Abstract
Lactose malabsorption is a common condition caused by reduced expression or activity of lactase in the small intestine. In such patients, lactose intolerance is characterized by abdominal symptoms (e.g. nausea, bloating, and pain) after ingestion of dairy products. The genetic basis of lactose malabsorption is established and several tests for this condition are available, including genetic, endoscopic, and H2-breath tests. In contrast, lactose intolerance is less well understood. Recent studies show that the risk of symptoms after lactose ingestion depends on the dose of lactose, lactase expression, intestinal flora, and sensitivity of the gastrointestinal tract. Lactose intolerance has recently been defined as symptoms developing after ingestion of lactose which do not develop after placebo challenge in a person with lactose maldigestion. Such blinded testing might be especially important in those with functional gastrointestinal diseases in whom self-reported lactose intolerance is common. However, placebo-controlled testing is not part of current clinical practice. Updated protocols and high-quality outcome studies are needed. Treatment options of lactose intolerance include lactose-reduced diet and enzyme replacement. Documenting the response to multiple doses can guide rational dietary management; however, the clinical utility of this strategy has not been tested. This review summarizes the genetic basis, diagnosis, and treatment of lactose malabsorption and intolerance.
Keywords: Blinded testing, FODMAP, genetic test, hydrogen breath test, lactose intolerance, lactase deficiency, lactose malabsorption, lactose maldigestion, irritable bowel syndrome, multiple-dose testing
Digestion of lactose
Lactose malabsorption refers to inefficient digestion of lactose due to reduced expression or impaired activity of the enzyme lactase (Box 1). After ingestion, lactose passes into the small intestine where it comes into contact with lactase at the intestinal brush border where it is hydrolysed into the monosaccharides glucose and galactose, which can be readily absorbed.1
Box 1.
Definition of important concepts
| Lactase non-persistence: In a majority of humans, the enzyme lactase at the jejunal brush border is reduced in activity after weaning. In some individuals, this reduced activity can cause symptoms after lactose ingestion. |
| Lactase persistence: Persistence of a high activity of the enzyme lactase into adulthood. This phenotype facilitates digestion of larger of amounts of lactose. |
| Lactose malabsorption: Inefficient digestion of lactose due to lactase non-persistence or other intestinal pathologies. |
| Lactose malassimilation: Inefficient absorption of lactose due to lactose malabsorption. |
| Lactose intolerance: Gastrointestinal symptoms in an individual with lactose malabsorption. |
Lactose is the main source of sugar from milk and milk products from all mammals except the sea lion. Inadequate lactase activity allows lactose to reach the large intestine. There, the gut flora provides a salvage pathway for lactose digestion by cleaving lactose into short-chain fatty acids (SCFA) and gas, mainly hydrogen (H2), carbon dioxide (CO2), and methane (CH4). Non-digested lactose can cause osmotic diarrhoea; products of its bacterial digestion can lead to secretory diarrhoea and gas distending the intestines, events that are likely to lead to clinical symptoms.2
Causes of lactase deficiency
The most frequent cause of lactose malabsorption is lactase non-persistence, a common condition in which lactase expression decreases during infancy. In contrast, congenital lactase deficiency due to complete lack of the enzyme is a rare condition that presents with severe symptoms in newborns. In addition, lactase malabsorption may be secondary to acquired conditions including small bowel bacterial overgrowth, infectious enteritis (i.e. giardiasis), or mucosal damage due to coeliac disease, inflammatory bowel disease, drugs, gastrointestinal surgery, short bowel syndrome, or radiation enteritis, conditions that lead to either reduction of absorptive capacity or downregulation of lactase expression in the small intestine.
Genetics of lactase non-persistence
Lactase activity changes during development. In most humans, lactase activity reaches a maximum in late pregnancy but declines after 2–3 years of age and reaches a stable low level at age 5–10, a process which might help weaning. However, a proportion of the human population, especially Caucasians from Northern Europe or northern European descent, retain high lactase levels during adulthood (lactase persistence). Both, lactase persistence and non-persistence (leading to lactose malabsorption) are thus normal human phenotypes.3
Prevalence of lactase persistence is high in most regions in Scandinavia, the British islands, and Germany (80–95%); however, this condition is observed in only 20–40% of Indian adults, 30% of Mexicans, 30% of African Americans, and <10% of adults in Southeast Asia4,5 (Figure 1). Lactase persistence is thought to be related to the development of farming during the last 10,000 years. The genetic polymorphism responsible for most cases of lactase persistence in Caucasian individuals is the −13910C/T variant (thereby, T at position 13910 upstream of the lactase gene within a putative regulatory DNA-region causes persistence; C leads to non-persistence).6 In contrast, in African tribes that herd cattle in Sudan, Kenya, and Tanzania), lactase persistence is mediated by the −14010*G, −13915*G or −13907*C polymorphism,3,7 and in Saudi Arabia by the −13915*G polymorphism.8 Thus, lactase persistence developed several times independently in human evolution in different areas of the world (for review, see Ingram et al.3).
