Abstract
Fistulizing Crohn’s disease represents an evolving, yet unresolved, issue for multidisciplinary management. Perianal fistulas are the most frequent findings in fistulizing Crohn’s disease. While enterocutaneous fistulas are rare, they are associated with considerable morbidity and mortality. Detailed evaluation of the fistula tract by advanced imaging techniques is required to determine the most suitable management options. The fundamentals of perianal fistula management are to evaluate the complexity of the fistula tract, and exclude proctitis and associated abscess. The main goals of the treatment are abscess drainage, which is mandatory, before initiating immunosuppressive medical therapy, resolution of fistula discharge, preservation of continence and, in the long term, avoidance of proctectomy with permanent stoma. The management of enterocutaneous fistulas comprises of sepsis control, skin care, nutritional optimization and, if needed, delayed surgery.
Keywords: Crohn’s disease, enterocutaneous fistula, perianal fistula, inflammatory bowel disease, anti-TNF therapy
Introduction
A fistula (Latin term for pipe) is defined as a chronic tract of granulation tissue between two epithelial lined surfaces.1 Fistula formation has been reported in 17–50% of patients with Crohn's disease in population-based studies.2,3 According to one epidemiologic study, 35% of the Crohn’s disease patients develop at least one fistula episode during the course of the disease. Of these fistulas, approximately two thirds are external (perianal 55%, enterocutaneous 6%) and one third are internal.2 The cumulative incidence of fistulizing Crohn’s disease is 21% after 1 year and increases to 50% after 20 years of diagnosis. Fistulizing episodes are reported to recur in one third of patients.2
Perianal fistulas in Crohn’s disease may originate from infected anal glands at the dentate line and/or penetration of fissures or ulcers in the anorectal wall.4 The prevalence of perianal fistulas varies according to disease location: 12% in patients with isolated ileal disease, 15% with ileocolonic disease, 41% with colonic disease and rectal sparing and 92% with colonic disease involving the rectum.3
Enterocutaneous fistulas are associated with significant morbidity and mortality due to septic complications, metabolic and electrolyte abnormalities, extensive skin damage and psychological disturbances. In a Canadian cohort, enterocutaneous fistulas in Crohn’s were reported to occur most commonly in areas of active luminal disease (77%) and, to a lesser extent (23%), at the anastomotic site after surgical resection, originating from an otherwise normal-appearing bowel.5 The latter should therefore be considered a surgical complication unrelated to Crohn’s disease.
Here we review the classification, diagnosis and multidisciplinary management of fistulizing Crohn’s disease in clinical practice.
Classification of perianal fistulas
Several classification systems are currently used in clinical practice to determine management strategy and to evaluate treatment efficacy.
The modified Parks classification was developed to avoid iatrogenic post-surgical incontinence, and supplies an anatomically precise description of the fistula tracts (superficial, intersphincteric, trans-sphincteric, suprasphincteric, extrasphincteric) in relation to the external anal sphincter.6 The Perianal Crohn’s Disease Activity Index is a functional index, which evaluates fistula discharge, type of perianal disease, induration, pain and restriction of activities, including sexual activities.7 The Fistula Drainage Assessment, used by several randomized controlled trials, was the first index to measure response to medical therapy, where a fistula is defined closed ‘when it no longer drains despite gentle finger compression’.8 An empiric approach to classifying perianal fistulas as ‘simple’ or ‘complex’ is also used to guide management; a simple fistula is low, has a single external opening, has no evidence of abscess, rectovaginal fistula or anorectal stricture, and may be associated with active rectal disease.1 A tract is defined as low when the internal opening is located in the lower third of the anal canal. A practical approach led to the development of a magnetic resonance imaging (MRI)-based scoring system to evaluate response to treatment. Remarkably, it includes both anatomical components and activity features of perianal fistulas.9
Imaging of perianal fistulas in Crohn’s disease
Detailed evaluation of the fistula tract is required to determine the optimal management strategy. A physical examination is conducted to assess the presence of perianal lesions (stenosis, fissure and ulcer, in particular), the number of external openings and active drainage. Endoscopic examination of the rectum is essential to detect active luminal disease. Imaging by rectal endosonography (EUS) and MRI yields information on the anatomy of the fistula tract, the relation to the external sphincter, the number of tracts (single or multiple), the location of internal (high or low) and external openings, and the presence of abscesses. The location of the internal opening of the fistula tract is especially important when Seton drainage or surgery is considered. Abscesses are relatively common findings, which often do not present as an emergency, but are found during imaging. They are typically lined with granulation tissue and filled with fluid.
