Abstract
Background
Bleeding and perforation are two major complications of gastric endoscopic submucosal dissection (ESD). There are only a few reports concerning gastric obstruction related to ESD in the stomach.
Objective
The aim of this study was to clarify the clinicopathological features of patients who experienced gastric obstruction after gastric ESD.
Methods
Clinicopathological data of 1878 patients who underwent gastric ESD from September 2002 to December 2010 were retrospectively reviewed. Data of lesion location, circumference, circumferential extent of ESD ulcer, specimen diameter, depth of cancer, ulcer findings within the lesion, curability of ESD, number of simultaneous lesions, and occurrence of post-operative bleeding and perforation were collected. The risk of gastric obstruction regarding lesion and procedure related factors were assessed, and treatment for these patients was studied.
Results
Gastric obstruction was observed in 2.5% of the patients (47/1878). Symptoms occurred in a median of 24 days after ESD. The incidence among patients with lesions in the upper part of the stomach was 4.7% (17/316), 0.36% (3/818) in the middle, and 3.8% (27/699) in the lower part. In relation to the circumferential extent, the incidence was 50% (33/66) among patients with a resection of >75% of the circumference. Stenosis was observed in 87% (41/47) of patients with gastric obstruction. Endoscopic balloon dilation was performed in 45 patients. Perforation due to EBD occurred in four patients; one was referred to surgery.
Conclusions
Patients with a wide resection of >75% of the circumference should be considered for early repeat endoscopy after ESD, and dilation should be performed with caution if found to have stenosis.
Keywords: Early gastric cancer, endoscopic balloon dilation (EBD), endoscopic resection, endoscopic submucosal dissection (ESD), stenosis
Introduction
Endoscopic submucosal dissection (ESD) is useful for treating superficial epithelial neoplasms of the gastrointestinal tract because of its high capability of en bloc resection.1 A distinctive feature of ESD is that a circumferential incision is made in the mucosa surrounding the lesion, followed by subsequent dissection of the submucosa beneath the lesion. This feature allows the operator to easily determine the area intended for resection. Therefore, ESD has been eagerly adopted in Japan for treating early gastric cancer. Lesions in areas previously considered difficult to treat using endoscopic mucosal resection (EMR), such as those involving the gastric cardia or pylorus, have now become targets for ESD. However, because of the widespread application of ESD to larger lesions and to those located in areas difficult to treat using EMR, the incidence of complications associated with ESD is reported to be higher than that with EMR.2
Bleeding and perforation are two major complications of gastric ESD. The incidence and management of most of these complications have been studied in detail.3–6 However, symptomatic stenosis is less frequently observed after gastric ESD, and there are only a few studies focusing on this complication.7–9 The circumferential extent of the resection has been reported as a risk of post-ESD stenosis. Still, the incidence of stenosis after gastric ESD remains unclear, and how to manage this complication is not established. The aim of this study was to clarify the risk factors of patients who developed gastric obstruction related to gastric ESD.
Materials and methods
Patients
Clinicopathological data of patients with gastric epithelial neoplasms who underwent ESD from September 2002 to December 2010 were retrospectively reviewed. Patients with lesions in a residual stomach or a gastric tube (n = 104), lesions treated by ESD with concomitant treatment such as photodynamic therapy (n = 26), recurrent lesions from a previous endoscopic resection (n = 78), and lesions not removed because of ESD cessation (n = 2) were excluded. Therefore, 1878 patients with 2314 lesions were included in this study (Figure 1). Patients who experienced gastric obstruction after ESD were selected. Clinicopathological data were retrieved from medical records, endoscopic reports, and histopathological reports.
Figure 1.
Flow chart of patients and lesions included in this study.
Indications for ESD
The general indications for ESD in our hospital are for lesions diagnosed as early gastric cancer, which meet the criteria for endoscopic resection proposed by the Japanese Gastric Cancer Society.10 These criteria include lesions with a preoperative diagnosis of differentiated-type intramucosal cancer without ulcer findings; differentiated-type intramucosal cancer no larger than 3 cm in diameter with ulcer findings; differentiated-type minute invasive submucosal cancer (invasion less than 500 µm below the muscularis mucosa) no larger than 3 cm in diameter; and undifferentiated-type intramucosal cancer no larger than 2 cm in diameter without ulcer findings. Curative resection was indicated for lesions that meet the above criteria with no lymphovascular infiltration and that were resected en bloc with negative margins.
