Abstract
Introduction
The wireless motility capsule (WMC) is a novel ambulatory technology that concurrently measures intraluminal pH, temperature, and pressure as it traverses the gastrointestinal tract.
Objectives
We aim to provide a concise summary of the WMC, detailing the procedure for its administration and the parameters it records. We also review the evidence that has validated the WMC against other methods currently regarded as ‘gold standard’.
Conclusions
The WMC offers a number of advantages over and above current techniques, especially with respect to patient tolerability, safety, and standardization. The WMC represents a considerable enhancement of the researchers’ and clinicians’ investigatory armamentarium. If this technology becomes widely adopted, coupled with international consensus upon the interpretation of physiological data derived therein, it may herald a new and exciting era in gastrointestinal physiology.
Keywords: Gastrointestinal dysmotility, motility measurement, wireless motility capsule
Introduction
Adequate motility is a prerequisite for the efficient transit of contents through the gastrointestinal (GI) tract. In a proportion of patients presenting with GI symptoms, dysmotility may be demonstrable using physiological tests. Any novel technique for investigating GI dysmotility must possess a number of fundamental characteristics. Firstly, it needs to be a method of measuring motor activity whose normal ranges are known and well circumscribed from those of disease. Secondly, it must be standardized, reproducible, and readily interpretable, and finally it must be acceptable to the patient particularly in terms of invasiveness. Whilst these characteristics may sound relatively straightforward to satisfy, it belies the reality that only relatively few techniques have become established in mainstream clinical practice, such as oesophageal manometry, 24-h pH-metry, colonic transit measures using radio-opaque markers (ROM), and gastric emptying scintigraphy (GES). The literature is awash with examples of techniques that have failed to become established outside of the environments of tertiary care and research centres.1,2 These failures have been largely as a consequence of not fulfilling these aforementioned principles.
Given the marked reduction in health-related quality of life and wider socioeconomic burden of primary and secondary disorders where GI dysmotility is prominent, there remains a clinical need for motility testing particularly in those patients who have an unsatisfactory response to empirical medical therapy.3,4 In modern clinical practice, motility testing is most commonly undertaken upon the region of the GI tract where the focus of abnormality is considered to ‘reside’, despite recent evidence suggesting to the contrary.5 For instance, in a proportion of patients with slow transit constipation, retarded gastric and/or small bowel emptying has been demonstrated rather than colonic dysmotility per se.6 Thus a comprehensive assessment of motility throughout the GI tract maybe beneficial as it may objectively inform diagnoses and guide further management decisions. The wireless motility capsule (WMC) provides a novel method of measuring whole and regional GI transit times, pH, and temperature in a single, ambulatory, minimally invasive manner that overcomes many of the methodological impediments that have hindered other approaches. In this review article, we shall describe the WMC, review the test procedure, and the motility measurements it provides, comparing and contrasting other techniques that are currently available. Finally, we shall conjecture how the WMC may lead to novel insights within the field of GI physiology research and clinical practice in the future.
Wireless motility capsule
The WMC system (SmartPill; Given Imaging, Israel) comprises of an indigestible single-use capsule, an external data receiver and display/analysis software (Figure 1). The WMC has dimensions of 26.8 mm × 11.7 mm (vis-à-vis the wireless capsule endoscopy (WCE; PillCam; Given Imaging, Israel) measuring 26 × 11 mm) and is capable of measuring temperature (range 25–49℃), pH (range 0.05–9.0) and pressure (range 0–350 mmHg) in the milieu adjacent to the sensors. The accuracy of the temperature sensor is ±0.5℃, pH sensor ±0.5 pH units, and pressure ±5 mmHg below 100 mmHg.7 In contrast to the WMC, the WCE encompasses a miniature, encapsulated video camera designed to image the entire small bowel, taking approximately 50,000–60,000 digital images per study.8 Currently, the main indications for a WCE study are the diagnosis of obscure GI bleeding and the investigation of small bowel Crohn’s disease.9
Figure 1.
