Abstract
Background
Cigarette smoking has been associated with an increased risk of oesophageal adenocarcinoma (OAC). However, the impact of smoking and more importantly smoking cessation on Barrett’s oesophagus (BO) is unclear.
Objective
The aim of the study is to evaluate the association between cigarette smoking and presence of BO in a large prospective cohort of patients with gastro-oesophageal reflux disease (GORD).
Methods
Patients presenting to the endoscopy unit for upper endoscopy completed a validated GORD questionnaire and information on demographics (age, gender, and ethnicity), cigarette smoking [status (current/past), amount (pack years) and duration of smoking cessation], clinical data [medication history, body mass index (BMI), and family history] and endoscopic findings [BO and hiatal hernia] were recorded. Cigarette smokers (current and past) and nonsmokers were compared using Fisher’s Exact test for categorical variables and Mann–Whitney test for continuous variables. Effects of cigarette smoking and smoking cessation on BO risk was assessed by stepwise logistic regression analysis.
Results
A total of 1056 patients were included in the analysis [mean age: 57.2 ± 12.7years, Caucasian 880 (83.3%), male 985 (93.3%), and mean BMI 29.6 (SD: ± 5.6)]. 827 (78.3%) were smokers and 229 (21.6%) were nonsmokers. 474 subjects (44.9%) had a previous history of smoking. Anytime smokers were more likely to have BO (adjusted OR: 3.3; 95 CI: 1.7–6.3; p < 0.01). Higher smoking burden (pack years) was associated with higher risk of BO in this GORD cohort (p for trend < 0.01). Duration of smoking cessation was inversely associated with risk of BO (p for trend: 0.01).
Conclusion
This study shows that smokers with reflux symptoms have about threefold higher risk of BO compared with nonsmokers, whereas discontinuing smoking is associated with a significant reduced risk. Smoking cessation appears to be a viable option to reduce BO risk in patients with reflux disease.
Keywords: GORD, Barrett’s oesophagus, cigarette smoking
Introduction
Barrett’s oesophagus (BO), the replacement of squamous mucosa by specialized intestinal columnar mucosa in the distal oesophagus, is an important risk factor for oesophageal adenocarcinoma (OAC).1 The rapidly rising incidence of OAC (>600% increase in the last 35 years) is attributed to growing population with gastro-oesophageal reflux disease (GORD), BO and possibly obesity.2 Moreover, effective screening programs for BO and OAC are limited by the lack of ability to predict the presence of BO or OAC using clinical prediction models. As a result, it is essential to identify the risk factors of BO, the only precursor lesion to OAC to improve the yield of screening programs.1,3,4 At present, advanced age, long standing reflux, male sex, Caucasian race, presence of hiatal hernia and obesity are well-known risk factors for BO.3 Most of these are non-modifiable risk factors for BO. Identifying a modifiable risk factor for BO can have an impact in reducing the burden of the disease with the ultimate goal of impacting the rising incidence and poor survival associated with OAC.
Cigarette smoking has been shown to be a risk factor for cancer within BO and this risk persists even after smoking cessation.5–7 However, its exact role in the metaplastic transformation, i.e. from squamous to intestinal epithelium, is not clear. Some studies have shown an association between cigarette smoking and BO,8 while others have concluded that no such association exists.9,10 Conflicting evidence may be due to lack of statistical power, lack of adequate controls and unknown confounding factors. Recently, Cook et al., in a pooled analysis of five included case-control studies, concluded cigarette smoking as a significant risk factor for BO among both reflux and non-reflux individuals.15 However, it was limited by lack of use confounders (hiatal hernia, ethnicity), wide heterogeneity study population and lack of standardized definition of BO and GORD.15 These limitations make it difficult to interpret the true effect of smoking (in terms of intensity and risk stratification) among those with GORD. Furthermore, the impact of smoking cessation on the risk of BO has not been evaluated. Understanding this relationship has significant implications in identifying the possible pathogenic role of tobacco on BO, risk stratification for screening GORD patients for BO, and finally risk modification for BO.
The aims of the current study were: 1) to evaluate the risk of BO in current, past and any time smokers with reflux disease; 2) to evaluate the association between smoking duration in terms of pack years and risk of BO; and 3) to identify the effects of smoking cessation on BO in GORD patients.
Methods
Study subjects
The present study was conducted at Veterans Affairs Medical Center, Kansas City, Missouri, after approval of the local Institutional Review Board. Patients presenting with reflux disease for index upper endoscopy were prospectively enrolled from January 2000 to December 2011. Prior to the upper endoscopy, these individuals completed a previously validated GORD questionnaire (GORQ questionnaire).11 Patients were included if they had heartburn or regurgitation for at least 6 months by GORQ questionnaire. Exclusion criteria were as follows: (1) prior endoscopic evaluation; (2) age <18 years; (3) prior history of oesophageal or gastric surgery; and (4) prior history of BO or OAC.