Fistulography and computed tomography
Fistulography and pelvic computed tomography are no longer considered adequate imaging modalities for perianal fistulizing Crohn’s disease owing to their low diagnostic accuracy, radiation exposure and lack of ability to evaluate the course of the tract in relation to anatomical structures.1 Advanced imaging procedures include MRI and rectal EUS, each having their pros and cons.
Anorectal EUS
EUS can be performed easily with limited costs, although overall diagnostic accuracy is highly dependent on the operator’s experience. A cylindrical transducer with high frequency and 360°-view supplies high diagnostic accuracy for simple fistulas; however, complex tracks may continue outside the view of the device. It has a good detection rate for internal openings. Hydrogen peroxide installation through the external opening facilitates identification of tracks and differentiation between active fistulas and fibrous scars, although the preferential flow of hydrogen peroxide may prevent visualizing fistula tracts to full extent. Introduction of the endoluminal device can be associated with discomfort and may become impossible when stenosis is present.
MRI
As MRI is performed with an external coil it supplies an unrestricted view of the extent of fistula tracts with high accuracy in both simple and complex fistulas when read by an expert (Figure 1). The intrinsic contrast of MRI results in easy differentiation between active and inactive disease, and simple identification of complications. Although external coil MRI is less appropriate for the detection of internal openings, sensitivity may be increased with an endoanal coil, which, in contrast, has a restricted view (similar to EUS) and availability, and its use may also be limited by patient tolerance.10 Dynamic contrast enhanced MRI is a promising new technique for monitoring disease activity, but currently it is still limited to research.11 MRI is associated with higher costs and limited accessibility for some institutions.
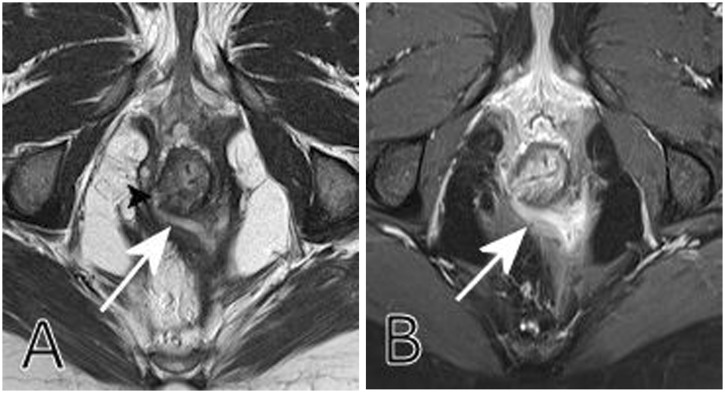
Figure 1.
(a) Axial T2-weighted turbo spin-echo shows a transsphincteric, horseshoe-shaped fistula (white arrow), with an internal opening (black arrowhead). (b) Axial fat saturated T1-weighted turbo spin-echo after intravenous contrast medium administration (same level as a). There is intense enhancement indicating extensive inflammation, but no abscess.
Data on the superiority of EUS and MRI are conflicting.12–14 The use of either technique is therefore determined by availability, expertise and preferences. In general, EUS can be used when low, simple tracks are expected, while MRI can be used in any patient and especially when the presence of complex fistula is suspected.14,15 (Table 1) Accurate determination of fistula course by MRI may decrease postoperative recurrence: recurrence was detected in 16% of cases where surgery was guided by MRI versus a recurrence rate of 57% in the absence of MRI.16
Table 1.
Rectal endosonography (EUS) and MRI in the imaging of perianal fistulas
| EUS | MRI | |
|---|---|---|
| Simple fistulas | +++ | +++ |
| Complex fistulas | ++ | +++ |
| High tracks/abscesses | ++ | +++ |
| Internal opening | +++ | ++ |
| Standard equipment | + | +++ |
| Costs | ++(+) | + |
| Availability | −/+++ | ++ |
| Comparison results | ++ | +++ |
Examination under anesthesia
Examination under anesthesia (EUA) consists of visual inspection, palpation and the passage of probes into fistula tracks. Experienced colorectal surgeons display up to 90% accuracy in detecting and classifying perianal fistulas. In addition to diagnostic benefit, the examination offers a possibility of immediate intervention for drainage or Seton placement.1
A prospective blinded study of 32 patients with suspected perianal Crohn’s disease showed that the accuracy of EUA, MRI and EUS were all excellent (91%, 87% and 91%, respectively). The combination of any two modalities increased the accuracy to 100%, and the combination of EUS or MRI with EUA changed surgical management in 10–15% of patients.13 Therefore, the combination of two modalities has been suggested as the optimal approach to evaluate fistula anatomy.
