Abstract
Background:
Since the publication of the Rome III criteria for functional dyspepsia (FD), the evidence about the efficacy of half-dose of proton pump inhibitors for dyspepsia symptoms have been limited.
Objective:
To examine the efficacy of lansoprazole for functional dyspepsia (FD) diagnosed with the Rome III criteria by the multicentre, double-blind, randomized, placebo-controlled study in Japan.
Methods:
A total of 54 FD participants were randomized to lansoprazole 15 mg once daily or placebo for a 4-week double-blind treatment period. The primary efficacy endpoint was an overall dyspeptic symptom relief rate evaluated by 5-point Likert scale scores. The alteration of dyspeptic symptom scores during the study period was also assessed.
Results:
At week 4, the overall dyspeptic symptom relief rates were higher in the lansoprazole group (30.4%) than in the placebo group (6.7%) (p = 0.045). The scores for epigastric pain (p = 0.045) and epigastric burning (p = 0.03) were significantly improved in the lansoprazole group compared to the placebo group, whereas the improvement of the scores for postprandial fullness (p = 0.81) and early satiation (p = 0.33) was not different between lansoprazole and placebo groups.
Conclusions:
Lansoprazole 15 mg ameliorates dyspeptic symptoms, particularly the epigastric pain syndrome-related symptoms of FD.
Keywords: Functional dyspepsia, lansoprazole, proton pump inhibitor, Rome III
Introduction
The Rome III consensus defined functional dyspepsia (FD) as the presence of epigastric pain or burning, postprandial fullness, or early satiation in the absence of either underlying organic disease detected by oesophagogastroduodenoscopy (OGD) or metabolic disease likely to explain the symptoms.1,2 The pathophysiological mechanisms in FD are diverse and include altered gastrointestinal motility, visceral hypersensitivity, Helicobacter pylori infection, psychosocial factors, and other causes.3,4 In Rome III, a subgroup classification of FD into postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) was proposed.
Among suggested treatments for FD, only antisecretory agents and H. pylori eradication therapy have been evaluated as evidence-based treatments.3,5,6 Acid suppression by proton pump inhibitors (PPIs) is considered to be a major treatment option for FD and is reportedly effective for ulcer-like or reflux-type dyspepsia, but not for dysmotility-type FD.7 Although several randomized placebo-controlled studies on the efficacy of PPIs for FD have been performed,8–11 the results of these studies are controversial.12 In addition, in a previous large-scale study involving cohorts with uninvestigated dyspepsia, the possible inclusion of cohorts with peptic ulcer disease or reflux oesophagitis may have caused overestimation of the efficacy of anti-secretory agents such as PPIs.13 Furthermore, double-blind, randomized, placebo-controlled trials to investigate the efficacy of PPIs for FD, but not for uninvestigated dyspepsia, using the Rome III criteria have been scarcely performed.
Differences in race or ethnicity may affect the efficacy of PPIs for FD. Higher rates of maximal and basal acid output have been reported in Europeans and Americans compared with Indians and Chinese.14,15 Japanese patients with FD generally have lower acid output than do Western patients, but the evidences about the efficacy of half dose of PPIs for dyspeptic symptoms have been limited even in this population.
The present multicentre, double-blind, placebo-controlled, parallel-group study investigated the efficacy of lansoprazole in treating each dyspepsia symptom as well as EPS or PDS symptom in a Japanese population with investigated FD.
Methods
Study design
This clinical trial (UMIN Clinical Trials Registry number: UMIN000001759; http://www.umin.ac.jp/ctr/) was conducted at 11 institutions in Japan. The study protocol was approved by the ethics committees of each centre, and written informed consent was obtained before subject enrolment. The first patient was enrolled in May 2010, and the last patient completed the trial in December 2011. The study was performed in accordance with the principles of the Declaration of Helsinki.