The following patient details were collected: 1) demographics – age, gender and race; 2) medication history – current aspirin and NSAID use; 3) family history of oesophageal disorders; 4) cigarette smoking history; 5) endoscopic details – date of procedure, presence of hiatal hernia, and BO; and 6) histological diagnosis of biopsies obtained during the procedure. Data on cigarette smoking history were obtained as follows: smokers could be current and past cigarette smokers. Current smokers were defined as those who were smoking currently or smoked within 1 year prior to endoscopy, while past smokers were those who quit smoking at least 1 year prior to the upper endoscopy. Nonsmokers were defined as those who have never smoked. Pack years of smoking were calculated by multiplying the number of pack of cigarettes per day an individual smoked on average, and number of years of smoking cigarettes.
Endoscopy procedure and biopsy protocol
Upper endoscopy was performed in a conventional manner by white light video endoscopy (Olympus GIF-180, GIF-160; Olympus America Inc, Center Valley, PA). The upper end of gastric folds was considered as gastro-oesophageal junction (GOJ) during the endoscopy. The squamo-columnar junction was identified by the salmon-colored columnar mucosa encroaching the pale white squamous mucosa. Columnar lined oesophagus was defined by the proximal displacement of the squamo-columnar junction above the GOJ.12 BO was defined by the presence of columnar lined oesophagus and intestinal metaplasia on histopathology. Presence of hiatal hernia was also noted.
All the biopsies were performed using standard forceps (Microvasive Endoscopy, Boston Scientific Corp., Natick, MA). Biopsy specimens were obtained from columnar-appearing mucosa that was detected in the distal oesophagus at a point clearly proximal to the GOJ. Biopsy protocol specified that 4-quadrant biopsy specimens at intervals of 2 cm be obtained from the columnar lined oesophagus.1,13
Histopathological evaluation
All biopsy specimens were fixed in formalin, embedded in paraffin wax, and stained with H&E. The diagnosis of BO was based on the presence of goblet cells in biopsy specimens obtained from the columnar lined oesophagus.
Study outcomes
The following were the study outcomes: 1) risk of BO among anytime, current and past smokers compared with nonsmokers; 2) assess dose-response in terms of smoking pack years and risk of BO; and 3) risk of BO following cigarette smoking cessation.
Data management and statistical analysis
All GORD patients undergoing upper endoscopy were entered into a database with unique identification numbers. All patient identifiers were deleted in compliance with the Health Information Portability and Accountability Act regulations. These de-identified patient data were entered into Microsoft Access for Windows (Microsoft Corp, Redmond, Washington) database. The distribution of study variables was tested using Shapiro–Wilk test and was found to be not normal. Fischer’s exact test was used to compare categorical variables such as race, gender, GORD symptoms (heartburn and acid regurgitation), and hiatal hernia between the smoking groups (any time, current and past) and the control group. Mann–Whitney U test was used to compare age, body mass index (BMI), duration of smoking (pack years), BO length and hiatal hernia length between these groups. When multiple comparisons were conducted, Bonferroni adjusted p values were calculated. Multivariable adjusted odds ratio and corresponding 95% confidence interval were calculated for the different cigarette smoking categories (anytime, current and past) using logistic regression models which included all significant variables for BO. Furthermore, BO risk among anytime smokers and those who quit cigarette smoking, based upon degree of exposure, was calculated. Analysis was done using SPSS version 20.0 (SPSS Inc., Chicago, IL).
Results
Patient characteristics
A total of 1056 GORD patients were enrolled [93.3% men, 83.3% Caucasian; mean age 57.2 (SD: ±12.7) years, mean BMI of 29.6 (SD: ±5.6) kg/m2]. Among the GORD patients, 956 (90.5%) had heartburn and 777 (73.6%) had acid regurgitation; 562 (53.2%) had heartburn duration >5 years (Table 1). Median duration of heartburn and acid regurgitation were 7.5 years (range: 0.5–20 years) and 3.5 years (range: 0.5–20 years), respectively. Of 827 (78.3%) smokers in the GORD cohort, 353 (42.6%) patients were current smokers while 474 (57.3%) patients were past smokers.
Table 1.