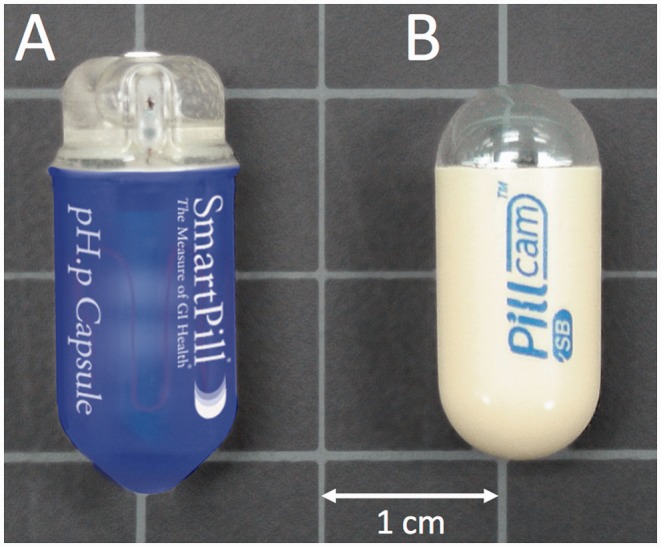
The external appearance of the wireless motility capsule (a), which records pH, temperature, and pressure in real time as it traverses the GI tract in comparison to a wireless endoscopy capsule (b). Image courtesy of Given Imaging.
In many respects, the WMC is not dissimilar to the WCE in that it is constructed of polyurethane and contains a battery that provides power to the capsule for at least 5 days.10 The WMC contains a high-frequency transmitter that broadcasts data in real time to an external receiver. The WMC has been validated for the objective measurement of gastric emptying time (GET), small bowel transit time (SBTT), and colonic transit time (CTT).11–13 The US Food and Drug Administration have approved the WMC for the measurement of GET for those in whom gastroparesis is suspected, the evaluation of CTT in patients with slow transit constipation, and the measurement of pH, pressure and temperature throughout the GI tract.14 These indications have been endorsed by the American and the European Neurogastroenterology and Motility Societies in a recently published position paper.15 WMC is not currently licensed for use in the paediatric population.
Wireless motility capsule test procedure
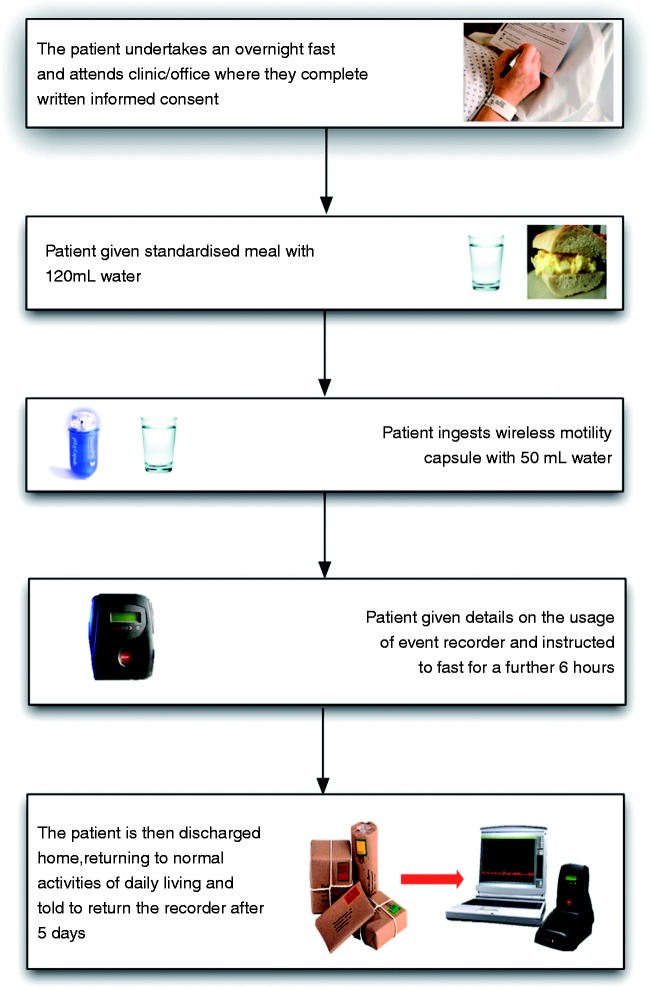
The WMC study can be performed in the outpatient/office setting after the patient has undergone an overnight fast and is schematically summarized in Figure 2. Drugs that influence GI motility such as prokinetics, antidiarrhoeals, and laxatives should be discontinued for at least 3 days prior to the test. Similarly, drugs that can modulate the acid/base balance within the GI tract, such as proton pump inhibitors, histamine receptor antagonists, and antacids, are recommended to be discontinued 7, 3, and 1 days, respectively, prior to the test.10 Although there is no absolute consensus, our own practice is to advise our patients to avoid these aforementioned medications during the WMC procedure. However, medication restrictions are ultimately at the investigators discretion.