Symptom assessment
The 5-point Likert dyspepsia severity scale was used to measure the severity of the four dyspepsia symptoms epigastric pain, epigastric burning, postprandial fullness, or early satiety (1, no complaints; 2, few complaints; 3, moderate complaints; 4, many complaints; and 5, serious complaints that significantly affect daily life). Symptom relief was defined as a response of 1 or 2 on the 5-point Likert scale of treatment with a response of 3, 4, or 5 at baseline. Gastrointestinal symptoms was also assessed using the Japanese version of the Gastrointestinal Symptom Rating Scale (GSRS),16 which included 15 scoring criteria: abdominal pain, heartburn, acid regurgitation, sucking sensations in the epigastrium, nausea and vomiting, borborygmus, abdominal distension, eructation, increased flatus, decreased passage of stools, increased passage of stools, loose stools, hard stools, urgent need for defecation, and feeling of incomplete evacuation. Scores on the 5-point Likert scale of dyspeptic symptoms and the GSRS were recorded once a week in the patients’ diaries.
Patients
Outpatients aged >20 years who had FD as defined by the Rome III classification were included in Japan. All patients underwent OGD within the last 6 months before inclusion. As an inclusion criteria, we recruited patients who had a 3-month history of one or more of the following symptoms such as bothersome postprandial fullness, early satiety, epigastric pain, or epigastric burning (patients with the scores more than 4 points for abdominal pain domain of GSRS or indigestion domain of GSRS or patients with the scores more than 5 points at least for one questionnaire related to abdominal pain or indigestion of GSRS) without any evidences of structural diseases such as peptic ulcer diseases, reflux oesophagitis, gastric cancer, and gastritis with multiple varioliform erosions. Here, epigastric pain and burning were recognized as a pain and burning sensation localized to the epigastrium, not extending up to the poststernal regions.
Patients scoring 1 or 2 on the 5-point Likert scale for all four dyspepsia symptoms at baseline were not eligible. Patients were excluded if they had a history of surgery in the upper gastrointestinal tract, severe liver, heart, or renal diseases, history of malignant diseases, history of H. pylori eradication therapy within the last 6 months, a diagnosis of irritable bowel syndrome, or current or past evidence of uncontrolled diabetes mellitus, psychosomatic disorders, or drug or alcohol abuse. Pregnant or breastfeeding women were also excluded. Patients with documented symptom induction or aggravation by self-recognized dysregulated food habits or acute psychological stress generated by a clear personal or social reason were excluded. Patients were not treated with PPIs, H2-receptor antagonists, antacids, prokinetics, non-steroidal anti-inflammatory drugs (NSAIDs), antidepressant drugs, anticholinergic agents (except for use as pretreatment for endoscopic examination), cholinergic agents, or tranquillizers within 1 week before study commencement. Patients who had experienced heartburn within 12 weeks before the baseline period were also excluded. Concomitant treatment with other PPIs, H2-receptor antagonists, antacids, prokinetics, NSAIDs, antidepressant drugs, anticholinergic agents, cholinergic agents, or tranquillizers was not allowed. Patients already receiving mucosal protectants were eligible for inclusion provided the investigator considered that a constant dose of such therapy was needed throughout the study.
Both H. pylori-negative and -positive patients were included. The presence of H. pylori infection was determined by serological evaluation for immunoglobulin G antibody to H. pylori. Patients in whom H. pylori had been successfully eradicated more than 6 months before the intervention were included in the H. pylori-negative group regardless of the serum levels of anti-H. pylori antibody.
Randomization and intervention
Eligible patients were sequentially randomized in a 1:1 ratio to a 4-week double-blind treatment with either lansoprazole 15 mg once daily or matching placebo according to a computer-generated random assignment program. The computer-generated randomization code was managed in the data management department of the Center for Clinical Research (CCR) at Keio University School of Medicine. Concealed allocation was assured by enciphered code kept in the Site Management Organizations for clinical trials (CMIC CMO, Shizuoka, Japan). After the completion of all data collection to the data management department of the CCR at Keio University School of Medicine, the code-key open was requested from CMIC CMO and then data was analysed. The appearance, packaging, and labelling of the lansoprazole and placebo capsules were identical to maintain blinding to investigators and patients. One 15-mg capsule of lansoprazole or placebo was taken daily after breakfast for 4 weeks. Treatment compliance was assessed by counting the returned unused capsules at the outpatient clinic visit; acceptable compliance was defined as an intake of 75% or more of the prescribed study medication. All patients were required to correctly complete a patient diary which is composed of 5-point Likert scale and GSRS questionnaire.