Clinical and endoscopic characteristics of GORD patients
| Patient Characteristics | GORD Cohort (N = 1056) |
|---|---|
| Mean age (years) ± SD | 57.2 ± 12.7 |
| Race: Caucasian/African American/Others | 880 (83.3%)/147 (13.9%)/29 (2.7%) |
| Male sex, n (%) | 985 (93.3) |
| Mean BMI(Kg/m2) ± SD | 29.6 ± 5.6 |
| Heart burn, n (%) | 956 (90.5) |
| Acid regurgitation, n (%) | 777 (73.6) |
| Heart burn duration >5 years, n (%) | 562 (53.2) |
| Smokers, n (%) | 827 (78.3) |
| Current smokers, n (%) | 353 (33.4) |
| Past smokers, n (%) | 474 (44.9) |
| Hiatal hernia, n (%) | 490 (46.3) |
| Mean Hiatal hernia length (cm) ± SD | 2.71 ± 1.38 |
| Mean BO length (cm) ± SD | 2.13 ± 2.57 |
| BO, n (%) | 153 (14.5) |
BMI: body mass index; BO: Barrett’s oesophagus; GORD: gastro-oesophageal reflux disease; SD: standard deviation.
At endoscopy, 153 (14.5%) patients had BO with mean BO length of 2.1 cm (SD: ±2.5 cm) and hiatal hernia was present in 490 (46.3%) of them, with a mean length of 2.7 cm (SD: ±1.4 cm) (Table 1).
Comparison of any time smokers and nonsmokers
On univariate analysis, smokers were comparable with nonsmokers with respect to mean age (p = 0.09), ethnicity (p = 0.39), average BMI (p = 0.06), proton pump inhibitor (PPI) use (p = 0.65) and GORD symptoms (heartburn: p = 0.28 and acid regurgitation: p = 0.14). Smokers when compared with nonsmokers, were more likely to be male (p = 0.03), more often had heartburn duration >5 years (p < 0.001) and had a higher prevalence of BO (17.0% vs. 5.2%; p < 0.001). Other endoscopic features (presence of hiatal hernia, hiatal hernia length and BO length) were comparable in both groups (Table 2).
Table 2.
Characteristics of smokers and nonsmokers
| Characteristics | Nonsmokers N = 229 | Smokers N = 827 | Smokers vs. Nonsmokers p value | Current Smokers N = 353 | Current Smokers vs. Nonsmokers p value | Past Smokers N = 474 | Past Smokers vs. Nonsmokers p value |
|---|---|---|---|---|---|---|---|
| Mean age (years) ± SD | 55.9 ± 14.1 | 57.6 ± 12.3 | 0.09 | 52.2 ± 10.4 | 0.001 | 61.6 ± 12.1 | <0.001 |
| Race: Caucasian, n (%) | 193 (84.6) | 687 (83.6) | 0.39 | 287 (81.8) | 0.22 | 400 (84.9) | 0.50 |
| Male sex, n (%) | 207 (90.4) | 778 (94.1) | 0.04 | 331 (93.8) | 0.34 | 447 (94.3) | 0.16 |
| Mean BMI (kg/m2) ± SD | 30.2 ± 5.9 | 29.4 ± 5.5 | 0.07 | 28.8 ± 5.8 | 0.01 | 29.9 ± 5.2 | 0.99 |
| Heart burn, n (%) | 209 (91.7) | 747 (90.1) | 0.29 | 331 (93.2) | 0.99 | 416 (87.8) | 0.30 |
| Acid regurgitation, n (%) | 174 (76.7) | 603 (72.8) | 0.14 | 258 (72.5) | 0.79 | 345 (73.1) | 0.95 |
| Duration of heartburn >5 years, n (%) | 96 (45.1) | 466 (60.3) | <0.001 | 211 (61.9) | <0.001 | 255 (59.0) | 0.002 |
| PPI use, n (%) | 123 (53.7) | 433 (52.3) | 0.65 | 177 (50.1) | 0.99 | 256 (54.0) | 0.99 |
| Mean smoking Intensity ± SD (pack years) | 0 (0) | 34.02 ± 28.9 | <0.001 | 33.6 ± 24.4 | <0.001 | 35.6 ± 31.9 | <0.001 |
| Hiatal hernia, n (%) | 104 (46.0) | 415 (50.4) | 0.14 | 166 (47.3) | 0.99 | 249 (52.6) | 0.30 |
| Mean HH length(cm) ± SD | 2.8 ± 1.6 | 2.7 ± 1.3 | 0.26 | 2.5 ± 1.2 | 0.09 | 2.8 ± 1.4 | 0.96 |
| BO, n (%) | 12 (5.2) | 141 (17.0) | <0.001 | 63 (17.7) | <0.001 | 78 (16.5) | <0.001 |
| Mean BO length(cm) ± SD | 1.4 ± 1.5 | 2.0 ± 2.6 | 0.32 | 2.0 ± 2.8 | 0.68 | 2.0 ± 2.5 | 0.60 |
BMI: body mass index; BO: Barrett’s oesophagus; HH: hiatal hernia; PPI: proton pump inhibitor; SD: standard deviation.
