol 2010; 53: 84–90 [DOI] [PubMed] [Google Scholar]
- 10.Mahmood U. Optical molecular imaging approaches in colorectal cancer. Gastroenterology 2010; 138: 419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoetker MS, Kiesslich R, Diken M, et al. Molecular in vivo imaging of gastric cancer in a human-murine xenograft model: targeting epidermal growth factor receptor. Gastrointest Endosc 2012; 76: 612–620 [DOI] [PubMed] [Google Scholar]
- 12.Hsiung P, Hsiung P, Hardy J, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med 2008; 14: 454–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect 2006; 114: 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadjipanayis CG, Machaidze R, Kaluzova M, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res 2010; 70: 6303–6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marten K, Bremer C, Khazaie K, et al. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology 2002; 122: 406–414 [DOI] [PubMed] [Google Scholar]
- 16.Fradet Y, Grossman HB, Gomella L, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J Urol 2007; 178: 68–73 [DOI] [PubMed] [Google Scholar]
- 17.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006; 7: 392–401 [DOI] [PubMed] [Google Scholar]
- 18.Berger AP, Steiner H, Stenzl A, et al. Photodynamic therapy with intravesical instillation of 5-aminolevulinic acid for patients with recurrent superficial bladder cancer: a single-center study. Urology 2003; 61: 338–341 [DOI] [PubMed] [Google Scholar]
- 19.Brand S, Wang TD, Schomacker KT, et al. Detection of high-grade dysplasia in Barrett’s esophagus by spectroscopy measurement of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence. Gastrointest Endosc 2002; 56: 479–487 [DOI] [PubMed] [Google Scholar]
- 20.Messmann H, Knüchel R, Bäumler W, et al. Endoscopic fluorescence detection of dysplasia in patients with Barrett’s esophagus, ulcerative colitis, or adenomatous polyps after 5-aminolevulinic acid-induced protoporphyrin IX sensitization. Gastrointest Endosc 1999; 49: 97–101 [DOI] [PubMed] [Google Scholar]
- 21.Messmann H, Kullmann F, Wild T, et al. Detection of dysplastic lesions by fluorescence in a model of colitis in rats after previous photosensitization with 5-aminolaevulinic acid. Endoscopy 1998; 30: 333–338 [DOI] [PubMed] [Google Scholar]
- 22.Kato S, Kawamura J, Kawada K, et al. Fluorescence diagnosis of metastatic lymph nodes using 5-aminolevulinic acid (5-ALA) in a mouse model of colon cancer. J Surg Res 2012; 176: 430–436 [DOI] [PubMed] [Google Scholar]
- 23.van Dam GM, Themelis G, Crane LMA, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med 2011; 17: 1315–1319 [DOI] [PubMed] [Google Scholar]
- 24.Goetz M, Wang TD. Molecular imaging in gastrointestinal endoscopy. Gastroenterology 2010; 138: 828–833.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding S, Eric Blue R, Chen Y, et al. Molecular imaging of gastric neoplasia with near-infrared fluorescent activatable probes. Mol Imaging 2012; 11: 507–515 [PMC free article] [PubMed] [Google Scholar]
- 26.Bird-Lieberman EL, Neves AA, Lao-Sirieix P, et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett’s esophagus. Nat Med 2012; 18: 315–321 [DOI] [PubMed] [Google Scholar]
- 27.Barrett T, Koyama Y, Hama Y, et al. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res 2007; 13: 6639–6648 [DOI] [PubMed] [Google Scholar]
- 28.Goetz M, Ziebart A, Foersch S, et al. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology 2010; 138: 435–446 [DOI] [PubMed] [Google Scholar]
- 29.Yang K, Zhang F, Tang H, et al. In-vivo imaging of oral squamous cell carcinoma by EGFR monoclonal antibody conjugated near-infrared quantum dots in mice. Int J Nanomed 2011; 6: 1739–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foersch S, Kiesslich R, Waldner MJ, et al. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut 2010; 59: 1046⊟1055–1046⊟1055 [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Zuo X, Li C, et al. In vivo molecular imaging of epidermal growth factor receptor in patients with colorectal neoplasia using confocal laser endomicroscopy. Cancer Lett 2012; 330: 200–207 [DOI] [PubMed] [Google Scholar]
- 32. Goetz M, Hoetker MS, Diken M, et al. In vivo molecular imaging with cetuximab, an anti-EGFR antibody, for prediction of response in xenograft models of human colorectal cancer. Endoscopy 2013 (in press) [DOI] [PubMed]
- 33. Atreya R, Neumann H, Neufert C, et al. In vivo molecular endomicroscopy predicts the therapeutic response to anti-TNF treatment in Crohn’s disease patients during a prospective clinical phase 1 study. 20th United European Gastroenterology Week, 20−24 October 2012, Amsterdam, The Netherlands.