Overall safety was assessed by the patients and investigators at the clinic visit and by telephone contact 30 days following termination of the study drug. Vital signs were evaluated at all study visits or at early termination.
Primary efficacy endpoint
The primary efficacy endpoint was the overall dyspeptic symptom relief rates at 4 weeks of treatment (last survey point) in an intention-to-treat (ITT) analysis. Patients who discontinued intervention were treated as censored cases in the ITT analysis, but excluded from the per-protocol (PP) population.
Secondary efficacy endpoints
Subgroup analysis of patients with both EPS and PDS, those with EPS alone, and those with PDS alone was conducted in ITT analysis. The mean scores in the 5-point dyspepsia Likert scales at 0, 1, 2, 3, and 4 weeks of treatment was assessed in the PP population. The average reduction in GSRS scores at 1, 2, 3, and 4 weeks of treatment compared with baseline was assessed in the PP population.
Sample size
According to the results of a previous study on omeprazole as a treatment for dyspepsia,8 symptom disappearance frequencies were expected to be 43.1% with lansoprazole and 26.0% with placebo. It was therefore originally projected that a sample size of 140 patients per treatment group, including enough patients to allow for the risk of loss to follow up, would be sufficient to detect a response rate difference of approximately 17% between the two treatment groups with 80% power at the 0.05 significance level on the basis of a two-sample, two-tailed normal approximation to the binomial test for equal proportions.
Statistical analyses
Baseline patient characteristics were compared between the lansoprazole and placebo groups using Pearson’s chi-squared test for categorical variables or an unpaired Student’s t-test for continuous variables.
The length of time required to relieve dyspeptic symptoms since the intervention started was determined based on the patients’ diaries. The changes of symptom relief rates with time were shown by Kaplan–Meier method and the differences between those in the lansoprazole group and in the placebo group were tested by the log-rank test.
The alteration of scores during the study period on the 5-point Likert scale and GSRS were compared between lansoprazole and placebo groups using a linear mixed model. We included group allocation (lansoprazole or placebo), time (weeks of treatments), and the interaction of group and time as fixed effects, and included the patient as a random effect into the linear mixed models. All statistical analyses were performed using SPSS version 18.0 for Windows (SPSS, Chicago, IL, USA). The data are expressed as mean ± standard deviation. Two-sided p-values were considered to be statistically significant at a level of <0.05.
Results
Patient disposition
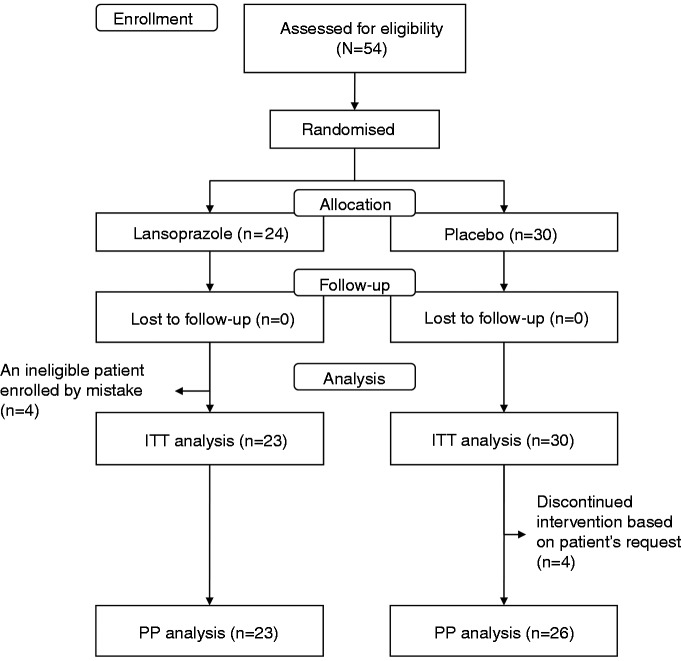
Because of slow patient recruitment, the trial was stopped prior to completion of enrolment. A total of 54 patients recruited from seven centres were randomized to receive either 15 mg lansoprazole (n = 24) or a placebo (n = 30) once a day for 4 weeks (Figure 1). Based on an enrolled population of 54, the detectable differences would be 38% at a 0.05 significance level and 80% power. In the placebo group, three patients took the test medication for only 1 week and one patient stopped the medication on day 6 because of epigastric pain. These four patients were included in the ITT population, but excluded from the PP population. In the lansoprazole group, one ineligible patient, whose 5-point Likert scale for all four dyspepsia symptoms at baseline were 2, was enrolled by mistake. This patient was excluded from both ITT and PP population. For PP analysis, 49 patients (23 in the lansoprazole group and 26 in the placebo group) were analysed.