Clinical course after ESD
ESD was performed using an insulated-tip knife (IT knife; KD-610 L; Olympus, Tokyo, Japan) or an IT knife-2 (KD-611 L; Olympus) as described previously.11–13 Written informed consent was obtained from all patients before ESD. According to the clinical pathway for ESD in our hospital, patients who underwent ESD uneventfully started a liquid diet intake 2 days after ESD and were discharged 4 days after ESD. All patients received intravenous omeprazole (40 mg) for 2 days followed by oral rabeprazole (10 mg) daily for 2 months after ESD. Follow-up oesophagogastroduodenoscopy (EGD) was scheduled 2 months after ESD and annually thereafter for asymptomatic patients to evaluate healing of the ESD ulcer or any other metachronous neoplastic lesions.
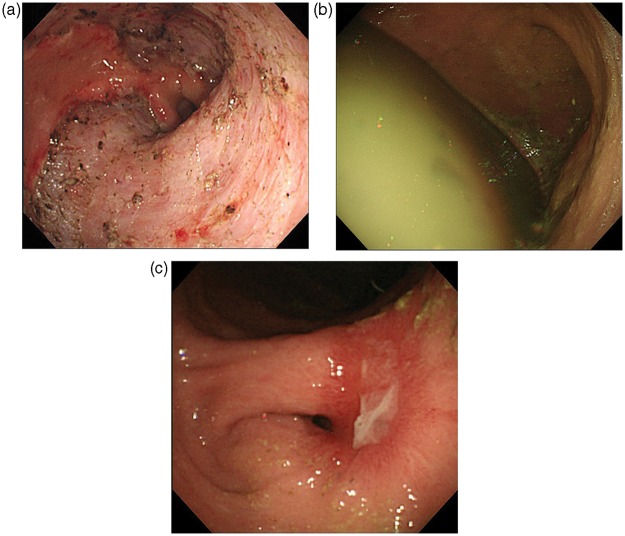
For patients with symptoms such as abdominal pain, fullness, dysphagia, or nausea, EGD was performed before the scheduled examination to evaluate any evidence of bleeding, stenosis, or presence of gastric residue (Figure 2). In this study, gastric obstruction due to ESD was defined as a condition of poor intake of food associated with findings of stenosis or massive gastric residue observed by EGD. Stenosis was defined as narrowing of the stomach to an extent that prevented a normal endoscope (9.8 mm in diameter, GIF-H260; Olympus) to pass through without endoscopic dilation.
Figure 2.
(a) A post-ESD ulcer immediately after resection from the gastric angle to the prepylorus. No immediate complication related to the ESD procedure was observed. Persistent early fullness and nausea developed. (b, c) Endoscopy performed 42 days after ESD showed massive gastric residue (b) and antral stenosis (c).
Data of lesion location, circumference, circumferential extent of ESD ulcer, specimen diameter, depth of cancer, ulcer findings within the lesion, curability of ESD, number of simultaneous lesions, and occurrence of post-operative bleeding and perforation were collected. The location of ulcers was described by UML classification: three portions are defined by subdividing the stomach by both lesser and greater curvature into three equal lengths: upper, middle, and lower.14 Upper included the cardia to the upper body, middle included middle body to gastric angulus, and lower included the gastric antrum to pylorus. Statistical analysis was done using chi-squared test for univariate analysis, and logistic regression was applied for multivariate analysis. A p-value less than 0.05 was considered statistically significant. All analyses were performed with Excel statistics 2012 (Social Survey Research Information, Tokyo, Japan.). This retrospective study was approved by the institutional review board of our hospital (24-J84-24-1-3).
Results
Demographic data
The patient population included 1452 men and 426 women. The median age was 71 years (range 27–93). The median diameter of gastric neoplasms was 18 mm (range 2–132).
Incidence of and factors affecting gastric obstruction
Gastric obstruction was observed in 2.5% of the patients (47/1878). The incidence of and factors related to gastric obstruction in patients after gastric ESD are shown in Table 1. The incidence among the location was 4.7% (17/316) in the upper, 0.36% (3/818) in the middle, and 3.8% (27/699) in the lower parts of the stomach. In relation to the circumferential extent of the ESD ulcer, gastric obstruction was observed in 0.1% of patients with a resection of <25% of the circumference (1/885), in 0.3% of cases with a resection of 25–50% (2/615), in 3.5% of cases with a resection of 50–75% (11/312), in 48% of cases with a resection of 75–99% (31/64), and in 100% of cases with whole circumference resection (2/2). In addition to location and circumferential extent, a specimen diameter of ≥40 mm and submucosal cancer were also related to gastric obstruction. The incidence was 5% among patients who were judged to have a non-curative resection according to the histopathological assessment after ESD. The circumference and ulcer findings of the lesion, the number of simultaneous lesions, and the occurrence of post-operative bleeding or perforation were not related to gastric obstruction.