Figure 1.
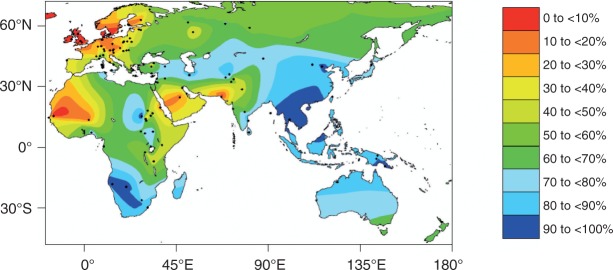
Interpolated frequency of the lactose malabsorption phenotype in the Eastern Hemisphere
Dots indicate locations of data collection. In regions with few data points (for instance Australia) this map is less reliable. Figure modified from Itan et al.5
Statistical calculations of genetic data estimated that selection for lactase persistence commenced relatively recently, during the last 10,000 years.3,9,10 Lactase persistence was beneficial for our ancestors by providing a clean source of fluid and a source of protein, fat, and carbohydrates.3 Lactase persistence generated a selective advantage of 1.5–19% in each generation.7,9 This indicates a strong selection pressure comparable to malaria resistance genes (2–5% for G6PD deficiency, 5–18% for sickle-cell trait) in various parts of the world.
The exact molecular mechanism for lactase persistence is unknown but factors enhancing gene transcription appear to be responsible by binding to a regulatory region 13,000 to 14,030 base pairs upstream of the lactase gene.3,11–13 In adult patients with homozygous lactase persistence, enzyme levels at the jejunal brush border are 10-times higher than for patients with homozygous non-persistence, heterozygous individuals showing intermediate levels.14
Definition of lactose intolerance
The lactose-rich diet in Western countries can cause symptoms in individuals with the lactase non-persistence phenotype. Typical gastrointestinal complaints are diarrhoea, nausea, bloating, borborygmi, and abdominal pain. A wide range of systemic problems have also been associated with lactose malabsorption including skin disease, rheumatological complaints, chronic fatigue, and failure to thrive in children,15 although the concept of ‘systemic lactose intolerance’ is still controversial. It is important to realize that lactose malabsorption (lactase non-persistence) is not equivalent or synonymous to lactose intolerance. Lactose malabsorption in many cases will not come to clinical attention. Development of symptoms depends on several individual factors including diet, oro-cecal transit time, distribution and fermentation capacity of gut flora,16 sensitivity towards chemical and mechanical stimulation of the gut, and psychological factors.17
A recent NIH conference defined lactose intolerance as ‘the onset of gastrointestinal symptoms following a blinded, single-dose challenge of ingested lactose by an individual with lactose malabsorption, which are not observed when the person ingests an indistinguishable placebo’.1 This new concept of lactose intolerance is provoking because it requires, in addition to evidence of lactase deficiency or lactose malabsorption, the development of symptoms after a blinded, placebo-controlled lactose challenge, which currently is not clinical practice.
Testing of lactose malabsorption
Typical symptoms of lactose intolerance are common among patients in a primary care setting but no consensus exists concerning which patients should be referred for further testing. In a recent meta-analysis, no specific complaint could predict lactose malabsorption, with sensitivities ranging from 0 to 90% and specificities ranging from 18 to 96% for symptoms such as bloating, diarrhoea, flatulence, and abdominal pain in individual studies.18 Self-reported milk intolerance was also of little value, showing sensitivities from 30 to 71% and specificities from 25 to 87%.18
Various options exist for diagnosing lactose malabsorption (Table 1). Testing of lactase activity in mucosal biopsies from the duodenum may be the reference standard19,20 and has the advantage that endoscopy and biopsy can also exclude other conditions that cause secondary lactose malabsorption (e.g. coeliac disease). Limitations are the patchy expression of lactase21 and the invasiveness of the test. Recently, an open-label trial in patients with self-reported intolerance of dairy products (excluding irritable bowel syndrome, IBS) reported that a commercial lactase assay was able to predict symptom improvement after implementation of an lactose-free diet more accurately than an H2-breath test (98 vs. 81%).22
Table 1.