Figure 2.
A schematic representation of the patient procedure for performing a wireless motility capsule study.
After obtaining written informed consent, the patient is asked to consume a standardized meal consisting of an egg sandwich (255 kcal, 2% fat, 1 g fibre) or a nutritionally equivalent SmartBar (260 kcal, 2% fat, 1 g fibre) (Given Imaging, Israel) taken with 120 ml of water, thereby initiating postprandial motility following the overnight fast.16 The patient is then instructed to swallow the WMC with the aid of 50 ml of water. Successful introduction of the WMC is confirmed through the patient reporting ingestion of the WMC in the absence of dysphagia and through an acidic pH being recorded on the small lightweight portable external recorder (suggesting passage of the WMC into the stomach), which is then given to the patient to wear during the duration of the study, typically for 1–5 days. The external data receiver also allows the patient to record, and thus electronically diarize, symptoms, meals, sleep, and bowel movements. In addition, a number of centres also ask patients to keep a written diary. During the study, when the external recorder is not being worn on the person, the patient should be instructed to keep the data receiver within 1.5 m of their abdomen as to avoid loss of data transfer from the WMC to the receiver. Body mass index can also influence data transfer as the body attenuates the signal and using the WMC in patients with a body mass index >35 kg/m2 may be problematic due to signal loss in overlying adipose tissue. This can be mitigated by keeping the recorder in close proximity to the patient’s abdomen especially during the first 6 h of the study when the capsule is in the stomach. Following successful ingestion, the patient can be discharged and may return home. They should avoid eating again for another 6 h in order that a fasting motility pattern is re-established and an accurate measurement of GET may be made. However, following this period, patients may resume their normal diet and activities of daily living, thus giving a prolonged and ambulatory measure of GI motility. However, the manufacturer advocates that patients avoid heavy physical exercise, such as abdominal scrunches or contact sports, for the duration of the investigation. The data receiver is then returned to the investigator after 5 days, where it is docked with a standard personal computer and the data is downloaded and analysed using a dedicated software package (MotiliGI; Given Imaging, Israel). Analysis is automated, although the investigator may perform a personal review of the data if needed. If the WMC has not excreted within 5 days, it is recommended that the patient returns to the unit so the ‘live’ monitoring feature of the system can be utilized and the data receiver batteries can be charged in the docking station. The ‘live’ monitoring feature allows the investigator to review the data to confirm or refute excretion of the WMC. If the receiver cannot establish telemetric contact with the WMC, or the temperature is recorded as being that of the ambient environment, this suggests that excretion has occurred. If uncertainty remains regarded capsule excretion, the investigator may decide to carry out a plain abdominal radiograph to confirm the location. If the WMC has been retained, administration of a stimulant laxative often results in a retained WMC being expelled.
Measurements derived from a wireless motility capsule study
Segmental and whole-gut transit times
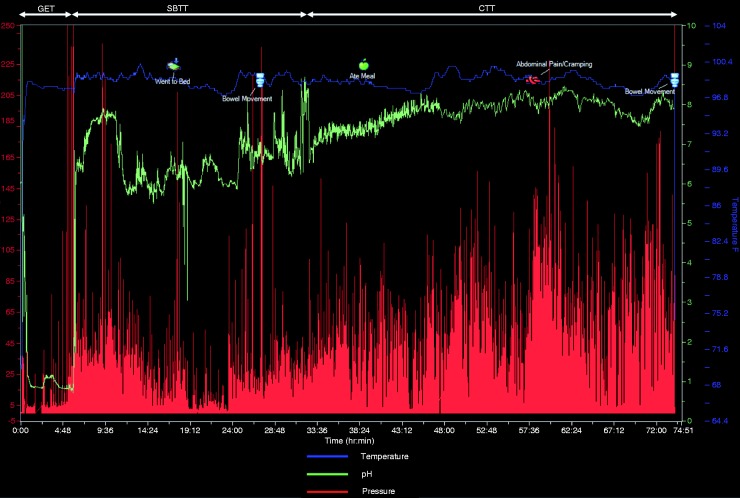
Through combining the interpretation of pH, time, pressure, and temperature data, a number of anatomical landmarks maybe defined around which regional transit times can be derived (Figure 3). Capsule ingestion time is marked by a sharp rise from ambient room temperature to that of the body. GET is defined as the time from ingestion of the WMC to an abrupt and sustained rise in pH by ≥2.0 units from the gastric baseline to pH ≥4, denoting the passage of the capsule from the acidic environment of the stomach to the relatively alkaline environment of the proximal small bowel.17 SBTT is defined as the time from exit from the stomach, as detailed above, to a sharp drop in pH of ≥1.5 units as the WMC traverses the ileo-caecal valve at least 30 min after entry into the small bowel from the stomach.18 This change in pH across the ileo-caecal valve is postulated to be due to alterations in the GI microbiota, where in the caecum there is heightened production of the short chain fatty acids through the process of fermentation.19 CTT is therefore defined based on entry of the WMC into the colon until expulsion/defaecation of the capsule from the body as denoted by a drop in the temperature as it enters the external environment. By combining the measurements of GET, SBTT, and CTT, whole-gut transit time (WGTT) may be derived. The normal values for a WMC study are detailed in Table 1.20
Figure 3.