Key messages for imaging perianal fistulas
To determine the optimal management strategy, detailed information is needed about the complexity and activity of the fistulas, accompanying abscesses, proctitis and relevant perianal lesions.
Rectal EUS and MRI are the preferred imaging techniques for perianal fistulas.
EUS is easy to use in the hands of an experienced operator with limited costs and is accurate in evaluating simple fistulas.
MRI is accurate in both simple and complex fistulas, and its use prevents postoperative recurrence.
The combination of any two modalities of EUA, EUS and MRI increases accuracy to 100%; therefore, a combination is suggested for optimal evaluation.
Management of perianal fistulizing Crohn’s disease
The main goals of treatment in perianal fistulizing Crohn’s disease are abscess drainage prior to initiating immunosuppressive therapy, resolution of fistula discharge, preservation of continence and avoidance of proctectomy with stoma. Most frequently, a fistula is preceded by a perianal abscess, which can drain spontaneously or requires intervention.
Medical management
Medical management is determined by the degree of active drainage and complexity of the fistulas, and the presence of associated proctitis. Corticosteroids and 5-aminosalicylic acid are not beneficial in the treatment of fistulizing disease. Uncontrolled case series and a single, underpowered randomized control trial indicate improvement of symptoms using the antibiotics metronidazole and ciprofloxacin; however, drainage resumes on cessation of treatment.17–20 Antibiotics are still advised as first-line therapy to reduce inflammation in the tissues surrounding fistulas. Additional thiopurin maintenance treatment has been shown to improve outcome.21 According to data from a randomized control trial and a meta-analysis, thiopurins result in fistula closure in 31% of patients compared with 6% of placebo-treated patients, and a decreased discharge in 54% of cases compared with 21% of placebo cases.22,23 Tacrolimus was shown to be effective in improving fistula drainage in a single randomized control trial with a short-term follow-up; however, it failed to induce fistula healing. Furthermore, tacrolimus use was limited by frequent toxicity.24 Data on cyclosporine come from uncontrolled case series with low patient numbers, and response to treatment is, generally, rapidly lost upon drug withdrawal.25 Several trials examined the benefit of anti-tumor necrosis factor (TNF) treatment for fistulizing Crohn’s disease. Infliximab 5 mg/kg, administered at weeks 0, 2 and 6, induced fistula closure in 46% of patients compared with 13% of placebo-treated patients at week 18.8 Maintenance treatment with infliximab was shown to be beneficial in the ACCENT II trial. Patients with actively draining fistulas received open-label treatment with infliximab 5 mg/kg at weeks 0, 2 and 6, and responders (69%) were then randomized to maintenance treatment with IFX 5 mg/kg every 8 weeks for 1 year. Complete fistula closure after 1 year was observed in 36% of patients versus 19% of those receiving placebo. The median duration of fistula closure in these responders was 40 weeks versus 23 weeks in the placebo group.26 Interestingly, in a small randomized control trial, ciprofloxacin was shown to further reduce fistula drainage in the short term, when administered together with infliximab.27 The long-term efficacy of adalimumab on fistula closure was evaluated in the CHARM study as a secondary endpoint. It was shown that 30% of patients with draining fistulas had complete closure versus 13% of the placebo-treated group at both weeks 26 and 56 of adalimumab maintenance treatment.28 Certolizumab pegol did not induce statistically significant fistula closure compared with placebo in randomized control trials.29,30
Surgical management
All fistulas are potential sources of sepsis; therefore, obtaining drainage is fundamental in all locations to prevent abscess formation. In the case of perianal fistulas non-cutting loose Seton drainage is the method of choice to maintain tract patency and to avoid recurrent abscesses. When a perianal abscess is, nevertheless, present, surgical drainage is essential. The timing of the removal of Seton drains varies between centers, as controlled data are lacking. The maintenance of drainage and the presence of a persisting tract should be weighed against each other. In general, Setons can be removed when fistula discharge reduces. If uncertainty remains, repeat imaging is advised. Further surgical procedures are only considered in the absence of associated proctitis, stenosis, significant ulcer or fissure. For symptomatic, simple superficial, low intersphincteric and selected low trans-sphincteric fistulas, fistulotomy or lay-open is a safe method of choice, and offers a 81–100% healing rate.31,32 In trans-sphincteric fistulas the ligation of intersphincteric fistula tract technique is a novel option if the tract has matured into a fibrotic tube with granulation tissue enabling ligation and transection.33 For the treatment of high fistulas, there are currently three further options: mucosal advancement flap procedure and filling of the perianal fistula tract with bioprosthetic plugs or glue. The mucosal advancement flap procedure is the most established method, which is suitable for high tracts, as well as for rectovaginal fistulas with a 64% (33–92%) success rate, although in the latter the recurrence rates are high.34 Bioprosthetic plugs of biologically absorbable porcine intestinal submucosa have been reported to induce 75–86% fistula closure.35–38 Although an open-label multicenter randomized control trial demonstrated initial success with fibrin glue (38% vs 16% of patients in clinical remission at week 8, fibrin glue vs