Summary of tests for lactose malabsorption and tolerance
| H2-breath test | Lactose tolerance test | Genetic test of –13910 C/T polymorphism | Lactase activity at jejunal brush border | |
|---|---|---|---|---|
| Test principle | Increase of H2 in respiratory air after lactose challenge | Increase of blood sugar after lactose challenge | Genetic Polymorphism 13910 upstream of lactase gene | Enzymatic activity of lactase enzyme in biopsy sample |
| Cut off | >20 ppm within 3 hours | <1.1 mmol/l within 3 hours | 13910C/C indicates lactase non-persistence | <17–20 IU/g |
| Availability | Good | Excellent | Variable | Rare |
| False positives (malabsorption incorrectly diagnosed) | Rapid GI-transit, small-intestinal bacterial overgrowth | Rapid GI-transit, impaired glucose tolerance | Rare (<5%) in Caucasians | Probably rare |
| False negatives (malabsorption wrongly excluded) | Non-H2-producers. Full colonic adaptation. | Fluctuations in blood sugar | All causes of secondary lactose malabsorption | Patchy enzyme expression |
| Secondary causes | Cannot be excluded, kinetic of H2-increase can be suggestive | Cannot be excluded | Cannot be excluded | Can be excluded (histopathology obtained at same procedure) |
| Assessment of symptoms/lactose tolerance | Possible | Possible | Not possible | Not possible |
| Comment | Method of choice for assessment of lactose malabsorption and intolerance | Rarely performed due to inferior sensitivity and specificity | Definitive test for lactase non-persistence in Caucasians. Less suitable in other populations. | Reference standard for detection of lactase deficiency (primary or secondary) |
| Not suitable in patients with intestinal disease at risk of secondary lactase deficiency. | ||||
| Cost | Low | Lowest | High | Highest |
Genetic tests establish lactase non-persistence in Caucasian patients and the −13910*T genotype correlated closely (86–95%) with other tests for lactose malabsorption in European countries.6,23,24 Since lactose malabsorption is a recessive condition, a heterozygous genotype has to be considered a negative test result. Current testing for the −13910*T genotype is of limited use in certain African, Arabic, or Asian subpopulations where lactase persistence may be linked to different polymorphisms, as already discussed. Future genetic tests will likely cover a range of genetic polymorphisms, potentially eliminating this limitation. Clearly, genetic tests will be negative in patients with secondary causes of lactase deficiency. Importantly, no information about clinical symptoms lactose intolerance is obtained during testing.
Lactose digestion can also be assessed by the lactose tolerance test25 and the H2-breath test.2,19,20 Both tests include an oral challenge with a standard dose of lactose (usually 20–50 g, corresponding to the lactose content of approximately 400–1000 ml cow milk). While the lactose tolerance test relies on an increase in blood glucose, the H2-breath test detects H2 produced by intestinal bacteria in expiratory air. The lactose tolerance test can be confounded by fluctuations of postprandial blood sugar in patients with impaired glucose tolerance or diabetes. The H2-breath test can be false-positive in the presence of small intestinal bacterial overgrowth.26 False-negative results for the H2-breath are observed for ‘H2-non-producers’, the 2–43% of individuals (<10% in most studies) in whom the bowel flora does not produce hydrogen.2 For these patients, test sensitivity can be improved by simultaneous measurement of methane.2 A related cause of false-negative results in some individuals may be ‘full colonic adaptation’ to lactose ingestion, in which repeated intake of lactose selectively favours the growth of colonic flora that rapidly ferment lactose without producing hydrogen.27,28 Currently, the genetic test (in Caucasians) and the H2-breath test are widely used in clinical practice. Studies in Caucasian populations without gastrointestinal comorbidity report a high degree of agreement for diagnosis of lactose malabsorption between the H2-breath test, intestinal lactase level, and genetic tests.14,23,24,29
Assessment of lactose intolerance
The presence of lactose malabsorption is necessary but is not sufficient to establish lactose intake as the cause of patient symptoms. Lactose intolerance is defined by the occurrence of typical symptoms (i.e. nausea, bloating, diarrhoea, borborygmi, abdominal pain) during the H2-breath test procedure. However, interpretation of patient reports may be complicated by placebo responses.