A typical tracing from a WMC recording.
Time along the X-axis, pressure on the Y1-axis (red line), and pH (green line) and temperature (blue line) on the Y2-axis. Gastric emptying time (GET), small bowel transit time (SBTT), and colonic transit time (CTT) are illustrated. Whole-gut transit time is derived from the addition of GET, SBTT, and CTT.
Table 1.
The normative reference data for whole-gut and regional transit times using the WMC
| Time | |
|---|---|
| Gastric emptying | ≥4 h suggests delayed gastric emptying |
| Small bowel transit | Normal range 2.5–6 h |
| Colonic transit | ≥59 h indicates delayed colonic transit |
| Whole-gut transit | Normal <73 h |
The data are a conglomeration from a number of validation studies of healthy subjects. Data kindly supplied by Given Imaging.19
Pressure measurements
In addition to deriving transit times, the WMC also directly measures intraluminal pressures within the GI tract, essentially acting as a free-floating single pressure transducer, recording both the amplitude and frequency of contractions. These data are then transformed, using the analysis software after download, into area under the curve (AUC) and a motility index (MI). MI is calculated as Ln(sum of amplitude × number of contractions +1).21 AUC and MI measurements facilitate both chronotropic and ionotropic assessments of GI motility although their exact role remains to be fully determined. Similarly, the interpretation of pressure parameters in the context of the diarized dietary data allows a degree of extrapolation as to physiological changes induced by the fed state.
Comparison of wireless motility capsule to other techniques
Currently, multiple techniques are used for the measurement of GI motility although their availability is variable. Table 2 summarizes these in comparison to the WMC in terms of their relative advantages and disadvantages.
Table 2.
Comparison of the various techniques, currently utilized, indicating their relative advantageous and disadvantageous features
| Technique | Area of the GI tract evaluated | Length of stay required in clinic/office | Invasiveness | Radiation exposure | Physiological conditions of measurement | Standardization of test | Measurement of propagating contractions | Availability | Cost (£) | Ease of interpretation of the result |
|---|---|---|---|---|---|---|---|---|---|---|
| Wireless motility capsule | Pan-GI | c.30 min | Low | No | Yes | Yes | No | Very limited | c.1500 | Relatively easy |
| Whole-gut scintigraphy | Pan-GI | c.8 h | Non-invasive | Yes | Yes | Yes | No | Very limited | c.1000 | Difficult |
| Gastric emptying scintigraphy | Stomach | c.5 h | Non-invasive | Yes | Yes | No | No | Widely | 722 | Moderate |
| 13C octanoic acid breath test | Stomach | c.4 h | Non-invasive | No | Yes | Yes | No | Very limited | c.600 | Relatively easy |
| Antroduodenal manometry | Distal stomach / proximal small bowel | c.4 h | High | No | No | No | Yes | Very limited | c.1000 | Difficult |
| Small bowel radiograph series | Small bowel | c.6 h | Non-invasive | Yes | No | No | No | Widely | 154 | Moderate |
| Radio-opaque marker study | Colon | c.30 min (once, twice, or three times depending on protocol used) | Low | Yes | Yes | No | No | Widely | 62 | Easy |
| Colonic manometry | Distal colon | c.6 h | High | No | No | No | Yes | Very limited | 599 | Difficult |
Invasiveness is defined according to whether a catheter (high) or an ingestible object (low) is introduced into the patient. Availability is defined as limited (largely available in tertiary care centres) or widely available (largely available in secondary care centres). The relative expense of each test is defined according to the UK National Health Service 2012–13 tariff/interprovider costs. Ease of interpretation of the test was considered easy if it was interpretable by a non-specialist clinician, moderate if interpretable by a trained gastroenterologist/physiologist, and difficult if subspecialist expertise were needed.