Figure 1.
Trial flow and patient disposition.
ITT, intention-to-treat; PP, per-protocol.
The two treatment groups were well balanced with respect to demographic and clinical characteristics at baseline in both the ITT population (Table 1) and the PP population (Supplementary Table S1, available online). In both lansoprazole and placebo groups, about two-thirds of the patients were women, about 80% were non-smokers, >60% were alcohol abstainers, >60% had both EPS and PDS, about 30% had PDS but not EPS, and <13% had only EPS. Here, more than 60% of patients had an overlap of EPS and PDS (Table 1) which was higher than that in the previous large-scale web survey in Japan.17,18 The presence of H. pylori infection was not significantly different (39.1% in lansoprazole arm, 20.0% in placebo arm, p = 0.13). The baseline 5-point Likert scales were also well balanced. The baseline score for postprandial fullness was the highest and that for epigastric burning was the lowest.
Table 1.
Characteristics of analysed patients (intention-to-treat population)
| Characteristic | Lansoprazole (n = 23) | Placebo (n = 30) | p-value |
|---|---|---|---|
| Age (years) | 54.7 ± 17.1 (22–81) | 54.6 ± 16.8 (21–82) | 0.97a |
| Sex | 0.82b | ||
| Men | 7 (30.4) | 10 (33.3) | |
| Women | 16 (69.6) | 20 (66.7) | |
| Smoking habits | 0.94b | ||
| Non-smokers | 19 (82.6) | 24 (80.0) | |
| Ex-smokers | 3 (13.0) | 4 (13.3) | |
| Current smokers | 1 (4.3) | 2 (6.7) | |
| Alcohol habits | 0.45b | ||
| Abstainers | 15 (65.2) | 18 (60.0) | |
| Social drinkers | 8 (34.8) | 10 (33.3) | |
| Heavy drinkers | 0 (0) | 2 (6.7) | |
| BMI (kg/m2) | 20.9 ± 1.9 | 21.0 ± 3.4 | 0.89a |
| Presence of H. pylori infection | 9 (39.1) | 6 (20.0) | 0.13b |
| Subgroup of functional dyspepsia | 0.41b | ||
| Overlap of EPS and PDS | 14 (60.9) | 20 (66.7) | |
| EPS alone | 3 (13.0) | 1 (3.3) | |
| PDS alone | 6 (26.1) | 9 (30.0) | |
| Baseline 5-point Likert scale | |||
| Epigastric pain | 3.48 ± 1.28 | 3.17 ± 1.51 | 0.43a |
| Epigastric burning | 2.61 ± 1.20 | 2.57 ± 1.22 | 0.90a |
| Postprandial fullness | 4.00 ± 1.00 | 4.17 ± 0.91 | 0.53a |
| Early satiety | 3.09 ± 1.51 | 3.23 ± 1.33 | 0.71a |
| Patients with symptom relief at week 4 | |||
| Overall | 7 (30.4) | 2 (6.7) | 0.045 |
| EPS and PDS | 4/14 (28.6) | 0/20 (0) | 0.02c |
| EPS alone | 0/3 (0) | 0/1 (0) | NAc |
| PDS alone | 3/6 (50.0) | 2/9 (22.2) | 0.53c |
Values are mean ± standard deviation (range) or n (%).
aStudent’s t-test; bPearson’s chi-squared test; clog-rank test.