Comparison of current smokers and nonsmokers
Current smokers were more likely to be younger (p = 0.001), had lower BMI (p = 0.01) and had duration of heartburn >5 years (p < 0.001). Patient demographics such as male gender (p = 0.34), race (p = 0.22), use of PPI (p = 0.99) and GORD symptoms (heartburn: p = 0.99 and acid regurgitation: p = 0.79) were similar in both groups. Current smokers had a higher prevalence of BO (17.7% vs. 5.2%; p < 0.001). Presence of hiatal hernia, hiatal hernia length and BO length were comparable among these groups (Table 2).
Comparison of past smokers and nonsmokers
Past smokers were significantly older (p < 0.001), more likely to have heartburn duration >5 years (p = 0.002) and had higher prevalence of BO compared with nonsmokers (16.5% vs. 5.2%; p < 0.001). Gender, race, BMI, GORD symptoms, PPI use and other endoscopic features were similar in past smokers and nonsmokers (Table 2).
Association of cigarette smoking and BO in subgroups
Among Caucasian individuals, anytime (OR: 3.6; 95% CI: 1.9–6.6; p < 0.01), current (OR: 3.7; 95% CI: 1.9–7.0; p < 0.01) and past (OR: 3.5; 95% CI: 1.8–6.7; p < 0.01) smokers had a higher risk of BO, while cigarette smoking did not appear to have significant association with BO risk among non-Caucasians (OR: not calculable; 95% CI: not calculable; p = 0.34). Among the male sex subgroup, anytime (OR: 3.4; 95% CI: 1.8–6.2; p < 0.01), current (OR: 3.5; 95% CI: 1.9–6.8; p < 0.01) and past (OR: 3.2; 95% CI: 1.7–6.1; p < 0.01) smoking were significantly associated with BO risk (Table 3). In the female subgroup, smoking was not significantly associated with BO (OR: not calculable; 95% CI: not calculable; p = 0.09). Irrespective of heartburn duration, presence of hiatal hernia, age and BMI, cigarette smokers had a higher risk for BO than the nonsmokers in this GORD cohort (Table 3).
Table 3.
Risk of BO on different subgroups
| Subgroups | Non-Smokers |
Any time smokers |
Current Smokers |
Past Smokers |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | BO(%) | N | BO(%) | p value | OR | N | BO(%) | p value | OR | N | BO(%) | p value | OR | |
| Age <60Yr | 128 | 3 (2.3) | 447 | 66 (14.8) | <0.001 | 7.2 (2.2–23.4) | 259 | 43 (16.6) | <0.01 | 8.3 (2.5–27.3) | 188 | 23 (12.2) | 0.001 | 5.8 (1.7–19.8) |
| Age ≥60 Yr | 89 | 9 (10.1) | 331 | 61 (18.4) | 0.07 | 2.0 (0.95–4.2) | 76 | 17 (22.4) | 0.03 | 2.5 (1.1–6.1) | 255 | 44 (17.3) | 0.12 | 1.8 (0.9–3.0) |
| Male Gender | 207 | 12 (8.3) | 771 | 132 (17.1) | <0.01 | 3.4 (1.8–6.2) | 330 | 59 (17.9) | <0.01 | 3.5 (1.9–6.8) | 441 | 73 (16.6) | <0.01 | 3.2 (1.7–6.1) |
| Female Gender | 22 | 0 (0) | 49 | 7 (14.3) | 0.09 | NC | 22 | 4 (18.2) | 0.1 | NC | 27 | 3 (6.1) | 0.24 | NC |
| Caucasian | 193 | 12 (6.2) | 680 | 131 (19.3) | <0.01 | 3.6 (1.9–6.6) | 286 | 56 (19.6) | <0.01 | 3.7 (1.9–7.0) | 394 | 75 (19.0) | <0.01 | 3.5 (1.8–6.7) |