observation-only, respectively), only 20% of patients were in clinical remission upon long-term follow-up.39 A prospective, randomized single-center trial failed to demonstrate improved outcome with fibrin glue when administered as an adjunct treatment to flap repair.40 Stem cell-based therapy has been investigated in a phase I and a phase II multicenter randomized controlled trial.41,42 In the phase II trial 49 patients were randomized to treatment with fibrin glue or fibrin glue and intrafistular stem cell treatment: fistula healing was observed in 16% versus 71%, respectively, at week 8. Upon long-term follow-up the recurrence rate was 17.6%. A major drawback of this study is that perianal fistulas were associated with Crohn’s disease in less than a third of the patients (14/49). In a small, uncontrolled cohort of 10 patients, sustained complete closure (7 cases) or incomplete closure (3 cases) of fistula tracks was seen after intrafistular injection of autologous bone marrow-derived mesenchymal stromal cells upon 12 months of follow-up.43 Failure to control perianal fistulas may ultimately result in a diverting stoma or in proctectomy with a permanent stoma. As these patients with debilitating disease are often on concomitant immunosuppression, it is notable that no relationship between the perioperative use of infliximab or immunosuppressants and the development of postoperative complications has been established.44 It shall also be taken into account that inflammatory bowel disease (IBD) patients are at increased risk of perioperative morbidity, with special regard to thrombotic complications; consequently, careful preparation and thrombosis prophylaxis is mandatory.45
Key messages for management of perianal fistulas
Optimal treatment requires close multidisciplinary teamwork of radiologists, gastroenterologists, surgeons and IBD specialist nursing staff.
The goals of treatment are resolution of fistula discharge, preservation of continence, avoidance of proctectomy and stoma.
Adequate abscess drainage is mandatory prior to immunosuppressive medical therapy.
The cornerstones of treatment are non-cutting Seton placement, antibiotics and anti-TNF therapy.
Further surgical procedures are only considered in the absence of proctitis.
Fistulotomy has high success rate for superficial and low intersphincteric fistulas.
Management of enterocutaneous fistulas in Crohn’s disease
Mortality rates in patients with enterocutaneous fistula remain disproportionately high ranging between 6% and 33%.46,47 Although the leading cause of death is sepsis, increased mortality has been associated with high initial fistula output, the presence of complications, co-morbidities, low serum albumin level and age.47–49 The general principles of management include extended conservative treatment and delayed surgery when necessary.
Medical management
Medical management can be divided into immediate, early and late care. Immediate care is focused on identification and aggressive treatment of intra-abdominal sepsis, correction of fluid and electrolyte disturbances, skin care and pain control.50 Early treatment should comprise initiation of nutritional support, control of fistula output, psychosocial assessment, and mobilization. Currently, there is no evidence to support the superiority of total enteral or parenteral nutrition.50–52 Total parenteral nutrition decreases gastrointestinal secretion, which might be advantageous in high output fistulas (with an output of more than 500 ml per 24 h), though there is no clear evidence for increased likelihood of spontaneous fistula closure. However, enteral nutrition prevents mucosal atrophy and bacterial translocation. Taking these into consideration, enteral nutrition is preferably applied, together with supplemental parenteral nutrition, if necessary, to avoid malnutrition. Fistuloclysis and reinfusion of fluid from the proximal fistula into a distal mucous fistula have both been described to have beneficial effects in case series.53 The role of immunonutrition with glutamin or arginine preparations remains controversial.50 Reduced fistula output can be achieved by restricted intake of hypo-osmolar fluids, intake of electrolyte mix with high concentrations of sodium and glucose, the use of antisecretory agents (such as proton pump inhibitors and somatostatin or octreotide) and by antimotility agents (loperamide, codeine).50 The value of somatostatin and analogues in the treatment of enterocutaneous fistulas is controversial.54,55 Fistula closure was evaluated in a recent meta-analysis, which showed that both somatostatin and octreotide increased the likelihood of fistula closure. However, enterocutaneous fistulas were mostly of postoperative origin; therefore, results may be of limited value with regard to Crohn’s patients.55 Weighing potential benefits against side effects has led many centres to exclude octreotide from standard management regime.56 There is a marked lack of randomised controlled trials on the effect of immunosuppressive treatment. Only a small subgroup of patients involved in the ACCENT II trial had enterocutaneous fistulas; however, no subgroup analysis has been performed.26 Small case series are controversial regarding the effect of infliximab treatment on enterocutaneous fistulas.57,58 It is suggestive that in cases where fistulas occur at areas of active luminal disease, immunosuppressive medication would have a beneficial effect; however, fistulas developing at anastomotic sites will not respond to such treatment. Late patient care should involve the anatomical assessment of the fistula and evaluation of gut length and quality. Only after all these issues have been adequately dealt with, and at least 2 months have been given for the fistula to close spontaneously, should operative treatment be contemplated.