Symptom relief was defined as a response of 1 or 2 on the 5-point Likert scale of treatment with a response of 3, 4, or 5 at baseline.
BMI, body mass index; EPS, epigastric pain syndrome; NA, could not be analysed; PDS, postprandial distress syndrome.
Dyspepsia symptom relief rates (ITT analysis)
In the ITT population, relief rates of overall dyspepsia symptoms at week 4 were significantly higher in the lansoprazole group (30.4%) than in the placebo group (6.7%) (Table 1 and Figure 2). Among patients with both EPS and PDS (n = 34), relief rates of overall dyspepsia symptoms at week 4 were also higher in the lansoprazole group (28.6%) than in the placebo group (0%) (Table 1). On the other hand, among patients with EPS alone (n = 4) or those with PDS alone (n = 15), relief rates of overall dyspepsia symptoms at week 4 were not statistically different between the lansoprazole group and the placebo group (Table 1).
Figure 2.
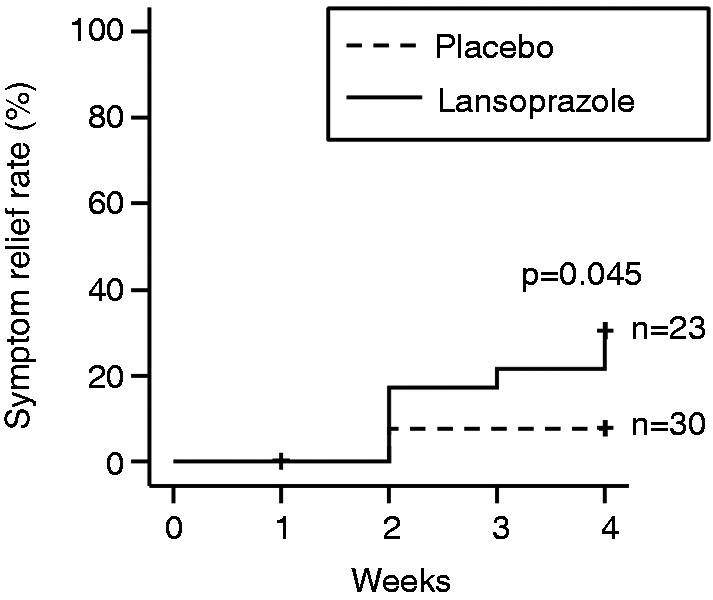
Overall symptom relief rates during the study period shown by the Kaplan–Meier method.
Overall symptom relief was defined as a response of 1 or 2 on the 5-point Likert scale of all dyspeptic symptoms. Patients who discontinued intervention were treated as censored cases.
Improvement of dyspepsia symptom score during the study period (PP analysis)
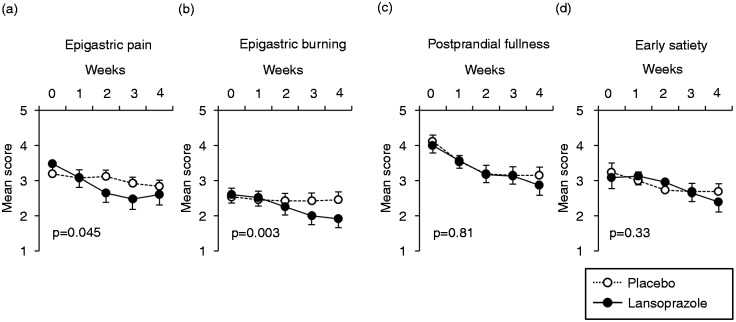
In the PP analysis, linear mixed model revealed that the epigastric pain score and the epigastric burning score were significantly reduced in the lansoprazole group as compared with the placebo group (Figure 3A, B). On the other hand, the alteration of scores for postprandial fullness and early satiation were not different between the lansoprazole and placebo groups (Figure 3C, D).
Figure 3.