Stomach
GES is considered to be the contemporaneous gold standard for diagnosing gastroparesis.22,23 This non-invasive quantitative method involves the patient consuming a 99 m technetium-labelled meal, following which gastric emptying is measured by scintigraphy. Whilst available in most secondary care centres, there has been a disappointing lack of standardization with particular differences including the constituent components of the test meal, patient positioning, and duration/frequency of scintigraphic imaging. Another technique, although limited to tertiary centres, is the breath testing method, which provides an indirect estimate of gastric emptying by utilizing a non-radioactive 13C isotope bound to a digestible substance. GES necessarily involves exposing the patient to a dose of radiation, albeit small, whilst both GES and breath testing methods require that the patient remains in situ in the clinic/office for a prolonged period of time often in the order of 6 h. Kuo et al.11 have performed a comparative head-to-head analysis of WMC and GES, assessing the discriminative value between 87 healthy subjects from 61 patients with known gastroparesis. These participants were simsultaneous studied with the WMC and GES. Following a period of fasting, subjects ingested the WMC and a 99 m technetium radiolabelled meal after which scintigraphic images were obtained every 30 min for 6 h.11 The diagnostic accuracy derived from the AUC between healthy subjects and patients with gastroparesis was 0.83, indicating good agreement.24 Using 300 min as a ‘cut off time’ for gastric emptying, the WMC demonstrated a sensitivity of 0.65 and specificity of 0.87 for making the diagnosis of gastroparesis. Based on these data, the WMC is an accurate method offering a non-radioactive, standardized, ambulatory alternative to GES. However, it must be noted that, since the WMC is an indigestible capsule, it may not be as ‘physiologically’ akin to a radiolabelled meal per se. To date, two important studies have examined the relationship of the emptying of a meal to the emptying of WMC, reporting a strong correlation between the two thus suggesting that differences between gastric emptying of a digestible vs. indigestible meal maybe small but further work in this area is warranted.25,26
Small bowel
There are a number of methods that are presently utilized to evaluate SBTT which include antroduodenal manometry (ADM), breath testing, a small bowel radiographic series, and whole-gut scintigraphy (WGS). However, many of these methods are cumbersome, invasive, lack standardization, and do not evaluate patients under normal, or near normal, physiological conditions. For instance, during ADM, a catheter that has from six to eight pressure transducers mounted along its length is introduced transnasally, often over a protracted period.27 Its routine clinical usage is therefore limited by a lack of patient tolerance in addition to the high degree of expertise needed to effectively interpret its results. In a study that aimed to determine whether solids empty from the stomach following resumption of phase-III migrating motor complexes (MMC), 15 healthy subjects underwent concurrent ADM and WMC.26 Pressure traces from the WMC showed high-amplitude phasic contractions before exit of the capsule into the small bowel, with gastric residence time correlating with the length of time at which the first phase-III MMC was re-established following feeding (r = 0.813; p < 0.01). Brun et al.28 further assessed the correlation of high-amplitude contractions measured by WMC with phase-III MMC determined by simultaneous measurements by ADM in 18 healthy controls. WMC detected 86% of MMC events measured by ADM with a negative predictive value of 99.9%. However, these studies utilized water-perfused catheter systems, which may be confounded by a number of factors inherent to the catheter itself namely that during prolonged recordings subjects may receive large amounts of water from the system perfusion, may feel uncomfortable, and that accurate evaluation of the pyloric pressures maybe limited as a result of catheter migration. The recent advent of solid-state high-resolution ADM catheters may address some of these difficulties in future.29
WGS can be used to estimate SBTT and this technique has been compared to WMC in a small study of 10 subjects, in which the two procedures were concurrently undertaken.30,31 It was shown that WMC measured small bowel contractions per min showed a positive correlation with percentage scintigraphic small bowel transit (r = 0.69; p = 0.05). However, further study comparing and contrasting WMC with ADM in clinical populations, and particularly in patients with suspected foregut motility, is needed to truly define the role of WMC in these situations. Moreover, it must be acknowledged that a significant current methodological limitation of WMC is its lack of ability to directly detect propagating peristaltic waves, and thus phase-III MMC, since the capsule acts as a single free-floating sensor.26