Table 1.
Incidence of and factors related to gastric obstruction in patients after gastric ESD
| Study population | p-value | |
|---|---|---|
| Total patients | 47/1878 (2.5) | |
| Location | ||
| Upper | 17/361 (5) | <0.05a |
| Middle | 3/971 (0.3) | |
| Lower | 28/811 (4) | |
| Circumference | ||
| Anterior | 4/370 (1) | NS |
| Posterior | 10/445 (2) | |
| Greater curvature | 9/284 (3) | |
| Lesser curvature | 24/779 (3) | |
| Circumferential extent | ||
| <1/4 | 1/885 (0.1) | <0.05a |
| 1/4–1/2 | 2/615 (0.3) | |
| 1/2–3/4 | 11/312 (3.5) | |
| ≥3/4–semicircular | 31/64 (48) | |
| Whole | 2/2 (100) | |
| Specimen diameter (mm) | ||
| <30 | 0/59(0) | <0.05a |
| 30–40 | 7/445 (1.5) | |
| ≥40 | 40/1374 (3) | |
| Depth | ||
| Mucosal cancer | 32/1499 (2) | 0.04a |
| Submucosal cancer | 15/379 (4) | |
| Ulcer findings | ||
| Present | 6/297 (2) | NS |
| Absent | 41/1581(2.6) | |
| Judgement of resection | ||
| Curative | 26/1450 (2) | <0.05a |
| Non-curative | 21/428 (5) | |
| No. of lesions | ||
| Single | 35/1583 (2) | 0.07 |
| Multiple | 12/295 (4) | |
| Post-operative bleeding | ||
| Yes | 6/181 (3) | NS |
| No | 41/1697 (2) | |
| Perforation | ||
| Yes | 5/94 (5) | NS |
| No | 42/1784 (2) |
Values are n/total (%). aChi-squared test. NS, not significant.
The results of multivariate analysis of factors related to gastric obstruction are shown in Table 2. Circumferential extent was only the factor related to gastric obstruction.
Table 2.
Results of multivariate analysis of factors related to gastric obstruction in patients after gastric ESD
| p-value | 95% CI | |
|---|---|---|
| Location (UML) | 0.37 | 0.52–1.27 |
| Circumference (APGL) | 0.10 | 0.95–1.61 |
| Circumferential extent | <0.0001 | 9.41–29.6 |
| Specimen size | 0.23 | 0.26–1.39 |
| Tumour depth | 0.37 | 0.31–1.54 |
| Ulcer findings | 0.42 | 0.28–1.68 |
| Curative resection | 0.58 | 0.37–1.74 |
| Number of lesions | 0.30 | 0.33–1.40 |
| Bleeding | 0.50 | 0.56–3.24 |
| Perforation | 0.07 | 0.92–6.39 |
APGL, anterior wall, posterior wall, greater curvature, lesser curvature; UML, upper, middle, lower.
Characteristics of patients with gastric obstruction
Among the 47 patients with gastric obstruction, 22 patients were judged to have undergone a non-curative ESD (Table 3). The main reason for a non-curative ESD was minute invasive submucosal cancer larger than 3 cm (six patients), massive invasive submucosal cancer (five patients), lymphovascular infiltration (four patients), mucosal cancer with ulceration larger than 3 cm (two patients), undifferentiated-type intramucosal cancer larger than 2 cm (two patients), and unclear or positive margins (three patients). The obstructive gastric symptoms occurred in a median of 24 days after ESD.
Table 3.
Characteristics of 47 patients with gastric obstruction after gastric ESD
| Study population | |
|---|---|
| Sex (male/female | 36/11 |
| Age (years, median, range) | 75 (46–86) |
| Time of symptom appearance after ESD (days, median, range) | 24 (9–60) |
| Curability of ESD | |
| Curative | 25 (53) |
| Non-curative | 22 (47) |
| Post-ESD stenosis | 41 (87) |
| Treatment | |
| EBD | 45 (96)a |
| Prokinetic drugs | 27 (57) |
| Naso-gastric tube | 3 (6) |
Values are n (%) unless otherwise stated.
Including four patients with no stenosis.
EBD, endoscopic balloon dilation; ESD,endoscopic submucosal dissection.