| Non-Caucasian | 35 | 0 (0) | 135 | 7 (5.2) | 0.34 | NC | 64 | 6 (9.4) | 0.09 | NC | 71 | 1 (1.4) | 0.99 | NC |
| BMI <30 | 128 | 9 (7.0) | 486 | 90 (18.5) | <0.01 | 3.0 (1.5–6.1) | 227 | 44 (19.4) | <0.01 | 3.2 (1.5–6.8) | 259 | 46 (17.8) | <0.01 | 2.9 (1.4–6.0) |
| BMI ≥30 | 100 | 3 (3.0) | 332 | 49 (14.8) | <0.01 | 5.6 (1.7–18.3) | 122 | 17 (13.9) | <0.01 | 5.2 (1.5–18.4) | 210 | 32 (15.2) | <0.01 | 5.8 (1.7–19.5) |
| HB ≥5 Yr | 96 | 5 (5.2) | 460 | 88 (19.2) | <0.01 | 4.3 (1.7–10.9) | 211 | 45 (21.3) | <0.01 | 4.9 (1.9–12.9) | 249 | 43 (17.2) | <0.01 | 3.8 (1.5–9.9) |
| HB <5 yr | 117 | 6 (5.1) | 307 | 37 (12.1) | 0.04 | 2.5 (1.1–6.2) | 129 | 14 (10.9) | 0.11 | 2.2 (0.83–6.0) | 177 | 23 (13.0) | 0.02 | 2.7 (1.1–7.0) |
| Presence of HH | 104 | 12 (11.5) | 413 | 99 (24) | <0.01 | 2.4 (1.2–4.5) | 167 | 42 (25.1) | <0.01 | 2.5 (1.3–5.2) | 246 | 57 (23.2) | 0.01 | 2.3 (1.2–4.5) |
| Absence of HH | 122 | 1 (0.8) | 407 | 43 (10.6) | <0.01 | 14.2 (1.9–104.9) | 183 | 21 (11.5) | <0.01 | 15.6 (2.0–118.2) | 224 | 22 (9.8) | <0.01 | 13.1 (1.7–99.0) |
HB: heartburn; HH: hiatal hernia; BMI: body mass index; BO: Barrett’s oesophagus; OR: odds ratio; CI: confidence interval; NC: not calculable.
Multivariate analysis
BO risk between smokers and nonsmokers and impact of smoking burden
On multivariate analysis, anytime smoking (adjusted OR: 3.30; 95% CI: 1.72–6.34; p < 0.001), current smoking (adjusted OR: 4.00; 95% CI: 1.96–8.15; p < 0.001) and past smoking (adjusted OR: 3.01; 95% CI: 1.51–6.03; p = 0.002) were independent predictors for BO after adjusting for age, gender, race, BMI, hiatal hernia and heartburn duration >5 years (Table 4). The reference group is the nonsmokers.
Table 4.
Risk of BO among smokers – multivariate logistic regression model for Barrett’s oesophagus
| Characteristic | Prevalence of BO (%) | OR | Adjusted OR* (95% CI) | p value |
|---|---|---|---|---|
| Any time Smoker | 17.0% | 7.2 (2.2–23.3) | 3.3 (1.7–6.3) | <0.001 |
| Current Smoker | 17.7% | 8.2 (2.5–27.2) | 4.0 (1.9–8.1) | <0.001 |
| Past Smoker | 16.5% | 5.8 (1.7–19.7) | 3.0 (1.5–6.0) | 0.002 |
| Nonsmokers | 5.2% | Referent | Referent | Referent |
BO: Barrett’s oesophagus; BMI: body mass index; CI: confidence interval; OR: odds ratio, *- Adjusted to age, gender, BMI, Caucasian race, hiatal hernia, heart burn duration >5 years.
The risk of BO among cigarette smokers of <10 pack years (adjusted OR: 2.96; 95% CI: 1.25–7.00; p = 0.013), 10–20 pack years (adjusted OR: 3.21; 95% CI: 1.44–7.16; p = 0.004) and >20 pack years (adjusted OR: 3.43; 95% CI: 1.73–6.83; p < 0.01) was significantly higher than nonsmokers. The risk of BO increased with higher pack years of cigarette smoking (p-trend <0.01) (Figure 1).
Figure 1.
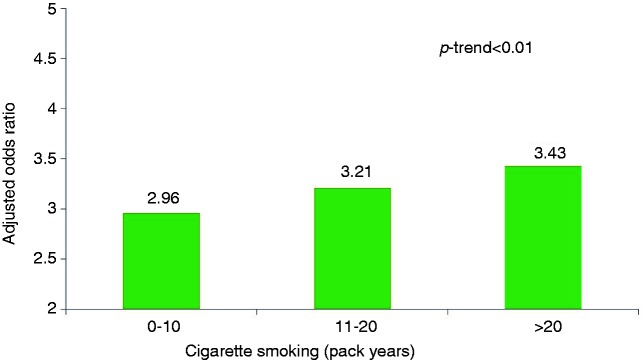
Risk for Barrett’s oesophagus by smoking duration (pack years).