Colon
In investigating patients with constipation, CTT has been proposed as a key investigation in delineating pathophysiology and informing management strategies.32 The gold standard for measuring CTT has been widely acknowledged to be WGS but it has not become generally adopted on account of the significant investments needed in time, equipment, and personnel.33 Therefore, WGS has not been widely adopted due to these constraints. Thus, CTT is therefore more commonly measured using ROM and is useful in delineating slow transit from normal transit.34 However, whilst WGS and CTT have been demonstrated to have a degree of comparability, variations between the two techniques are more marked as recording times are prolonged.35 Camilleri et al.13 studied 158 patients who underwent simultaneous measurement of CTT using ROM and WMC. The CTT differed between the two methods, with the median ROM time being 55 h vs. 43.5 h for the WMC. The positive agreement for delayed transit between ROM and WMC was approximately 80%, with negative agreement for detecting normal transit was 91% representing an overall agreement of 91%.13 While ROM studies are straightforward, inexpensive, and easily administered, difficulties in interpretation of the markers can lead to erroneous calculations and conclusions being drawn. As Szarka and Camilleri note,36 there is ‘substantial radiation exposure with this method and the need for multiple imaging every 30 minutes for up to 8 hours, which is inconvenient for the patient as well as the staff.’ These deficiencies are also compounded by a lack of standardization and variation in the protocols used.
Whole-gut transit time
In comparison to WGS, the WMC may offer a more readily adoptable investigation, particularly in secondary care centres where access to the appropriate nuclear medicine imaging maybe limited. In a small study, Maqbool et al.31 have compared WGS and WMC WGTT. In this study, the mean WGTT for the WMC was 36.2 ± 15.1 h. Total isotopic excretion time, a proxy maker of WGTT, was significantly longer than that measured by the WMC as the nuclear medicine measure represents the excretion of the entire quantity of radioactivity. Nevertheless, there was a good correlation between these variables (r = 0.79, p = 0.01) albeit when the data was adjusted for the presence of a single outlying subject who had very slow transit measured by scintigraphy (c.140 h) relative to WMC transit (23.5 h).
Contraindications and adverse events related to the wireless motility capsule
WMC testing is contraindicated in patients with a history of dysphagia, swallowing disorders, or in those with known or suspected strictures along the length of the GI tract due to the higher risks of capsule impaction and retention. The WMC is also contraindicated in patients with gastric bezoars, diverticulitis, Crohn’s disease, or those within 3 months of GI surgery or who have an implanted medical device (e.g. cardiac pacemaker). In the prototypical stages of the development of WMC technology, there were 36 instances of equipment or software failure in 495 patients (7.2%). In addition, of these, three further patients were unable to swallow the capsule.37 In the post-marketing analysis of c.5000 procedures, equipment failure has been reported to be in the order of 0.8%.10 The most serious adverse event related to WMC testing is retention of the capsule and thus, potentially, GI obstruction. This is defined as inability to confirm passage from the body 5 days after administration. Twenty cases of capsule retention have been reported; five of these were in the stomach, two in the small bowel, and the remainder in the colon. Of these, oesophagogastroduodenoscopy was successful in extracting five WMC from the upper GI tract, whilst 14 capsules passed spontaneously and, in the final case, a prokinetic agent was administered to aid passage. In the two cases of small bowel retention, caused by undiagnosed small bowel tumours, surgical intervention was also required. In addition, currently magnetic resonance imaging is contraindicated for WMC study. Our personal recommendation is that if there are clinical concerns regarding possible strictures/obstructions within the GI tract, then another investigation should be considered.