Self-reported food intolerance is reported by a large proportion of the community in population-based studies, with frequency rates ranging from 9.5 to 25%30–32 and is even higher in patients with functional gastrointestinal diseases such as IBS.33,34 However, when subjected to controlled and blinded testing, only 25–40% of these patients react to the offending food.30,32 Similarly, an open label challenge with 240 ml of milk induced symptoms in 59% of patients with lactose malabsorption35 but had no significant effects in a placebo-controlled challenge of subjects that self-reported severe lactose intolerance (indeed nine out of 30 patients did not have lactose malabsorption on breath testing).36 These findings demonstrate a high frequency of placebo response to lactose in patients referred for investigation of digestive symptoms. Such a response might still impact on patient well being and could be referred to as ‘functional lactose intolerance’ (Box 1). These studies also demonstrate that blinded testing would increase the specificity of diagnosis and guide more rational dietary management of lactose intolerance. Double-blind placebo-controlled challenges for testing of food allergies has been criticized;37 however, these arguments do not apply to lactose intolerance for which procedures can easily be standardized and anaphylactic reactions will not occur.
Further, multiple-dose testing in a controlled and blinded manner may have additional advantages. Clear evidence of a dose–response effect eliminates any doubts about cause and effect and this approach can also define individual lactose tolerance thresholds and guide dietary management since patients often dramatically underestimate the amount of lactose they could safely ingest. Recently a double-blinded, randomized, three-way cross-over comparison of lactose tolerance testing at 10, 20, and 40 g lactose was performed in patients with diarrhoea-predominant IBS (IBS-D) and controls in a Chinese population with lactase deficiency.34 The proportion of participants with an objective increase of at least 20 ppm H2 increased progressively with lactose dose and was similar in both groups (Table 2). In contrast, the proportion of IBS-D patients reporting typical symptoms of lactose intolerance was significantly higher than for healthy subjects at every dose. Furthermore, IBS-D patients reported multiple symptoms and more severe symptoms compared to healthy subjects. These findings confirm that symptoms of lactose intolerance are very rare at the low, 10 g lactose doses in healthy subjects and are uncommon even in IBS patients with lactase deficiency. Such multiple-dose testing with a dose below normal symptom threshold (10 g), a dose reflecting normal intake at a single meal (20 g), and a ‘positive control’ (40 g) provides comprehensive information that explains the causes of symptoms and guides clinical management in a given patient.
Table 2.
Blinded multiple dose testing for lactose intolerance
| 10 g lactose | 20 g lactose | 40 g lactose | ||
|---|---|---|---|---|
| Proportion of individuals with positive H2 breath test >20 ppm | Controls | 35 | 87 | 93 |
| IBS-D patients | 42 | 80 | 92 | |
| Proportion of individuals with typical symptoms | Controls | 3 | 22 | 73 |
| IBS-D patients | 18* | 47** | 85* |
Values are %. Results of a recent blinded, placebo controlled three-way crossover trial.34
p < 0.05, **p < 0.01 compared to healthy controls.
IBS, diarrhoea-predominant irritable bowel syndrome.
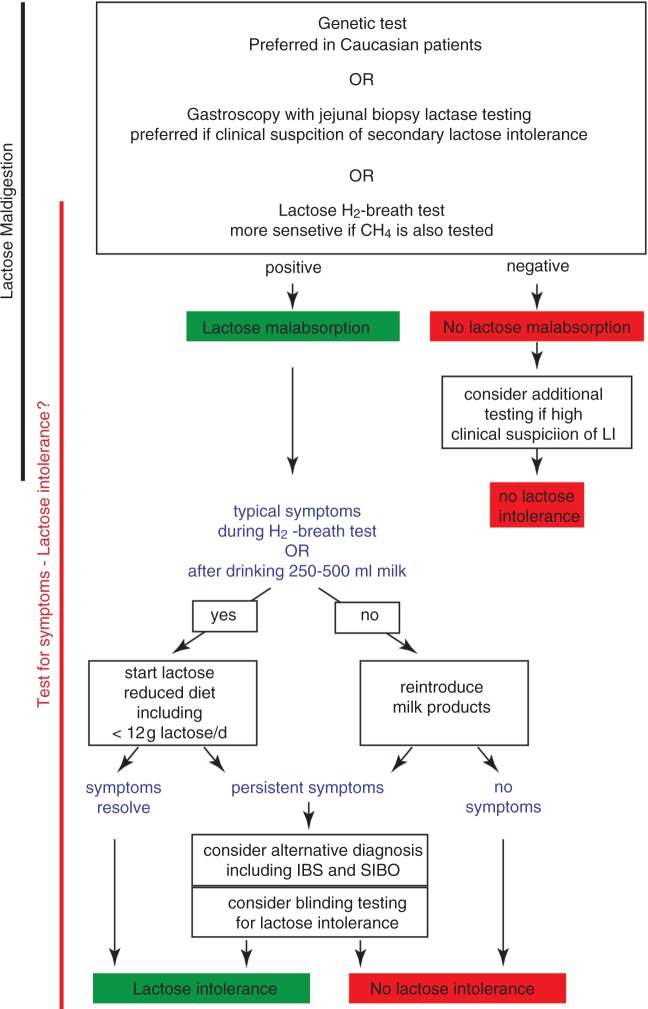
In current practice, lactose tolerance testing is performed as an open label procedure during the H2 breath test. The negative predictive value of these tests is high and lack of symptoms after a 40–50 g lactose challenge excludes lactose intolerance.38,39 In contrast, if symptoms develop, the possibility of a ‘placebo-response’ should not be ignored especially in patients without lactose malabsorption and those with functional gastrointestinal disease or self-reported food intolerance or allergies. Currently, placebo-controlled or multiple-dose testing is not performed outside research studies and would be difficult to implement in clinical practice. One solution to this practical issue would be, after confirming lactase deficiency in the laboratory, to perform blinded testing at multiple doses in the home environment. This approach could facilitate widespread implementation of appropriate dietary management rather than absolute exclusion of lactose intake. A diagnostic algorithm for lactose malabsorption and intolerance is provided in Figure 2.