Effect of smoking cessation on the risk of BO
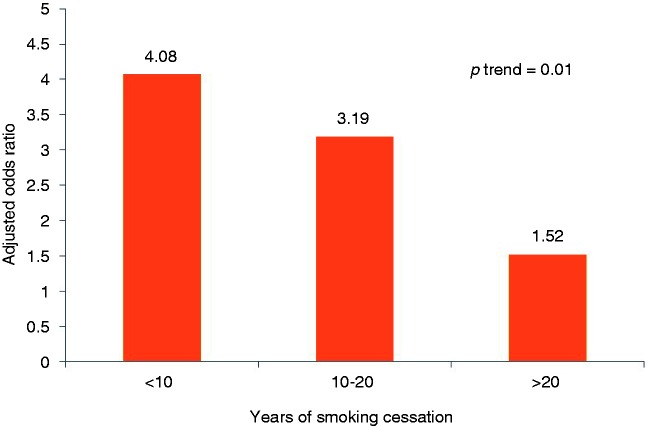
Past smokers who quit smoking within 10 years (adjusted OR: 4.08; 95% CI: 1.78–9.33; p = 0.001) and for 10–20 years (adjusted OR: 3.19; 95% CI: 1.34–7.59; p = 0.009) had a significant risk of BO, while those who quit smoking for >20 years did not have significant risk for BO (adjusted OR: 1.52; 95% CI: 0.62–3.68; p = 0.08). There was a progressive decrease in the risk of BO as the duration of smoking cessation increased (adjusted OR: 0.95 per year; 95% CI: 0.92–0.98,) with a significant trend (p-trend = 0.01) (Figure 2).
Figure 2.
Risk for Barrett’s oesophagus after smoking cessation.
Discussion
In this large cohort of GORD patients referred for upper endoscopy, the risk of BO among smokers was explored. Results from this case-control study demonstrate that current smoking (adjusted OR: 4.00; 95%CI: 1.96–8.15), past smoking (adjusted OR: 3.01; 95% CI: 1.51–6.03) and anytime smoking (adjusted OR: 3.30; 95% CI: 1.72–6.34) were independent predictors for BO. The risk for BO increased progressively with higher pack years of anytime smoking in this GORD cohort, suggesting a cumulative exposure risk (p-trend <0.01). Similarly, the risk for BO development decreased in a linear fashion with duration of smoking cessation (p-trend = 0.01). To our knowledge, this is the first study to show an impact of smoking cessation on BO risk. However, this risk for BO persisted for at least 20 years even after smoking cessation.
Although studies in the past have shown an association between smoking and BO, the literature is not entirely cohesive. In a study of 615 patients, Edelstein et al. showed an association between cigarette smoking and BO among GORD patients (adjusted OR: 2.8; 95% CI: 1.1–7.0).14 A recent pooled data analysis by Cook et al. revealed subjects with BO were significantly more likely to have ever smoked cigarettes than GORD controls (adjusted OR, 1.67) and general population (adjusted OR, 1.61). This study also showed an increase in the OR with increasing cumulative impact of smoking measured in pack years. The authors note that the OR of BO is 1.5 in patients with <15 and 15–29 pack years and 2 in those with 30 or more pack years when compared with those who never smoked. Although this study has a lower OR compared with ours, the effect may be due to the heterogeneity of the pooled data. The risk for BO increased in those with history of cigarette smoking and reflux disease (adjusted OR: 16.47; 95% CI: 10.73–25.29) compared with the control population.15The synergy between GORD and cigarette smoking for BO risk indicates the need for aggressive modification of this habit among these high-risk individuals. On the contrary, others studies have not found any association between cigarette smoking and risk for BO in GORD. An Irish study group (FINBAR) reported no BO risk attributable to cigarette smoking in a case-control study (p = 0.40). However, the study included only patients with BO length of ≥3 cm.6 A study by Gerson et al. found no association between cigarette smoking and BO on multivariate analysis (adjusted OR:1.33; 95% CI: 0.89–1.98; p = 0.16), and this may be due to a smaller sample size.10
Irrespective of age, BMI, duration of reflux and presence of hiatal hernia, cigarette smokers had higher risk of BO. Interestingly cigarette smokers did not have higher risk for BO among female gender and non-Caucasians. This is could be explained by limited sample size involving female gender and non-Whites in our study.