Future directions
Given that aberrancies of GI sensorimotor activity contribute, directly or indirectly, to a significant number of common clinical entities and account for significant expenditure, it is entirely appropriate that patients should expect a punctilious evaluation. However, given the piecemeal nature of GI physiology services in many centres, this ideal is rarely delivered.38 There are a plethora of underlying reasons for this but notably include marked variations in the differences in interpretation of physiological data in the context of the presenting symptoms, availability of techniques, coupled with fragmentary training and revalidation. In attempting to redress some of these difficulties with the introduction of the WMC, there is much that can be garnered from the experience encountered with high-resolution oesophageal pressure topography. This technology, now widely available, has combined high-resolution oesophageal manometry with pressure topography in the form of spatiotemporal plots, which has helped to refine, and to a degree, redefine both the clinical field and research agenda. This latter point has largely been as a consequence of the considerable foresight of the pioneers of this technique by forming a multinational iterative process that has developed a practical classification for the objective definition oesophageal motility disorders based on specific metrics, known as the Chicago classification.39 In order for the wider field of GI physiology to advance in a similar fashion, the development of a similar process of pattern recognition for data derived from the WMC is urgently needed. For instance, fast Fourier transformation spectral analysis of pressure data the combination of pH and pressure data have provided novel insights into landmark identification and gastroparesis, respectively.40,41 Moreover, WMC is beginning to demonstrate novel pathophysiological insights into prevalent disorders such as small intestinal bacterial overgrowth and bloating.19,42
Conclusions
The WMC is a novel and exciting technology that allows the measurement of GI motility in a convenient, acceptable, relatively noninvasive, physiological setting without needed to expose the patient to ionizing radiation. Nonetheless, the WMC is not without its disadvantages in that currently it remains confined to specialist centres and the absolute clinical utility of pressure and contractility measures remains to be fully determined. However the WMC does represent an exciting, readily adoptable, and acceptable technology for defining GI motility and transit.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
ARH is a paid instructor for Given Imaging. The other authors declare no conflict of interest.
References
- 1.Jonderko K, Kasicka-Jonderko A, Krusiec-Swidergol B, et al. How reproducible is cutaneous electrogastrography? An in-depth evidence-based study. Neurogastroenterol Motil 2005; 17: 800–809 [DOI] [PubMed] [Google Scholar]
- 2.Scott SM. Manometric techniques for the evaluation of colonic motor activity: current status. Neurogastroenterol Motil 2003; 15: 483–513 [DOI] [PubMed] [Google Scholar]
- 3.Ouyang A, Locke GR 3rd. Overview of neurogastroenterology-gastrointestinal motility and functional GI disorders: classification, prevalence, and epidemiology. Gastroenterol Clin North Am 2007; 36: 485–498, vii [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil 2008; 20(Suppl 1): 121–129 [DOI] [PubMed] [Google Scholar]
- 5.Lin HC, Prather C, Fisher RS, et al. Measurement of gastrointestinal transit. Dig Dis Sci 2005; 50: 989–1004 [DOI] [PubMed] [Google Scholar]
- 6.Shahid S, Ramzan Z, Maurer AH, et al. Chronic idiopathic constipation: more than a simple colonic transit disorder. J Clin Gastroenterol 2012; 46: 150–154 [DOI] [PubMed] [Google Scholar]
- 7. Glen Imaging. Motility monitoring. Available at: www.smartpillcorp.com (2009)
- 8.Lee HG, Choi MK, Shin BS, et al. Reducing redundancy in wireless capsule endoscopy videos. Comput Biol Med 2013; 43: 670–682 [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellence. Wireless capsule endoscopy for investigation of the small bowel. London: NICE, 2004.
- 10.Saad RJ, Hasler WL. A technical review and clinical assessment of the wireless motility capsule. Gastroenterol Hepatol 2011; 7: 795–804 [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther 2008; 27: 186–196 [DOI] [PubMed] [Google Scholar]
- 12.Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: comparison of the SmartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci 2009; 54: 2167–2174 [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Thorne NK, Ringel Y, et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil 2010; 22: 874–882, e233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Food and Drink Administration. Smartpill GI Monitoring System, version 2.0. Market approval notification. Silver Springs, MD: Department of Health and Human Services, FAO, 2009.
- 15.Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil 2011; 23: 8–23 [DOI] [PubMed] [Google Scholar]
- 16.Kuo B, Maneerattanaporn M, Lee AA, et al. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig Dis Sci 2011; 56: 2928–2938 [DOI] [PubMed] [Google Scholar]
- 17.Evans DF, Pye G, Bramley R, et al. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988; 29: 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarate N, Mohammed SD, O’Shaughnessy E, et al. Accurate localization of a fall in pH within the ileocecal region: validation using a dual-scintigraphic technique. Am J Physiol Gastrointest Liver Physiol 2010; 299: G1276–G1286 [DOI] [PubMed] [Google Scholar]
- 19.Hobson A, Mohammed SD, Dukes G, et al. Caecal pH measurement is an objective biomarker of excessive fermentation in patients with bloating and distension. Gut 2013; 62: A133–A134 [Google Scholar]
- 20. Imaging G. Normal values for gastrointestinal transit using SmartPill. 2013.