Figure 2.
Diagnostic algorithm for diagnosing lactose malabsorption and lactose intolerance
Diagnosis of lactose malabsorption is a pre-requisite for diagnosis of lactose intolerance (LI). Tests of malabsorption (genetic, biopsy) should be followed by a test of intolerance before lactose restriction is recommended. Blinded testing at multiple doses in the home setting may help guide rational dietary management; however, protocols still need to be established. LI, lactose intolerance; IBS, irritable bowel syndrome; SIBO, small intestinal bacterial overgrowth.
Treatment of lactose intolerance
Treatment of lactose intolerance should not be aimed at reducing malabsorption but rather at improving digestive symptoms. Reduction of lactose intake (Table 3) rather than exclusion is recommended because, as noted above, in blinded studies most patients with self-reported lactose intolerance can ingest at least 12 g lactose (equivalent to 250 ml milk) without experiencing symptoms36,40,41 and taken with other foods, up to 18 g lactose can often be tolerated.41 It should be emphasized that the amount of lactose in tablets (<500 mg) is very unlikely to cause gastrointestinal complaints under any circumstances,42 whereas symptoms after intake of small amounts of dairy products should raise the suspicion of a true food allergy to cow's milk protein. Observational studies report improvement of abdominal complaints, with lactose restriction in up to 85% of IBS patients with lactose malabsorption;43,44 however, in randomized controlled trials, no significant improvement has been found with dietary or enzyme-replacement treatment.45,46 This lack of efficacy in well-designed trials may be because lactose is only one of many poorly digestible, fermentable carbohydrates and dietary fibres in the diet.47 Therefore, even in patients with lactase deficiency, lactose restriction will rarely provide complete symptom relief. Consistent with this hypothesis, a recent controlled trial of a diet low in FODMAPs (Fermentable Oligo-, Di- and Mono-saccharides And Polyols) reported symptom improvement in 86% of IBS patients, compared to 49% for a standard dietary intervention.48
Table 3.
Lactose and calcium content of different milk products
| Serving size | Lactose | Calcium | |
|---|---|---|---|
| Milk, regular | 1cup, 250 ml | 12 g | 285 mg |
| Milk, reduced fat | 1cup, 250 ml | 13 g | 340 mg |
| Yoghurt, regular | 200 g | 9 g | 340 mg |
| Yoghurt, reduced fat | 200 g | 12 g | 420 mg |
| Cheese, cheddar | 30 g | 0.02 g | 260 mg |
| Cheese, creamed cottage | 30 g | 0.1 g | 22 mg |
| Butter | 1 teaspoon | 0.03 g | 1 mg |
| Ice cream | 2 scoops, 50 g | 3 g | 55 mg |
Substantial amounts of lactose can also be found in processed meat including sausages, chocolate and ready-made meals. Hard cheese, for instance cheddar and parmesan, contain only minute amounts of lactose. Table modified from.58
Lactase enzyme replacement is another option although this changes the taste of the food when mixed with the dairy products because glucose and galactose produced by lactose digestion are sweeter than the original sugar. A variety of preparations are available over the counter but may not be equally effective.49 Another strategy involves probiotics that alter the intestinal flora and may have beneficial effects in IBS patients that persist even after treatment.50 Finally, although lactase expression is not upregulated by lactose ingestion, tolerance may be induced by repeated lactose dosing due to adaptation of the intestinal flora.41 Further studies are required to provide high-quality evidence to support/compare the efficacy of these strategies.41
In summary, restricting lactose intake is sensible treatment for lactose intolerance but patients should be reassured that absolute exclusion is unnecessary. If patients are unwilling to reduce milk intake, then lactase supplementation is appropriate. If typical abdominal symptoms persist despite these measures, then a trial of a diet low in FODMAPs may be more effective.