Stenosis was observed in 87% of the patients (41/47). The stenosis was due to scarring in all patients. Endoscopic balloon dilation (EBD) was performed in 45 patients (96%), including four patients in whom stenosis was not detected but the symptoms were present. The dilation was performed by using a Controlled Radial Expansion Wireguided balloon dilator (Boston Scientific, Tokyo, Japan). The balloon was gradually inflated from 15 to 18 mm in diameter according to the degree of the stenosis. The goal of treating post-ESD stenosis was based on both symptomatic relief of dysphagia and the passage of a normal endoscope (9.8 mm in diameter) without any resistance. The interval between dilations was 1 week in most cases; however, there were a few cases with severe stenosis who underwent dilation twice a week.
The treatment duration for EBD was 8 weeks, and the median number of EBD procedures per patient was 3 (Table 4). Complications due to EBD included bleeding, which required endoscopic haemostasis in one patient. Perforation occurred in four patients, one of whom underwent emergency surgery. For patients with gastric obstruction, a median of 9 weeks was required before normal diet was resumed. Three patients were hospitalized and required nasogastric tube drainage. In addition, 27 patients received prokinetic drugs such as mosapride or domperidone. Prokinetic drugs were administered in anticipation of promotion of gastric outflow and to decrease gastric residue. Additional surgery due to refractory stenosis after multiple EBD was performed in one patient.
Table 4.
Treatment outcomes in 47 patients with gastric obstruction after gastric ESD
| Study population | |
|---|---|
| Recovery to a normal diet (weeks, median, range) | 9 (2–56) |
| Treatment duration of EBD (weeks, median, range) | 8 (1–50) |
| EBD per patient (n, median, range) | 3 (1–25) |
| Complication due to EBD (n) | |
| Bleeding | 1 |
| Perforation | 4 |
| Reason for surgery after ESD (n) | |
| Non-curative ESD | 12 |
| Stenosis | 1 |
| EBD perforation | 1 |
EBD, endoscopic balloon dilation; ESD,endoscopic submucosal dissection.
Discussion
The incidence of gastric obstruction after gastric ESD in the study sample was 2.5%. The related factor from multivariate analysis was a wide resection of ≥75% of the circumferential extent. Stenosis was the main cause (87%) of gastric obstruction.
In the literature, only three Japanese studies have reported stenosis caused by gastric ESD. Tsunada et al.7 reported an incidence of 0.9% (5/532); stenosis in all cases was located in the antrum. Coda et al.8 reported an incidence of 0.8% (15/1819); stenosis in all cases was located in either the antrum or the cardia. Iizuka et al.9 reported an incidence of 1.9% (6/308), one case in the cardia and the other five cases in the antrum. The incidence of stenosis observed in the current study was 2% (41/1878), which was compatible with the results of these previous studies. The related risk factor of resecting more than 75% of the circumferential extent was also similar to those in previous reports.7–9
However, in the current study, six patients without stenosis developed gastric obstruction after a gastric ESD. Among patients with stenosis, even after the stenosis was treated with multiple dilation treatments (median duration 8 weeks), more time was required to resume normal oral intake (median duration 9 weeks). Transient motility dysfunction was thus suspected in patients with gastric obstruction after ESD with or without stenosis. This was determined from supporting evidence obtained by EGD, which revealed the presence of massive gastric residue, and the fact that many patients required post-operative treatment with prokinetic drugs.
Suspected motility dysfunction after gastric ESD has been reported previously. Lian et al.15 reported a case of gastroparesis following ESD for a 10-cm lesion located in the gastric incisure. Although no evidence of gastric outlet obstruction was noted, the patient required enteral feeding, gastrointestinal decompression, prokinetic agents, and antianxiety medications until a normal eating pattern was restored approximately 6 weeks later. Tanaka et al.16 reported a case of Wernicke–Korsakoff syndrome 4 months after a widespread ESD in the gastric antrum. Antral stenosis occurred and the patient was treated with intravenous hyperalimentation and multiple balloon dilation that required 2 months for recovery. Takeuchi17 reported a case of gastroparesis following a widespread ESD extending from the upper gastric body to the antrum. Although multiple EBD was performed and prokinetics were administered, the patient required at least 4 times of hospitalization during the following year after ESD. Finally, the patient underwent a distal gastrectomy.17 These reports indicate that not only stenosis but motility dysfunction may also cause gastric obstruction after gastric ESD.
The mechanism of motility dysfunction remains unclear. Hypoperistalsis due to deformity and stenosis after a widespread resection, transient damage of vagal nerves from the burning effect during the ESD procedure, or fibrosis and adhesions close to the wound may possibly affect gastric motor function. As in this study, most cases could be treated using multiple EBD in addition to prokinetics for approximately 2 months.