The present study showed that both current and past cigarette smoking were significantly associated with an increased risk of BO in the GORD cohort. In contrast, a Dutch study found past cigarette smoking to be significantly associated with BO (adjusted OR: 1.33, 95% CI: 1.00–1.77), but not current cigarette smoking. These results were potentially confounded by unmeasured variables such as reflux disease and hiatal hernia – well-known predictors of BO.16 Thus it appears that, irrespective of the time of cigarette smoking, smokers should be considered at high risk for BO.
The strength of our study is two-fold. One, we demonstrate a strong dose–response relationship between smoking measured in pack years and the odds of developing BO, validating the pooled analysis by Cook et al.15 Two, we also show that the risk of BO is diminished 20 years after stopping smoking, further buttressing the association between smoking and BO. Since longer smoke cessation usually correlates inversely to the pack years; it could be a possible reason to find an inverse trend with risk of BO. However, in the past smoking cessation has been reported to be beneficial in reducing the mortality due to coronary artery disease and lung cancer.17,18 Prospective studies would be needed to confirm the findings of the present study. If proven, use of smoking cessation programs could be an attractive option for reducing the burden of this premalignant condition as it is one of the few modifiable risk factors.
The precise mechanism by which smoking leads to this metaplastic change in GORD is unknown. However, some prior studies have illustrated that smoking was significantly associated with increased acid exposure in the distal oesophagus measured by ambulatory oesophageal 24 h pH monitoring.19,20 The excess distal oesophageal acid exposure could be due to reduction of basal lower oesophageal sphincter tone by nicotine, frequent transient lower oesophageal sphincter relaxations, impaired oesophageal acid clearance and/or impaired gastric emptying.21 A study from the United Kingdom reported that cigarette smoking was associated with higher DNA damage to the oesophageal squamous cells (p < 0.01) and also to the Barrett’s mucosa (p < 0.05).22 In a rat model, smoking has been shown to act independently and synergistically with reflux to increase the risk of BO by over expression of NF-kB and the COX-2 pathway.23 Further studies are required to define the pathway by which cigarette smoking influences the development of BO.
Despite the important results from this study, it has some limitations. There could be selection bias, as patients with higher burden of GORD symptoms may be more likely to be referred for endoscopy, but we have no reason to suspect that this will affect the relative prevalence of GORD and BO patients. Since the study population included primarily white veteran males and the majority were smokers, the results may not be generalizable to the general population. A control group of asymptomatic patients was not included, but this should not affect our overall conclusion that smoking increases the risk of BO prevalence and smoking cessation decreases that risk. BO biopsies have a sampling error for detection of intestinal metaplasia, thus misclassifying BO patients as GORD. Oesophageal pH monitoring was not performed to confirm the diagnosis of GORD, but a validated questionnaire was used.11 Although the prevalence of current and past smokers are higher in this cohort, most of the prior studies on veterans had similar range of current and past smokers.24–26
In summary, although current guidelines do not recognize cigarette smoking as a risk factor for the development of BO, the present study indicates smoking (including past and current) as an independent risk factor for the development of BO.1,13 The risk of BO significantly increases with the intensity of smoking (pack years), whereas smoking cessation of 20 years or more diminishes this risk. These results suggest cigarette smoking as a strong risk factor for BO, and hence smoking could be used as one of the risk stratification tools for BO screening among GORD patients. Similarly, smoking cessation would be a feasible option as a risk modification strategy among GORD patients.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is is no conflict of interest.
Author contributions
Conception and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript: Gokulakrishnan Balasubramanian; Conception and design, performing endoscopic procedures, interpretation of data and critical revision of the manuscript for important intellectual content: Maria Giacchino, Vijay Kanakadandi, Srinivas Gaddam, Mandeep Singh, Sachin B Wani, Neil Gupta, Amit Rastogi, and Ajay Bansal; acquisition of data, and administrative and technical support: April D Higbee and Maria Giacchino; statistical support: Gokulakrishnan Balasubramanian and Neil Gupta; study concept and design, acquisition of data, performing endoscopic procedures, and critical revision of the manuscript for important intellectual content: Prateek Sharma. Results of this study were presented in part at the Digestive Disease Week 2012, San Diego.