Long-term effects of lactose restriction
Although restricting dietary lactose may improve gastrointestinal complaints, long-term effects of a diet free of dairy products may be of concern.51 Dairy products are the major source of calcium in many individuals (Table 3). Current guidelines suggest a daily calcium intake of 1000 mg per day for adults, 1300 mg for adolescents, and 1200 mg for people over 50 years.52 Drinking three cups of milk per day has been recommended to obtain this amount.52 It has been shown that adults and adolescents with a homozygous −13910*C genotype or self-reported milk intolerance consume less milk or lactose and less calcium than controls.51,53 There is also some evidence that a low intake of dairy products is associated with a higher risk of fractures in women, although much less so in men.51,54 Similarly, the −13910*C genotype was a risk factor for osteoporosis in some studies51,55,56 but not in others.51,57 No study has addressed the safety and effectiveness of calcium replacement for patients with lactose intolerance; however, it seems reasonable to recommend increasing calcium intake from other sources in patients that restrict intake of dairy products and to have a low threshold for calcium replacements in the presence of other risk factors for osteoporosis.
Conclusions
Progress has been made in our understanding of lactose malabsorption and intolerance. Sensitive and specific tests for lactase deficiency are now available. Diagnosis of lactose intolerance is less definitive as it requires concurrent assessment of lactose digestion and abdominal symptoms. Due to high rates of self-reported intolerance of dairy products and the fact that even patients with lactase deficiency can often tolerate up to 20 g lactose, such tests may be best performed as a series of blinded procedures at multiple doses. This approach provides information that can guide rational dietary management; however, the practical implementation of such testing is still unresolved. Once the optimal diagnostic method has been established, studies will be needed to assess the long-term outcome of dietary interventions.
Sources and selection criteria
We identified randomized controlled trials for diagnosis and treatment of lactose malabsorption and intolerance. This research had been done by an NIH evidence report51 and others;18,41 however, high-quality, evidence-based guidelines were not available. We identified additional studies through Medline using search terms ‘lactose malabsorption’, ‘lactose maldigestion’, ‘lactose intolerance’, ‘intolerance AND dairy OR milk’, and also examined our own database for appropriate publications that tackle these issues.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. Brannon PM, Carpenter TO, Fernandez JR, et al. NIH consensus development conference statement: lactose intolerance and health. NIH Consens State Sci Statements 2010; 27: 1–27. PMID: 20186234. [PubMed]
- 2.Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther 2009; 29(Suppl 1): 1–49 [DOI] [PubMed] [Google Scholar]
- 3.Ingram CJ, Mulcare CA, Itan Y, et al. Lactose digestion and the evolutionary genetics of lactase persistence. Hum Genet 2009; 124: 579–591 [DOI] [PubMed] [Google Scholar]
- 4.Scrimshaw NS, Murray EB. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am J Clin Nutr 1988; 48: 1079–1159 [DOI] [PubMed] [Google Scholar]
- 5.Itan Y, Jones BL, Ingram CJ, et al. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol Biol 2010; 10: 36–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enattah NS, Sahi T, Savilahti E, et al. Identification of a variant associated with adult-type hypolactasia. Nat Genet 2002; 30: 233–237 [DOI] [PubMed] [Google Scholar]
- 7.Tishkoff SA, Reed FA, Ranciaro A, et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet 2007; 39: 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imtiaz F, Savilahti E, Sarnesto A, et al. The T/G 13915 variant upstream of the lactase gene (LCT) is the founder allele of lactase persistence in an urban Saudi population. J Med Genet 2007; 44: e89–e89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bersaglieri T, Sabeti PC, Patterson N, et al. Genetic signatures of strong recent positive selection at the lactase gene. Am J Hum Genet 2004; 74: 1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coelho M, Luiselli D, Bertorelle G, et al. Microsatellite variation and evolution of human lactase persistence. Hum Genet 2005; 117: 329–339 [DOI] [PubMed] [Google Scholar]