A useful acronym to represent conservative treatment is ‘SNAP’, which represents management of sepsis and skin care, nutritional support, definition of intestinal anatomy and development of a surgical procedure.
Surgical management
Early surgery should be restricted to the treatment of otherwise unmanageable intra-abdominal sepsis and hemorrhage.51 Successful surgery requires resection of the affected abdominal wall, the fistula tract and the affected bowel segment, which is associated with lower recurrence rate compared with over-sewing.59 The re-anastomosed bowel should be separated from the abdominal wall if possible, with omental fat. For complex fistulas often a significant portion of the abdominal wall needs to be resected. In these cases a human acellular dermal matrix has been reported to successfully reconstruct the defect.60 In the only prospective study, where one third of the patients with enterocutaneous fistulas (mostly postoperative) had Crohn’s disease, successful healing was reported to be 63%.61 Clinical trials are currently being performed to evaluate the effect of stem cell injections on healing of enterocutaneous fistula.62 In conclusion, owing to the intrinsic bowel pathology and the poor quality of the abdominal wall due to multiple abdominal operations, the recurrence rate remains high.59
Key messages for management of enterocutaneous fistulas
Optimal treatment requires close multidisciplinary teamwork of radiologists, gastroenterologists, surgeons, dietician and a dedicated stoma nurse.
A useful acronym to represent the conservative treatment is ‘SNAP’, which represents management of sepsis and skin care, nutritional support, definition of intestinal anatomy and development of a surgical procedure.
Surgery should be delayed until both local and systemic conditions have been optimized.
Successful surgery requires the resection of the bowel associated to the fistula.
Recurrence rates remain high after surgery.
Conclusions
Management of patients with fistulizing Crohn’s disease remains a considerable challenge. In spite of a growing medical and surgical treatment repertoire, randomized control trials with fistula healing as a primary endpoint are scarce. Therefore, management protocols mainly rely on retrospective reviews from large specialist centres. All agree that optimal patient management for fistulizing Crohn’s disease require close multidisciplinary teamwork.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
KG, RK, JJ and DH have no conflicts of interest to declare. JS has received consultancy fee from Robarts Clinical Trials. SG served as a member of advisory board for NPS Pharmaceuticals in 2011 and Covidien in 2012 and received lecture fees from Fresenius, Baxter and Convatec. GD'H has received consultancy fees from Abbott Laboratories, Actogenix, Centocor, Cosmo, Engene, Ferring Pharmaceuticals, GlaxoSmithKline, Jansen Biologics, Millenium Pharmaceuticals, MSD, Novonordisk, PDL Biopharma, Pfizer, SetPoint, Shire, Sigmoid Pharma Ltd, Takeda, Teva, Tillotts Pharma, UCB; research grants from Abbott Laboratories, Jansen Biologics, Given Imaging, MSD, DrFalk Pharma, Photopill; speaking honoraria from Abbott Laboratories, Jansen Biologics, Tillotts, Tramedico, Ferring, MSD, UCB, Norgine and Shire.