The alteration of mean scores in 5-point dyspepsia Likert scales during the study period.
The differences between symptoms of patients in the lansoprazole group and in the placebo group were assessed using the linear mixed models. We included group allocation (lansoprazole or placebo), time (weeks of treatments), and the interaction of group and time as fixed effects, and included the patient as a random effect into the linear mixed models. Data represent means ± SEM.
Average reduction in GSRS scores (PP analysis)
In the PP analysis, there was no significant difference in each of the 14 GSRS scores between the two groups during the whole test period except the score for hard stools (Supplementary Figure S1). Linear mixed model showed that the score for hard stools was significantly reduced in the lansoprazole group as compared with the placebo group (Supplementary Figure S1).
Safety assessment
Only mild symptoms were reported during the test period, including one case of constipation, one of abdominal pain, one of epigastric pain, one of purpura at the lower right extremities, and one tingling sensation of the tongue. Among these symptoms, constipation, abdominal pain, and purpura at the lower right extremities were related to the medication. In two cases (epigastric pain and purpura), test drug administration was stopped at symptom onset.
Discussion
The present study was a multicentre, double-blind, randomized, placebo-controlled, parallel comparative study on the efficacy of lansoprazole (15 mg/day) for the treatment of Rome III-based FD. Because of the lower-than-projected sample size caused by slow recruitment of patients, the data obtained here are exploratory. However, the overall dyspeptic symptom relief rates were significantly higher in the lansoprazole group (30.4%) than in the placebo group (6.7%) at week 4 (Table 1, Figure 2). The scores for epigastric pain and epigastric burning, symptoms related to EPS, were significantly improved in the lansoprazole group (Figure 3A, B), whereas the improvement of the scores for postprandial fullness and early satiation, symptoms related to PDS, was not different between lansoprazole and placebo groups (Figure 3C, D). These results suggest the effect of lansoprazole for the treatment of FD, particularly in patients with EPS-related symptoms. Although a previous investigation showed that the benefit of PPIs over placebo was confined to patients with FD with ‘ulcer-like’ and ‘reflux-like’ dyspepsia using the Rome II criteria,7 the present study showed the first evidence under Rome III classification that lansoprazole can improve especially EPS-related symptoms.
Our systematic review of randomized clinical trials of the efficacy of PPIs for treating FD revealed that efficacy assessments are inconsistent.12 Treatment was effective in one omeprazone-based trial7 and one lansoprazole-based trial,8 but ineffective in one lansoprazole-based trial9 and one esomeprazole-based trial.10 These inconsistencies may stem from differences in ethnicities. In one cohort, for example, most subjects were Chinese and would therefore have lower acid output than that of a Western population; the effect of PPIs on symptom relief may thus be limited. Furthermore, because these four randomized controlled trials used the Rome II classification for diagnosis of FD, there was great heterogeneity in the subject population. Another issue caused by such heterogeneity may be the lack of general and consensus measures for evaluating dyspeptic symptoms.
In the present study, the Rome III classification was strictly used for diagnosis2 after excluding structural diseases by OGD. This allowed for definitive patient selection according to the internationally approved FD diagnostic criteria. Despite examination in Japanese individuals, who have lower acid output than Westerners, similar to Chinese individuals, the present study clearly showed a significant benefit of lansoprazole for the relief of overall dyspeptic symptom and particularly the EPS-related symptoms.
Reflux (erosive) oesophagitis was strictly excluded by the endoscopic inspection and only a moderate or severe FD based on Rome III classification was included in the present study. However, the overlap between FD and nonerosive reflux disease is reported to be very frequent,19 it would not be completely avoided for the inclusion of nonerosive reflux disease patients with FD in the present study.
A recent study showed that long-term PPI treatment might increase bowel symptoms. Patients with non-erosive reflux disease complained of bloating, flatulence, abdominal pain, and diarrhoea at rates of 43, 17, 7, and 2%, respectively, after 8 weeks of treatment with esomeprazole (20 mg).20 In the present study, significant amelioration of hard stools was shown in the lansoprazole group, although no significant difference between the lansoprazole and placebo groups was found in the other 14 GSRS scores. The improvement of hard stools might be attributed as intestinal bacterial overgrowth.20,21 The other concerns include that diarrhoea and microscopic colitis might be associated with PPI use.22 In the present study, however, the scores for loose stools and for increased passage of stools were not different between the lansoprazole group and the placebo group.