- 11.Lewinsky RH, Jensen TG, Moller J, et al. T–13910 DNA variant associated with lactase persistence interacts with Oct-1 and stimulates lactase promoter activity in vitro. Hum Mol Genet 2005; 14: 3945–3953 [DOI] [PubMed] [Google Scholar]
- 12.Olds LC, Ahn JK, Sibley E. 13915*G DNA polymorphism associated with lactase persistence in Africa interacts with Oct-1. Hum Genet 2011; 129: 111–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen TG, Liebert A, Lewinsky R, et al. The -14010*C variant associated with lactase persistence is located between an Oct-1 and HNF1alpha binding site and increases lactase promoter activity. Hum Genet 2011; 130: 483–493 [DOI] [PubMed] [Google Scholar]
- 14.Enattah NS, Kuokkanen M, Forsblom C, et al. Correlation of intestinal disaccharidase activities with the C/T–13910 variant and age. World J Gastroenterol 2007; 13: 3508–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews SB, Waud JP, Roberts AG, et al. Systemic lactose intolerance: a new perspective on an old problem. Postgrad Med J 2005; 81: 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Fox M, Cong Y, et al. Lactose intolerance in patients with chronic functional diarrhoea: the role of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2010; 31: 892–900 [DOI] [PubMed] [Google Scholar]
- 17.Tomba C, Baldassarri A, Coletta M, et al. Is the subjective perception of lactose intolerance influenced by the psychological profile? Aliment Pharmacol Ther 2012; 36: 660–669 [DOI] [PubMed] [Google Scholar]
- 18.Jellema P, Schellevis FG, van der Windt DA, et al. Lactose malabsorption and intolerance: a systematic review on the diagnostic value of gastrointestinal symptoms and self-reported milk intolerance. QJM 2010; 103: 555–572 [DOI] [PubMed] [Google Scholar]
- 19.Newcomer AD, McGill DB, Thomas PJ, et al. Prospective comparison of indirect methods for detecting lactase deficiency. N Engl J Med 1975; 293: 1232–1236 [DOI] [PubMed] [Google Scholar]
- 20.Metz G, Jenkins DJ, Peters TJ, et al. Breath hydrogen as a diagnostic method for hypolactasia. Lancet 1975; 1: 1155–1157 [DOI] [PubMed] [Google Scholar]
- 21.Maiuri L, Raia V, Potter J, et al. Mosaic pattern of lactase expression by villous enterocytes in human adult-type hypolactasia. Gastroenterology 1991; 100: 359–369 [DOI] [PubMed] [Google Scholar]
- 22. Furnari M, Bonfanti D, Parodi A, et al. A comparison between lactose breath test and quick test on duodenal biopsies for diagnosing lactase deficiency in patients with self-reported lactose intolerance. J Clin Gastroenterol 2012; 47: 148–152. [DOI] [PubMed]
- 23.Hogenauer C, Hammer HF, Mellitzer K, et al. Evaluation of a new DNA test compared with the lactose hydrogen breath test for the diagnosis of lactase non-persistence. Eur J Gastroenterol Hepatol 2005; 17: 371–376 [DOI] [PubMed] [Google Scholar]
- 24.Krawczyk M, Wolska M, Schwartz S, et al. Concordance of genetic and breath tests for lactose intolerance in a tertiary referral centre. J Gastrointestin Liver Dis 2008; 17: 135–139 [PubMed] [Google Scholar]
- 25.Arola H. Diagnosis of hypolactasia and lactose malabsorption. Scand J Gastroenterol Suppl 1994; 202: 26–35 [DOI] [PubMed] [Google Scholar]
- 26.Nucera G, Gabrielli M, Lupascu A, et al. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2005; 21: 1391–1395 [DOI] [PubMed] [Google Scholar]
- 27.Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am J Clin Nutr 1996; 64: 232–236 [DOI] [PubMed] [Google Scholar]
- 28.Szilagyi A, Cohen A, Vinokuroff C, et al. Deadaption and readaptation with lactose, but no cross-adaptation to lactulose: a case of occult colonic bacterial adaptation. Can J Gastroenterol 2004; 18: 677–680 [DOI] [PubMed] [Google Scholar]
- 29.Pohl D, Savarino E, Hersberger M, et al. Excellent agreement between genetic and hydrogen breath tests for lactase deficiency and the role of extended symptom assessment. Br J Nutr 2010; 104: 900–907 [DOI] [PubMed] [Google Scholar]