References
- 1.Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB. AGA technical review on perianal Crohn's disease. Gastroenterology 2003; 125(5): 1508–1530 [DOI] [PubMed] [Google Scholar]
- 2.Schwartz DA, Loftus EV, Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, et al. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology 2002; 122(4): 875–880 [DOI] [PubMed] [Google Scholar]
- 3.Hellers G, Bergstrand O, Ewerth S, Holmstrom B. Occurrence and outcome after primary treatment of anal fistulae in Crohn's disease. Gut 1980; 21: 525–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes LE. Surgical pathology and management of anorectal Crohn's disease. J R Soc Med 1978; 71: 644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poritz LS, Gagliano GA, McLeod RS, MacRae H, Cohen Z. Surgical management of entero and colocutaneous fistulae in Crohn's disease: 17 year's experience. Int J Colorectal Dis 2004; 19(5): 481–485 [DOI] [PubMed] [Google Scholar]
- 6.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg 1976; 63(1): 1–12 [DOI] [PubMed] [Google Scholar]
- 7.Irvine EJ. Usual therapy improves perianal Crohn's disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol 1995; 20(1): 27–32 [PubMed] [Google Scholar]
- 8.Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, Van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999; 340(18): 1398–1405 [DOI] [PubMed] [Google Scholar]
- 9.Van AG, Vanbeckevoort D, Bielen D, Coremans G, Aerden I, Noman M, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn's disease. Am J Gastroenterol 2003; 98(2): 332–339 [DOI] [PubMed] [Google Scholar]
- 10.Halligan S, Bartram CI. MR imaging of fistula in ano: are endoanal coils the gold standard? AJR Am J Roentgenol 1998; 171(2): 407–412 [DOI] [PubMed] [Google Scholar]
- 11.Horsthuis K, Lavini C, Bipat S, Stokkers PC, Stoker J. Perianal Crohn disease: evaluation of dynamic contrast-enhanced MR imaging as an indicator of disease activity. Radiology 2009; 251(2): 380–387 [DOI] [PubMed] [Google Scholar]
- 12.Orsoni P, Barthet M, Portier F, Panuel M, Desjeux A, Grimaud JC. Prospective comparison of endosonography, magnetic resonance imaging and surgical findings in anorectal fistula and abscess complicating Crohn's disease. Br J Surg 1999; 86(3): 360–364 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz DA, Wiersema MJ, Dudiak KM, Fletcher JG, Clain JE, Tremaine WJ, et al. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn's perianal fistulas. Gastroenterology 2001; 121(5): 1064–1072 [DOI] [PubMed] [Google Scholar]
- 14.Sahni VA, Ahmad R, Burling D. Which method is best for imaging of perianal fistula? Abdom Imaging 2008; 33: 26–30 [DOI] [PubMed] [Google Scholar]
- 15.Ziech M, Felt-Bersma R, Stoker J. Imaging of perianal fistulas. Clin Gastroenterol Hepatol 2009; 7: 1037–1045 [DOI] [PubMed] [Google Scholar]
- 16.Buchanan G, Halligan S, Williams A, Cohen CR, Tarroni D, Phillips RK, et al. Effect of MRI on clinical outcome of recurrent fistula-in-ano. Lancet 2002; 360(9346): 1661–1662 [DOI] [PubMed] [Google Scholar]
- 17.Bernstein LH, Frank MS, Brandt LJ, Boley SJ. Healing of perineal Crohn's disease with metronidazole. Gastroenterology 1980; 79(2): 357–365 [PubMed] [Google Scholar]
- 18.Brandt LJ, Bernstein LH, Boley SJ, Frank MS. Metronidazole therapy for perineal Crohn's disease: a follow-up study. Gastroenterology 1982; 83(2): 383–387 [PubMed] [Google Scholar]
- 19.Thia KT, Mahadevan U, Feagan BG, Wong C, Cockeram A, Bitton A, et al. Ciprofloxacin or metronidazole for the treatment of perianal fistulas in patients with Crohn's disease: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis 2009; 15(1): 17–24 [DOI] [PubMed] [Google Scholar]
- 20.Jakobovits J, Schuster MM. Metronidazole therapy for Crohn's disease and associated fistulae. Am J Gastroenterol 1984; 79(7): 533–540 [PubMed] [Google Scholar]
- 21.Dejaco C, Harrer M, Waldhoer T, Miehsler W, Vogelsang H, Reinisch W. Antibiotics and azathioprine for the treatment of perianal fistulas in Crohn's disease. Aliment Pharmacol Ther 2003; 18: 1113–1120 [DOI] [PubMed] [Google Scholar]