In general, the placebo effect is relatively large in the evaluation of FD symptom relief.23 According to our previous systematic review, although significant improvements were shown in all observational studies for the efficacy of PPIs on FD symptoms, improvements in placebo-controlled studies were limited.12 The placebo effect in the present cohorts was smaller than previously reported,23 possibly due to the enrolment of FD patients only with moderate to severe symptoms recruited mainly from the tertiary care institute. In addition, our results showed that, in the placebo group, the scores for epigastric pain or burning scores were hardly improved, while the score for postprandial fullness or early satiety was remarkably improved. This suggests that the placebo effect is limited for the relief of EPS symptoms compared with PDS symptoms.
The primary limitation of the present study is the lack of statistical power as a result of small enrolment numbers. The results of the present study should thus be considered exploratory. In addition, contingency could not be eliminated because of the limited enrolment. Further investigation with sufficient statistical power will be necessary to confirm and extend these results.
In conclusion, the results of the present study suggest the effectiveness of lansoprazole for treatment of FD, particularly EPS-related symptoms. Lansoprazole appears to provide specific benefit in terms of relief of epigastric burning and pain. These results are valuable with respect to serving as a platform for further studies on the effects of lansoprazole for treatment of Rome III-based FD.
Acknowledgements
The authors thank the investigators of the study group and their patients for their contributions: Keio University Hospital, Nihon University Surugadai Hospital, Kyorin University Hospital, Nippon Medical School Hospital, National Tokyo Medical Center, Tokyo Saiseikai Central Hospital, Eiju General Hospital, Tsuzuki Clinic, Nagoya City University Hospital, Aichi Medical University Hospital, and Kawasaki Medical University Hospital. We thank Prof Yuji Sato, Dr Naoki Tomotsugu, and Ms Mami Okada, Center of Clinical Research, Keio University School of Medicine, for their outstanding contribution as a central research office of the present study.
Funding
This study was partially supported by the grant from Takeda Pharmaceutical Co Ltd.
Conflict of interest
Hidekazu Suzuki has received research grants from AstraZeneca K.K., Daiichi-Sankyo Co., Otsuka Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Astellas Pharm Inc. Takeshi Kamiya has received research grants from AstraZeneca K.K., Daiichi-Sankyo Co., Takeda Pharmaceutical Co. Ltd., and Astellas Pharm Inc. Seiji Futagami has received research grants from AstraZeneca K.K. and Takeda Pharmaceutical Co. Ltd. Choitsu Sakamoto has received grant/research support from Astellas Pharma Inc., AstraZeneca, Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Takeda Pharmaceutical Co., Ltd. Shin'ichi Takahashi has received research grants from Takeda Pharmaceutical Co. Ltd. Takashi Joh has received research grants from AstraZeneca K.K., Daiichi-Sankyo Co., Takeda Pharmaceutical Co. Ltd., Astellas Pharm Inc., Mitsubishi Tanabe Pharma Co., and MSD Co. Hiroaki Kusunoki, Yasuharu Yamaguchi, Toshihiro Nishizawa, Eisuke Iwasaki, Juntaro Matsuzaki, Keiko Asakura, have no conflicts to declare.
