Abstract
Background
There is growing evidence that indigo carmine chromoendoscopy is effective for the in vivo diagnosis of colonic polyps. However, the impact of colonoscope resolution on diagnostic accuracy has not been investigated.
Objective
We aimed to compare the effectiveness of in vivo diagnosis of small colonic polyps using indigo carmine dye spray with standard-definition and high-definition colonoscopes.
Methods
Procedures were performed using Fujinon colonoscopes and EPX 4400 processor. Fujinon standard-definition (SD) and high-definition (HD) colonoscopes were used, with the endoscopist blinded to colonoscope definition. Polyps <10 mm were assessed using 0.2% indigo carmine dye spray, with the predicted diagnosis recorded. In each case the kind of colonoscope (SD or HD) was recorded. Polyps were removed and sent for histological analysis, with the pathologist blinded to the diagnosis made by the endoscopist. The predicted diagnosis was compared with the true histology to calculate the accuracy, sensitivity and specificity of in vivo assessment using either SD or HD scopes.
Results
In total 237 polyps <10 mm in size were examined. There was no statistically significant difference for any of the measured parameters between SD and HD assessments, with an accuracy, sensitivity and specificity of 89%, 91% and 87% with SD colonoscopes and 92%, 96% and 84% with HD colonoscopes.
Conclusions
The accuracy of in vivo assessment of small colonic polyps with indigo carmine dye spray is excellent with standard-definition colonoscopes and is not improved with high-definition colonoscopes.
Keywords: Colonic polyp, in vivo diagnosis, indigo carmine
Introduction
There has been considerable interest in the in vivo diagnosis of colonic polyps, with studies suggesting that such techniques could be used as an alternative to conventional histology.1,2 The American Society of Gastrointestinal Endoscopy (ASGE) have recently released guidelines for proposed standards for in vivo diagnosis in place of conventional histology,3 making the prospect of such techniques being used in mainstream practice a realistic proposition. Furthermore, there is a growing body of evidence which suggests that this would be a cost-effective approach.4–6 However, there has been very little published describing the impact of colonoscope resolution on the accuracy of this technique. Most of the research has been conducted using high-definition (HD) equipment. Whilst there has been a growth in the availability of HD colonoscopes, which are increasingly being used for screening procedures and for the examination of colonic polyps, such equipment requires a significant initial financial investment, and are more expensive to purchase than conventional colonoscopes. This can act as a significant barrier to the adoption of in vivo diagnosis.
There has been one study which compared accuracy of in vivo diagnosis using white light and the electronic imaging modality FICE using standard-definition (SD) and HD colonoscopes.7 This found that whilst endoscope definition made no difference to the white light diagnosis, which was inadequate for in vivo assessment, there was a significant reduction in sensitivity for neoplasia when assessments were made using FICE. This is important as it raises an important question regarding the applicability in vivo diagnosis when using older equipment.
Indigo carmine is a surface dye which is widely used for in vivo diagnosis of colonic polyps. There have been studies which suggest it can be used effectively with SD colonoscopes.8 However, the results were relatively poor, with a sensitivity and specificity of 82%, below the levels expected for effective in vivo diagnosis. It is unclear whether this is a result of endoscope resolution or due to another factor. It is therefore uncertain whether there is a benefit from using it with HD colonoscopes when making an assessment with indigo carmine dye spray.
Aims of the study
We aimed to compare the accuracy of in vivo diagnosis of small colonic polyps using indigo carmine dye spray with SD and HD colonoscopes.
Methods
The study had ethical approval (REC No. 09/H0501/94) and was registered with the European clinical trials database Eudra CT 2009-016742-10 and with clinical trials.gov NCT01182623.
This was a prospective double-blinded (endoscopist and pathologist) observational study where 237 consecutive polyps were assessed by a single endoscopist (PB) who was trained and experienced in in vivo diagnostic methods. Patients were all faecal occult blood-positive referrals for bowel cancer screening colonoscopy on a standard screening list where SD and HD colonoscopes were routinely used. Exclusion criteria were a diagnosis of inflammatory bowel disease, familial polyp syndromes or poor bowel preparation, all of which could influence surface pattern assessment.
All of the procedures were performed using Fujinon colonoscopes. These were equipped with either standard-definition EC530 (SD) 410,000 pixel CCD or high-definition EC530 (HD) 650,000 pixel Super CCD chips. The type of colonoscope (HD or SD) was allocated randomly by the nursing staff on a basis of availability and was not influenced by the endoscopist, who was blinded to the type of colonoscope being used. Each colonoscope was allocated a unique identification number through which its resolution could be identified. This was recorded prospectively and not unblinded until the end of the study. Optical magnification, if available, was not used. Images were viewed on a flat screen Sony 24-inch WUXGA LCD monitor (LMD-2450 MD) capable of 1080i resolution. The SD and HD equipment had all identifying markers removed, and all equipment appeared outwardly identical in all respects. All procedures were performed on routine Bowel Cancer Screening programme (BCSP) lists which were not reduced for the purposes of the study. No additional time was allowed for assessments for the purposes of the study.