EBD was performed in patients with stenosis in this study and in previous reports.7–9,16,17 In the current series, perforation occurred in four of 45 patients (9%). Iizuka et al.9 reported perforation in one of six patients, and Tsunada et al.7 reported perforation in two of four patients. In another study, perforation due to EBD for stenosis after esophageal ESD was reported in seven of 76 patients (9%).18 The relatively high perforation rate due to EBD for post-ESD stenosis may be because of severe deformity, thinness of the mucosal wall, and the need for multiple EBD sessions. Therefore, EBD for post-ESD stenosis should be performed with caution. In addition, other measures to decrease the risk of stenosis, such as prophylactic EBD or steroid use, should be studied in the future.19,20
This study was a retrospective, single-centre study. However, the results were based on data from a single ESD database, which included detailed clinical and pathological information. In addition, the number of cases in our study was large in comparison to previous reports.
In conclusion, patients with a wide resection of ≥75% of the circumference are considered as a high risk of post-ESD stenosis or gastric obstruction. Early follow up by EGD after ESD should be considered, and dilation should be performed with caution if found to have stenosis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol 2008; 14: 2962–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lian J, Chen S, Zhang Y, et al. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc 2012; 76: 763–770 [DOI] [PubMed] [Google Scholar]
- 3.Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2005; 17: 54–58 [Google Scholar]
- 4.Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection – an analysis of risk factors. Endoscopy 2008; 40: 179–183 [DOI] [PubMed] [Google Scholar]
- 5.Miyahara K, Iwakiri R, Shimoda R, et al. Perforation and postoperative bleeding of endoscopic submucosal dissection in gastric tumors: analysis of 1190 lesions in low- and high-volume centers in Saga, Japan. Digestion 2012; 86: 273–280 [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Moon JS, Youn YH, et al. Management of the complications of endoscopic submucosal dissection. World J Gastroenterol 2011; 17: 3575–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67: 979–983 [DOI] [PubMed] [Google Scholar]
- 8.Coda S, Oda I, Gotoda T, et al. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41: 421–426 [DOI] [PubMed] [Google Scholar]
- 9.Iizuka H, Kakizaki S, Sohara N, et al. Stricture after endoscopic submucosal dissection for early gastric cancers and adenomas. Dig Endosc 2010; 22: 282–288 [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver.3). Gastric Cancer 2011; 14: 113–123 [DOI] [PubMed] [Google Scholar]
- 11.Ono H, Hasuike N, Inui T, et al. Usefulness of a novel electrosurgical knife, the insulation-tipped diathermic knife-2, for endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2008; 11: 47–52 [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Ono H, Hasuike N, et al. Endoscopic submucosal dissection of early gastric cancer. Digestion 2008; 77: 23–28 [DOI] [PubMed] [Google Scholar]
- 13.Kakushima N, Ono H, Tanaka M, et al. Endoscopic submucosal dissection using the insulated-tip knife. Tech Gastrointest Endosc 2011; 13: 63–69 [Google Scholar]
- 14.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14: 101–112 [DOI] [PubMed] [Google Scholar]
- 15.Lian J, Chen S, Zhang Y, et al. Gastroparesis following endoscopic submucosal dissection for gastric cancer. Endoscopy 2011; 43: E369–E369 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Hasuike N. A case of gastric dysfunction after a gastric ESD. (Wernicke-Korsakoff syndrome). In: Oyama T, Ono H. (eds). Complications of ESD: how we treated severe cases, Tokyo: Nankodo Co., 2012, pp. 104–107 in Japanese [Google Scholar]
- 17.Takeuchi M. A case of gastric dysfunction after a gastric ESD. (a case that did not recover by internal therapy). In: Oyama T, Ono H. (eds). Complications of ESD: How we treated severe cases, Tokyo: Nankodo Co., 2012, pp. 108–112 in Japanese [Google Scholar]
- 18.Takahashi H, Arimura Y, Okahara S, et al. Risk of perforation during dilation for esophageal strictures after endoscopic resection in patients with early squamous cell carcinoma. Endoscopy 2011; 43: 184–189 [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto S, Kobayashi M, Takeuchi M, et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc 2011; 74: 1389–1393 [DOI] [PubMed] [Google Scholar]
- 20.Isomoto H, Yamaguchi N, Nakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol 2011; 11: 46–46 [DOI] [PMC free article] [PubMed] [Google Scholar]