References
- 1.Talley NJ, Stanghellini V, Heading RC, et al. Functional gastroduodenal disorders. Gut 1999; 45(Suppl 2): II37–II42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006; 130: 1466–1479 [DOI] [PubMed] [Google Scholar]
- 3.Suzuki H, Nishizawa T, Hibi T. Therapeutic strategies for functional dyspepsia and the introduction of the Rome III classification. J Gastroenterol 2006; 41: 513–523 [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013; 10: 168–174 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Inadomi JM, Hibi T. Japanese herbal medicine in functional gastrointestinal disorders. Neurogastroenterol Motil 2009; 21: 688–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loyd RA, McClellan DA. Update on the evaluation and management of functional dyspepsia. Am Fam Physician 2011; 83: 547–552 [PubMed] [Google Scholar]
- 7.Wang WH, Huang JQ, Zheng GF, et al. Effects of proton-pump inhibitors on functional dyspepsia: a meta-analysis of randomized placebo-controlled trials. Clin Gastroenterol Hepatol 2007; 5: 178–185 quiz 40 [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ, Meineche-Schmidt V, Pare P, et al. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials (the Bond and Opera studies). Aliment Pharmacol Ther 1998; 12: 1055–1065 [DOI] [PubMed] [Google Scholar]
- 9.Peura DA, Kovacs TO, Metz DC, et al. Lansoprazole in the treatment of functional dyspepsia: two double-blind, randomized, placebo-controlled trials. Am J Med 2004; 116: 740–748 [DOI] [PubMed] [Google Scholar]
- 10.Wong WM, Wong BC, Hung WK, et al. Double blind, randomised, placebo controlled study of four weeks of lansoprazole for the treatment of functional dyspepsia in Chinese patients. Gut 2002; 51: 502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Zanten SV, Armstrong D, Chiba N, et al. Esomeprazole 40 mg once a day in patients with functional dyspepsia: the randomized, placebo-controlled ‘ENTER’ trial. Am J Gastroenterol 2006; 101: 2096–2106 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Okada S, Hibi T. Proton-pump inhibitors for the treatment of functional dyspepsia. Therap Adv Gastroenterol 2011; 4: 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, et al. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in Helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol 2005; 100: 1477–1488 [DOI] [PubMed] [Google Scholar]
- 14.Feldman M, Richardson CT, Lam SK, et al. Comparison of gastric acid secretion rates and serum pepsinogen I and II concentrations in Occidental and Oriental duodenal ulcer patients. Gastroenterology 1988; 95: 630–635 [DOI] [PubMed] [Google Scholar]
- 15.Lam SK, Hasan M, Sircus W, et al. Comparison of maximal acid output and gastrin response to meals in Chinese and Scottish normal and duodenal ulcer subjects. Gut 1980; 21: 324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Masaoka T, Sakai G, et al. Improvement of gastrointestinal quality of life scores in cases of Helicobacter pylori-positive functional dyspepsia after successful eradication therapy. J Gastroenterol Hepatol 2005; 20: 1652–1660 [DOI] [PubMed] [Google Scholar]
- 17. Matsuzaki J, Suzuki H, Asakura K, et al. Classification of functional dyspepsia based on concomitant bowel symptoms. Neurogastroenterol Motil 2012; 24: 325–e164. [DOI] [PMC free article] [PubMed]
- 18. Matsuzaki J, Suzuki H, Fukushima Y, et al. High frequency of overlap between functional dyspepsia and overactive bladder. Neurogastroenterol Motil 2012; 24: 821–827. [DOI] [PubMed]
- 19.Talley NJ. Functional (non-ulcer) dyspepsia and gastroesophageal reflux disease: one not two diseases? Am J Gastroenterol 2013; 108: 775–777 [DOI] [PubMed] [Google Scholar]
- 20.Compare D, Pica L, Rocco A, et al. Effects of long-term PPI treatment on producing bowel symptoms and SIBO. Eur J Clin Invest 2011; 41: 380–386 [DOI] [PubMed] [Google Scholar]
- 21.Lombardo L, Foti M, Ruggia O, et al. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8: 504–508 [DOI] [PubMed] [Google Scholar]
- 22.Keszthelyi D, Jansen SV, Schouten GA, et al. Proton pump inhibitor use is associated with an increased risk for microscopic colitis: a case-control study. Aliment Pharmacol Ther 2010; 32: 1124–1128 [DOI] [PubMed] [Google Scholar]
- 23.Enck P, Vinson B, Malfertheiner P, et al. The placebo response in functional dyspepsia–reanalysis of trial data. Neurogastroenterol Motil 2009; 21: 370–377 [DOI] [PubMed] [Google Scholar]