Polyps were identified with white light endoscopy and cleaned of debris prior to assessment using 10–20 ml of water with 2 ml of 10% simethicone. Lesion size was assessed using the open jaws of the biopsy forceps. Polyps >10 mm in size were excluded from analysis. Small (<4 mm) rectal hyperplastic polyps were excluded as these are considered easy to assess and of no clinical significance, and could potentially bias the results of the study. Morphology was recorded using the Paris classification system. Indigo carmine dye spray was then applied at a concentration of 0.2%. This was applied directly by flushing 10 ml of dye down the biopsy channel followed by 20 ml of air. The mucosa was deflated and then reinflated to ensure even coverage and excess dye suctioned. The endoscopist assessed the lesions by examination of surface pit patterns as described by Kudo et al.9 Patterns I and II were defined as hyperplastic, III-s, III-L and IV as adenoma and V as cancer. The diagnosis made by the endoscopist (neoplastic (adenoma + cancer) vs. non-neoplastic (hyperplastic)) was recorded and the polyp then removed and sent for histological analysis by an expert gastrointestinal pathologist blinded to the diagnosis made by the endoscopist.
The study was prospectively powered. The assumptions were made that 70% of polyps found are neoplastic, that the true sensitivity for neoplasia with indigo carmine using HD endoscopes would be between 90–99%, and that the true sensitivity for neoplasia using SD endoscopes would be between 80–90%. With 80% power (assuming a 5% significance level and phi coefficient of 0.2) to demonstrate a 15% absolute difference in the accuracy for neoplasia between HD and SD endoscopy 152 neoplastic polyps would need to be assessed in total, with 76 in each group, requiring a total sample of 218 polyps (neoplastic + hyperplastic) to produce significant results.
IBM SPSS-18 for Windows was used for statistical calculations, and analysis was performed on a per lesion basis. Pearson’s uncorrected chi test was used for the comparison between SD and HD results. The accuracy for the correct diagnosis of neoplasia using indigo carmine for both SD and HD endoscopes was compared with histology and 95% confidence intervals calculated.
Results
In total, 150 patients underwent colonoscopy using either SD or HD colonoscopes; 80 patients were examined using SD colonoscopes (group A), with 70 patients examined with HD colonoscopes (Group B). Group A (SD): 51 patients were found to have 114 polyps<10 mm in size. Group B (HD): 39 patients were found to have 123 polyps <10 mm in size.
Among the patients with polyps, 58/90 were male. Mean age was 65 (range 60–75). The size and morphology of the polyps are shown in Table 1.
Table 1.
Size and morphology of polyps
| Group A (SD) | Group B (HD) | |
|---|---|---|
| No. of polyps | 114 | 123 |
| Mean size (mm) | 5.0 | 4.3 |
| Range (mm) | 1–10 | 1–10 |
| No. <5 mm | 69 | 96 |
| Morphology | ||
| Pedunculated | 5 | 10 |
| Sessile | 59 | 73 |
| Flat | 50 | 40 |
| Location | ||
| Caecum | 9 | 8 |
| Ascending | 13 | 13 |
| Transverse | 29 | 19 |
| Descending | 4 | 13 |
| Sigmoid/rectum | 59 | 70 |
| Histology | ||
| Hyperplastic | 37 | 45 |
| Tubular adenoma | 54 | 67 |
| Villous adenoma | 0 | 1 |
| Tubulovillous adenoma | 22 | 9 |
| Adenocarcinoma | 1 | 1 |
More polyps were found using HD colonoscopes, with a mean of 3.1 polyps per patient in the HD limb vs. 2.2 polyps per patient in the SD limb. This difference was not statistically significant (p = 0.175).
Accuracy, sensitivity and specificity, negative predictive value and positive predictive value for assessments with indigo carmine chromoendoscopy using SD and HD endoscopes was performed. This is shown in Table 2. There was no statistically significant difference for any of the measured parameters between SD and HD assessments (see Figure 1). A breakdown of the observed pit patterns for each histology type for SD and HD assessments is shown in Table 3.
Table 2.
Accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for indigo carmine using HD and SD endoscopes
| HD | SD | p value | |
|---|---|---|---|
| Accuracy (95% C.I.) | 92.0% (88–95) | 89.0% (85–93) | 0.51 |
| Sensitivity (95% C.I.) | 96.2% (91–99) | 90.9% (85–94) | 0.317 |
| Specificity (95% C.I.) | 84.4% (75–89) | 87.1% (73–95) | 1.000 |
| PPV (95% C.I.) | 91.5% (86–94) | 94.6% (89–98) | 0.54 |
| NPV (95% C.I.) | 92.7% (82–98) | 79.4% (67–87) | 0.17 |
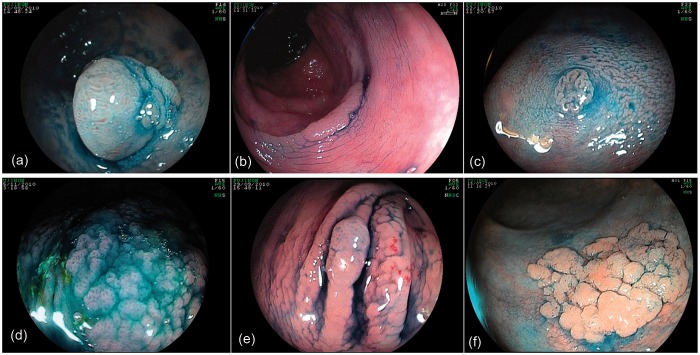
Figure 1.
A selection of polyps examined with high definition (a,b,c) and standard definition (d,e,f) colonoscopes. Note the visible differences are minimal.
Table 3.
Pit pattern observed for each histology type for HD and SD assessments
| HD |
SD |
|||
|---|---|---|---|---|
| Kudo pattern | Hyperplastic | Neoplastic | Hyperplastic | Neoplastic |
| I | 26 | 3 | 25 | 5 |
| II | 12 | 0 | 6 | 2 |
| III-s | 6 | 37 | 4 | 41 |
| III-L | 1 | 35 | 2 | 25 |
| IV | 0 | 2 | 0 | 2 |
| V | 0 | 1 | 0 | 2 |
Rescope intervals
Surveillance intervals were calculated using BSG10 and ASGE11 guidelines. There were five patients who were excluded from analysis due to the coexistence of larger polyps which would have required histology. It was found that when using HD endoscopes surveillance intervals were set correctly in 34/34 = 100% of patients with both BSG and ASGE standards. With SD endoscopes surveillance intervals were set correctly in 48/51 = 94% of patients with ASGE guidelines and 49/51 = 96% patients using British Society of Gastroenterology (BSG) guidelines. There were no statistically significant differences observed (see Table 4).
Table 4.
Surveillance intervals using BSG and ASGE guidelines
| HD | SD | p value | |
|---|---|---|---|
| BSG surveillance intervals | 34/34 100% | 49/51 96% | 0.661 |
| ASGE surveillance intervals | 34/34 100% | 48/51 94% | 0.401 |
Discussion
This study demonstrates that HD endoscopes offer no benefits for the purposes of in vivo diagnosis when using indigo carmine dye spray. Accuracy is excellent with both modalities of assessment, meeting the standards outlined in the ASGE guidelines. This is important, as it contrasts the results previously published with white light and the electronic imaging modality FICE, where HD colonoscopes offered a significant improvement in sensitivity for neoplasia.7
Whilst it is likely that in time HD colonoscopes will replace SD equipment, currently most units are managing with a mix of old and new equipment. This makes the question of how endoscope resolution impacts on capacity to make an in vivo diagnosis very relevant, with endoscopists faced with the decision as to whether the equipment in their department is adequate for task. We feel that this data is reassuring that in vivo diagnosis can be safely performed using relatively modest equipment. Indigo carmine is a cheap, universally available agent which is easy and safe to use. We believe that a safe approach to in vivo diagnosis would be to make a diagnosis based on indigo carmine dye spray if HD equipment is unavailable.
It is important in the current era of austerity to be critical regarding the equipment we purchase. HD colonoscopes still carry a significantly higher price than SD colonoscopes, and we have to be able to justify our purchasing decisions. Decisions as to whether all equipment in a unit needs to be changed are potentially very expensive. We feel our data contributes to this by offering a potential route for making an in vivo diagnosis with SD equipment.
The key strengths of our study are that all assessments were performed in real time by a single endoscopist highly skilled in in vivo diagnosis. Therefore we are unlikely to be underreporting accuracy, and this is likely to represent a true representation of what can be achieved with indigo carmine and SD colonoscopes. It is a well-powered study with limited ability for bias.
The key weakness of our study is that it is a single centre, single endoscopist experience. As such we cannot be certain how transferrable these results are to others. It may be that less experienced endoscopists would find increased benefit from HD colonoscopes, where the patterns may appear clearer. This would be a good area for further study.