References
- 1.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011; 140: 1084–1091 [DOI] [PubMed] [Google Scholar]
- 2.Lagergren J. Controversies surrounding body mass, reflux, and risk of oesophageal adenocarcinoma. Lancet Oncol 2006; 7: 347–349 [DOI] [PubMed] [Google Scholar]
- 3.Sharma P. Clinical practice. Barrett's esophagus. N Engl J Med 2009; 361: 2548–2556 [DOI] [PubMed] [Google Scholar]
- 4.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005; 97: 142–146 [DOI] [PubMed] [Google Scholar]
- 5.Coleman HG, Bhat S, Johnston BT, et al. Tobacco smoking increases the risk of high-grade dysplasia and cancer among patients with Barrett's esophagus. Gastroenterology 2012; 142: 233–240 [DOI] [PubMed] [Google Scholar]
- 6.Anderson LA, Watson RG, Murphy SJ, et al. Risk factors for Barrett's oesophagus and oesophageal adenocarcinoma: Results from the FINBAR study. World J Gastroenterol 2007; 13: 1585–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: A pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010; 102: 1344–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith KJ, O'Brien SM, Green AC, et al. Current and past smoking significantly increase risk for Barrett's esophagus. Clin Gastroenterol Hepatol 2009; 7: 840–848 [DOI] [PubMed] [Google Scholar]
- 9.Kubo A, Levin TR, Block G, et al. Cigarette smoking and the risk of Barrett's esophagus. Cancer Causes Control 2009; 20: 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerson LB, Ullah N, Fass R, et al. Does body mass index differ between patients with Barrett's oesophagus and patients with chronic gastro-oesophageal reflux disease? Aliment Pharmacol Ther 2007; 25: 1079–1086 [DOI] [PubMed] [Google Scholar]
- 11.Locke GR, Talley NJ, Weaver AL, et al. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc 1994; 69: 539–547 [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: The Prague C & M criteria. Gastroenterology 2006; 131: 1392–1399 [DOI] [PubMed] [Google Scholar]
- 13.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol 2008; 103: 788–797 [DOI] [PubMed] [Google Scholar]
- 14.Edelstein ZR, Bronner MP, Rosen SN, et al. Risk factors for Barrett's esophagus among patients with gastroesophageal reflux disease: A community clinic-based case-control study. Am J Gastroenterol 2009; 104: 834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook MB, Shaheen NJ, Anderson LA, et al. Cigarette smoking increases risk of Barrett's esophagus: An analysis of the Barrett's and Esophageal Adenocarcinoma Consortium. Gastroenterology 2012; 142: 744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steevens J, Schouten LJ, Driessen AL, et al. A prospective cohort study on overweight, smoking, alcohol consumption, and risk of Barrett's esophagus. Cancer Epidemiol Biomarkers Prev 2011; 20: 345–358 [DOI] [PubMed] [Google Scholar]
- 17.Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: A randomized clinical trial. Ann Intern Med 2005; 142: 233–239 [DOI] [PubMed] [Google Scholar]
- 18.Hjermann I, Holme I, Leren P. Oslo Study Diet and Antismoking Trial. Results after 102 months. Am J Med 1986; 80: 7–11 [DOI] [PubMed] [Google Scholar]
- 19.Kadakia SC, Kikendall JW, Maydonovitch C, et al. Effect of cigarette smoking on gastroesophageal reflux measured by 24-h ambulatory esophageal pH monitoring. Am J Gastroenterol 1995; 90: 1785–1790 [PubMed] [Google Scholar]
- 20.Waring JP, Eastwood TF, Austin JM, et al. The immediate effects of cessation of cigarette smoking on gastroesophageal reflux. Am J Gastroenterol 1989; 84: 1076–1078 [PubMed] [Google Scholar]
- 21.Pandolfino JE, Kahrilas PJ. Smoking and gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol 2000; 12: 837–842 [DOI] [PubMed] [Google Scholar]
- 22.Olliver JR, Hardie LJ, Gong Y, et al. Risk factors, DNA damage, and disease progression in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev 2005; 14: 620–625 [DOI] [PubMed] [Google Scholar]
- 23.Aiyer HS, Li Y, Harper N, et al. Molecular changes in the esophageal epithelium after a subchronic exposure to cigarette smoke in the presence of bile-acid reflux. Inhal Toxicol 2011; 23: 304–311 [DOI] [PubMed] [Google Scholar]
- 24.Kamath AS, Vaughan Sarrazin M, Vander Weg MW, et al. Hospital costs associated with smoking in veterans undergoing general surgery. J Am Coll Surg 2012; 214: 901–908 [DOI] [PubMed] [Google Scholar]
- 25.McKinney WP, McIntire DD, Carmody TJ, et al. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep 1997; 112: 212–217 [PMC free article] [PubMed] [Google Scholar]
- 26.Park SY, Zhu K, Potter JF, et al. Health-related characteristics and dietary intakes of male veterans and non-veterans in the Multiethnic Cohort Study (United States). J Mil Veterans Health 2011; 19: 4–9 [PMC free article] [PubMed] [Google Scholar]