- 22.Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn's disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med 1980; 302: 981–987 [DOI] [PubMed] [Google Scholar]
- 23.Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med 1995; 123(2): 132–142 [DOI] [PubMed] [Google Scholar]
- 24.Sandborn WJ, Present DH, Isaacs KL, Wolf DC, Greenberg E, Hanauer SB, et al. Tacrolimus for the treatment of fistulas in patients with Crohn's disease: a randomized, placebo-controlled trial. Gastroenterology 2003; 125(2): 380–388 [DOI] [PubMed] [Google Scholar]
- 25.Sandborn WJ. A critical review of cyclosporine therapy in inflammatory bowel disease. Inflamm Bowel Dis 1995; 1: 48–63 [Google Scholar]
- 26.Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004; 350(9): 876–885 [DOI] [PubMed] [Google Scholar]
- 27.West RL, van der Woude CJ, Hansen BE, Felt-Bersma RJ, van Tilburg AJ, Drapers JA, et al. Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn's disease with infliximab: a double-blind placebo-controlled study. Aliment Pharmacol Ther 2004; 20(11–12): 1329–1336 [DOI] [PubMed] [Google Scholar]
- 28.Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 2007; 132(1): 52–65 [DOI] [PubMed] [Google Scholar]
- 29.Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, et al. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med 2007; 357(3): 228–238 [DOI] [PubMed] [Google Scholar]
- 30.Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OO, Hanauer SB, McColm J, et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med 2007; 357(3): 239–250 [DOI] [PubMed] [Google Scholar]
- 31.Scott HJ, Northover JM. Evaluation of surgery for perianal Crohn's fistulas. Dis Colon Rectum 1996; 39(9): 1039–1043 [DOI] [PubMed] [Google Scholar]
- 32.Halme L, Sainio AP. Factors related to frequency, type, and outcome of anal fistulas in Crohn's disease. Dis Colon Rectum 1995; 38(1): 55–59 [DOI] [PubMed] [Google Scholar]
- 33. van Onkelen RS, Gosselink MP and Schouten WR. Ligation of the intersphincteric fistula tract in low transsphincteric fistula: A new technique to avoid fistulotomy. Colorectal Dis. Epub ahead of print 13 September 2012. [DOI] [PubMed]
- 34.Soltani A, Kaiser AM. Endorectal advancement flap for cryptoglandular or Crohn's fistula-in-ano. Dis Colon Rectum 2010; 53(4): 486–495 [DOI] [PubMed] [Google Scholar]
- 35.Ellis CN, Rostas JW, Greiner FG. Long-term outcomes with the use of bioprosthetic plugs for the management of complex anal fistulas. Dis Colon Rectum 2010; 53(5): 798–802 [DOI] [PubMed] [Google Scholar]
- 36.Chung W, Ko D, Sun C, Raval MJ, Brown CJ, Phang PT. Outcomes of anal fistula surgery in patients with inflammatory bowel disease. Am J Surg 2010; 199(5): 609–613 [DOI] [PubMed] [Google Scholar]
- 37.O'Connor L, Champagne BJ, Ferguson MA, Orangio GR, Schertzer ME, Armstrong DN. Efficacy of anal fistula plug in closure of Crohn's anorectal fistulas. Dis Colon Rectum 2006; 49(10): 1569–1573 [DOI] [PubMed] [Google Scholar]
- 38.Schwandner O, Stadler F, Dietl O, Wirsching RP, Fuerst A. Initial experience on efficacy in closure of cryptoglandular and Crohn's transsphincteric fistulas by the use of the anal fistula plug. Int J Colorectal Dis 2008; 23(3): 319–324 [DOI] [PubMed] [Google Scholar]
- 39.Grimaud JC, Munoz-Bongrand N, Siproudhis L, Abramowitz L, Senejoux A, Vitton V, et al. Fibrin glue is effective healing perianal fistulas in patients with Crohn's disease. Gastroenterology 2010; 138(7): 2275–2281 [DOI] [PubMed] [Google Scholar]
- 40.Ellis CN, Clark S. Fibrin glue as an adjunct to flap repair of anal fistulas: a randomized, controlled study. Dis Colon Rectum 2006; 49(11): 1736–1740 [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 2005; 48(7): 1416–1423 [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 2009; 52(1): 79–86 [DOI] [PubMed] [Google Scholar]
- 43.Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut 2013; 60(6): 788–798 [DOI] [PubMed] [Google Scholar]
- 44.Colombel JF, Loftus EV, Jr, Tremaine WJ, Pemberton JH, Wolff BG, Young-Fadok T, et al. Early postoperative complications are not increased in patients with Crohn's disease treated perioperatively with infliximab or immunosuppressive therapy. Am J Gastroenterol 2004; 99(5): 878–883 [DOI] [PubMed] [Google Scholar]