It is important to understand that this data relates to lesion characterization only. Lesion detection is a fundamentally important issue, and it is only when lesions are identified that they can be appropriately managed. The effect of colonoscope definition on lesion detection is unclear, with studies producing mixed results.12–15 Our data did not show a statistically significant difference in lesion detection between SD and HD colonoscopes (p = 0.175). However, it is important to stress that the study was not powered to investigate lesion detection, and therefore this should be interpreted with caution. We feel further studies are needed to investigate this important area.
In summary, this study demonstrates that the accuracy of in vivo assessment of small colonic polyps with indigo carmine is excellent with SD colonoscopes, with no improvement seen when using HD colonoscopes. This provides the endoscopist with evidence of an accurate method of lesion assessment using SD equipment.
Acknowledgements
Dr G Longcroft-Wheaton: first author of the article. Responsible for study design, data collection, analysis and interpretation. James Brown: scientific review of study design. Dr David Cowlishaw: pathologist reporting on the specimens. Mr Bernard Higgins: Power calculations and statistical analysis. Professor Pradeep Bhandari MD, FRCP: Conception and study design. Endoscopist performing the procedures.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare that there is no conflict of interest.
References
- 1.Kato S, Fujii T, Koba I, et al. Assessment of colorectal lesions using magnifing colonoscopy and mucosal dye spraying: Can significant lesions be distinguished? Endoscopy 2001; 33: 306–310 [DOI] [PubMed] [Google Scholar]
- 2.Fu KL, Sano Y, Kato S, et al. Chromoendoscopy using indigo carmine dye spraying with magnifying observation is the most reliable method for differential diagnosis between non-neoplastic and neoplastic colorectal lesions: A prospective study. Endoscopy 2004; 36: 1089–1093 [DOI] [PubMed] [Google Scholar]
- 3.Rex D, Kahl C, O'Brien M, et al. The American Society of Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminuitive colorectal polyps. Gastrointest Endosc 2011; 73: 419–422 [DOI] [PubMed] [Google Scholar]
- 4.Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastrienterol Hepatol 2010; 8: 865–869 [DOI] [PubMed] [Google Scholar]
- 5.Kessler WR, Imperiale TF, Klein RW, et al. A quantitative assessment of the risks and cost savings of forgoing histologic examination of diminuitive polyps. Endoscopy 2011; 43: 683–691 [DOI] [PubMed] [Google Scholar]
- 6.Longcroft-Wheaton GR, Higgins B, Bhandari P. Flexible spectral imaging color enhancement and indigo carmine in neoplasia diagnosis during colonoscopy: A large prospective UK series. Eur J Gastroenterol Hepatol 2011; 23: 903–911 [DOI] [PubMed] [Google Scholar]
- 7.Longcroft-Wheaton G, Brown J, Cowlishaw D, et al. High definition vs. standard definition colonoscopy in the characterization of small colonic polyps: Results from a randomized trial. Endoscopy 2012; 44: 905–910 [DOI] [PubMed] [Google Scholar]
- 8.Elsen GM, Kim CY, Fleischer DE, et al. High-resolution chromoendoscopy for classifying colonic polyps: a multicenter study. Gastrointest Endosc 2002; 55: 687–694 [DOI] [PubMed] [Google Scholar]
- 9.Kudo S, Tamura S, Nakajima T, et al. Diagnosis of colorectal tumourous lesions by magnifying endoscopy. Gastrointest Enodsc 1996; 44: 8–14 [DOI] [PubMed] [Google Scholar]
- 10.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate to high risk groups. Gut 2010; 59: 666–689 [DOI] [PubMed] [Google Scholar]
- 11.Davila RE, Rajan E, Baron TH, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc 2006; 63: 546–557 [DOI] [PubMed] [Google Scholar]
- 12.Buchner AM, Shahid MW, Heckman MG, et al. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol 2010; 8: 364–370 [DOI] [PubMed] [Google Scholar]
- 13.Burke CA, Choure AG, Sanaka MR, et al. A comparison of high-definition versus conventional colonoscopes for polyp detection. Dig Dis Sci 2010; 55: 1716–1720 [DOI] [PubMed] [Google Scholar]
- 14.East JE, Stavrindis M, Thomas-Gibson S, et al. A comparative study of standard vs. high definition colonoscopy for adenoma and hyperplastic polyp detection with optimized withdrawal technique. Aliment Pharmacol Ther 2008; 28: 768–776 [DOI] [PubMed] [Google Scholar]
- 15.Tribonias G, Theodoropoulou A, Konstantinidis K, et al. Comparison of standard vs. high-definition, wide-angle colonoscopy for polyp detection: A randomized controlled trial. Colorectal Dis 2010; 12(10 Online): e260–e266 [DOI] [PubMed] [Google Scholar]