- 21.Tran K, Brun R, Kuo B. Evaluation of regional and whole gut motility using the wireless motility capsule: relevance in clinical practice. Therap Adv Gastroenterol 2012; 5: 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camilleri M. Gastrointestinal problems in diabetes. Endocrinol Metab Clin North Am 1996; 25: 361–378 [DOI] [PubMed] [Google Scholar]
- 23.Farmer AD, Kadirkamanathan SS, Aziz Q. Diabetic gastroparesis: pathophysiology, evaluation and management. Br J Hosp Med (Lond) 2012; 73: 451–456 [DOI] [PubMed] [Google Scholar]
- 24.Kestler HA. Calculation and display of confidence bounds for receiver operator characteristics. Methods Inf Med 1999; 38: 74–74 [PubMed] [Google Scholar]
- 25.Kuo B, Maneerattanaporn M, Lee AA, et al. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig Dis Sci 2011; 56: 2928–2938 [DOI] [PubMed] [Google Scholar]
- 26.Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil 2008; 20: 311–319 [DOI] [PubMed] [Google Scholar]
- 27.Byrne KG, Quigley EM. Antroduodenal manometry: an evaluation of an emerging methodology. Dig Dis 1997; 15(Suppl 1): 53–63 [DOI] [PubMed] [Google Scholar]
- 28.Brun R, Michalek W, Surjanhata BC, et al. Comparative analysis of phase III migrating motor complexes in stomach and small bowel using wireless motility capsule and antroduodenal manometry. Neurogastroenterol Motil 2012; 24: 332–e165 [DOI] [PubMed] [Google Scholar]
- 29.Patcharatrakul T, Gonlachanvit S. Technique of functional and motility test: how to perform antroduodenal manometry. J Neurogastroenterol Motil 2013; 19: 395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonapace ES, Maurer AH, Davidoff S, et al. Whole gut transit scintigraphy in the clinical evaluation of patients with upper and lower gastrointestinal symptoms. Am J Gastroenterol 2000; 95: 2838–2847 [DOI] [PubMed] [Google Scholar]
- 31.Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: comparison of the SmartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci 2009; 54: 2167–2174 [DOI] [PubMed] [Google Scholar]
- 32.Tack J, Muller-Lissner S, Stanghellini V, et al. Diagnosis and treatment of chronic constipation – a European perspective. Neurogastroenterol Motil 2011; 23: 697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maurer AH, Krevsky B. Whole-gut transit scintigraphy in the evaluation of small-bowel and colon transit disorders. Semin Nucl Med 1995; 25: 326–338 [DOI] [PubMed] [Google Scholar]
- 34.Rao SS, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging 2010; 5: 163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cremonini F, Mullan BP, Camilleri M, et al. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther 2002; 16: 1781–1790 [DOI] [PubMed] [Google Scholar]
- 36.Szarka LA, Camilleri M. Methods for the assessment of small-bowel and colonic transit. Semin Nucl Med 2012; 42: 113–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saad RJ, Hasler WL. A technical review and clinical assessment of the wireless motility capsule. Gastroenterol Hepatol (NY) 2011; 7: 795–804 [PMC free article] [PubMed] [Google Scholar]
- 38.Parkman HP, Orr WC. The gastrointestinal motility laboratory. Gastrointest Endosc Clin North Am 2009; 19: 171–184, viii [DOI] [PubMed] [Google Scholar]
- 39.Kahrilas PJ, Ghosh SK, Pandolfino JE. Esophageal motility disorders in terms of pressure topography: the Chicago Classification. J Clin Gastroenterol 2008; 42: 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon S, Shea B, Kuo B. Use of additional wireless motility capsule (WMC) parameters improves gastrointestinal landmark identification. Gastroenterology 2013; 144: S740–S740 [Google Scholar]
- 41.Parkman HP, Regan K, McCadden K, et al. Characterization of rapid gastric emptying using the wireless motility capsule. Gastroenterology 2013; 144: S847–S847 [Google Scholar]
- 42.Roland BC, Pasricha PJ, Ciarleglio MM, et al. Potential role for ileocecal valve dysfunction and small intestinal dysmotility in bacterial overgrowth as assessed by wireless motility capsule. Gastroenterology 2013; 144: S730–S730 [Google Scholar]