- 45.Wallaert JB, De Martino RR, Marsicovetere PS, Goodney PP, Finlayson SR, Murray JJ, et al. Venous thromboembolism after surgery for inflammatory bowel disease: are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum 2012; 55(11): 1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez D, Zibari G, Aultman D, McMillan R, Mancini MC, Rush BM, et al. The outcome of intestinal fistulae: the Louisiana State University Medical Center–Shreveport experience. Am Surg 1998; 64(3): 252–254 [PubMed] [Google Scholar]
- 47.Mawdsley JE, Hollington P, Bassett P, Windsor AJ, Forbes A, Gabe SM. An analysis of predictive factors for healing and mortality in patients with enterocutaneous fistulas. Aliment Pharmacol Ther 2008; 28(9): 1111–1121 [DOI] [PubMed] [Google Scholar]
- 48.Campos AC, Andrade DF, Campos GM, Matias JE, Coelho JC. A multivariate model to determine prognostic factors in gastrointestinal fistulas. J Am Coll Surg 1999; 188(5): 483–490 [DOI] [PubMed] [Google Scholar]
- 49.Hollington P, Mawdsley J, Lim W, Gabe SM, Forbes A, Windsor AJ. An 11-year experience of enterocutaneous fistula. Br J Surg 2004; 91(12): 1646–1651 [DOI] [PubMed] [Google Scholar]
- 50.Lloyd DA, Gabe SM, Windsor AC. Nutrition and management of enterocutaneous fistula. Br J Surg 2006; 93(9): 1045–1055 [DOI] [PubMed] [Google Scholar]
- 51.Levy E, Frileux P, Cugnenc PH, Honiger J, Ollivier JM, Parc R. High-output external fistulae of the small bowel: management with continuous enteral nutrition. Br J Surg 1989; 76(7): 676–679 [DOI] [PubMed] [Google Scholar]
- 52.Li J, Ren J, Zhu W, Yin L, Han J. Management of enterocutaneous fistulas: 30-year clinical experience. Chin Med J (Engl) 2003; 116(2): 171–175 [PubMed] [Google Scholar]
- 53.Teubner A, Morrison K, Ravishankar HR, Anderson ID, Scott NA, Carlson GL. Fistuloclysis can successfully replace parenteral feeding in the nutritional support of patients with enterocutaneous fistula. Br J Surg 2004; 91(5): 625–631 [DOI] [PubMed] [Google Scholar]
- 54.Hesse U, Ysebaert D, de HB. Role of somatostatin-14 and its analogues in the management of gastrointestinal fistulae: clinical data. Gut 2001; 49Suppl 4iv11–iv21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahbour G, Siddiqui MR, Ullah MR, Gabe SM, Warusavitarne J, Vaizey CJ. A Meta-analysis of outcomes following use of somatostatin and its analogues for the management of enterocutaneous fistulas. Ann Surg 2012; 256(6): 946–954 [DOI] [PubMed] [Google Scholar]
- 56.Gabe SM, Shaffer JL, Forbes A, Holst M, Irtun O, Klek S, et al. The management of patients with high output enterocutaneous fistulae: a European survey. Clin Nutr 2012; 7(14): OP033–OP033 [Google Scholar]
- 57.Poritz LS, Rowe WA, Koltun WA. Remicade does not abolish the need for surgery in fistulizing Crohn's disease. Dis Colon Rectum 2002; 45(6): 771–775 [DOI] [PubMed] [Google Scholar]
- 58.Fries W, La MG, Costantino G, Repici A, Mazziotti S, Navarra G. Combined approach with biologics and surgery for enterocutaneous fistulas in Crohn's disease. Inflamm Bowel Dis 2013; 17(2): 671–673 [DOI] [PubMed] [Google Scholar]
- 59.Lynch AC, Delaney CP, Senagore AJ, Connor JT, Remzi FH, Fazio VW. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg 2004; 240(5): 825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taner T, Cima RR, Larson DW, Dozois EJ, Pemberton JH, Wolff BG. Surgical treatment of complex enterocutaneous fistulas in IBD patients using human acellular dermal matrix. Inflamm Bowel Dis 2009; 15(8): 1208–1212 [DOI] [PubMed] [Google Scholar]
- 61.Datta V, Engledow A, Chan S, Forbes A, Cohen CR, Windsor A. The management of enterocutaneous fistula in a regional unit in the United Kingdom: a prospective study. Dis Colon Rectum 2010; 53(2): 192–199 [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Olmo D, Herreros D, Pascual M, Pascual I, De La Quintana P, Trebol J, et al. Treatment of enterocutaneous fistula in Crohn's Disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. Int J Colorectal Dis 2009; 24(1): 27–30 [DOI] [PubMed] [Google Scholar]