- 30.Young E, Stoneham MD, Petruckevitch A, et al. A population study of food intolerance. Lancet 1994; 343: 1127–1130 [DOI] [PubMed] [Google Scholar]
- 31.Locke GR, 3rd, Zinsmeister AR, Talley NJ, et al. Risk factors for irritable bowel syndrome: role of analgesics and food sensitivities. Am J Gastroenterol 2000; 95: 157–165 [DOI] [PubMed] [Google Scholar]
- 32.Gelincik A, Buyukozturk S, Gul H, et al. Confirmed prevalence of food allergy and non-allergic food hypersensitivity in a Mediterranean population. Clin Exp Allergy 2008; 38: 1333–1341 [DOI] [PubMed] [Google Scholar]
- 33.Teufel M, Biedermann T, Rapps N, et al. Psychological burden of food allergy. World J Gastroenterol 2007; 13: 3456–3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Deng Y, Chu H, et al. Prevalence, presentation and effects on intake of dairy products of lactose intolerance in healthy subjects and patients with irritable bowel syndrome. J Clin Gastroenterol Hepatol 2013; 11: 262–268 [DOI] [PubMed] [Google Scholar]
- 35.Bayless TM, Rothfeld B, Massa C, et al. Lactose and milk intolerance: clinical implications. N Engl J Med 1975; 292: 1156–1159 [DOI] [PubMed] [Google Scholar]
- 36.Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med 1995; 333: 1–4 [DOI] [PubMed] [Google Scholar]
- 37.Asero R, Fernandez-Rivas M, Knulst AC, et al. Double-blind, placebo-controlled food challenge in adults in everyday clinical practice: a reappraisal of their limitations and real indications. Curr Opin Allergy Clin Immunol 2009; 9: 379–385 [DOI] [PubMed] [Google Scholar]
- 38.Beyerlein L, Pohl D, Delco F, et al. Correlation between symptoms developed after the oral ingestion of 50 g lactose and results of hydrogen breath testing for lactose intolerance. Aliment Pharmacol Ther 2008; 27: 659–665 [DOI] [PubMed] [Google Scholar]
- 39.Hermans MM, Brummer RJ, Ruijgers AM, et al. The relationship between lactose tolerance test results and symptoms of lactose intolerance. Am J Gastroenterol 1997; 92: 981–984 [PubMed] [Google Scholar]
- 40.Savaiano DA, Boushey CJ, McCabe GP. Lactose intolerance symptoms assessed by meta-analysis: a grain of truth that leads to exaggeration. J Nutr 2006; 136: 1107–1113 [DOI] [PubMed] [Google Scholar]
- 41.Shaukat A, Levitt MD, Taylor BC, et al. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med 2010; 152: 797–803 [DOI] [PubMed] [Google Scholar]
- 42.Montalto M, Gallo A, Santoro L, et al. Low-dose lactose in drugs neither increases breath hydrogen excretion nor causes gastrointestinal symptoms. Aliment Pharmacol Ther 2008; 28: 1003–1012 [DOI] [PubMed] [Google Scholar]
- 43.Bohmer CJ, Tuynman HA. The effect of a lactose-restricted diet in patients with a positive lactose tolerance test, earlier diagnosed as irritable bowel syndrome: a 5-year follow-up study. Eur J Gastroenterol Hepatol 2001; 13: 941–944 [DOI] [PubMed] [Google Scholar]
- 44.Vernia P, Ricciardi MR, Frandina C, et al. Lactose malabsorption and irritable bowel syndrome. Effect of a long-term lactose-free diet. Ital J Gastroenterol 1995; 27: 117–121 [PubMed] [Google Scholar]
- 45.Parker TJ, Woolner JT, Prevost AT, et al. Irritable bowel syndrome: is the search for lactose intolerance justified? Eur J Gastroenterol Hepatol 2001; 13: 219–225 [DOI] [PubMed] [Google Scholar]
- 46.Lisker R, Solomons NW, Perez Briceno R, et al. Lactase and placebo in the management of the irritable bowel syndrome: a double-blind, cross-over study. Am J Gastroenterol 1989; 84: 756–762 [PubMed] [Google Scholar]
- 47.Gibson PR, Shepherd SJ. Personal view: food for thought–western lifestyle and susceptibility to Crohn's disease. The FODMAP hypothesis. Aliment Pharmacol Ther 2005; 21: 1399–1409 [DOI] [PubMed] [Google Scholar]
- 48.Staudacher HM, Whelan K, Irving PM, et al. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet 2011; 24: 487–495 [DOI] [PubMed] [Google Scholar]
- 49.Ramirez FC, Lee K, Graham DY. All lactase preparations are not the same: results of a prospective, randomized, placebo-controlled trial. Am J Gastroenterol 1994; 89: 566–570 [PubMed] [Google Scholar]
- 50.Almeida CC, Lorena SL, Pavan CR, et al. Beneficial effects of long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr Clin Pract 2012; 27: 247–251 [DOI] [PubMed] [Google Scholar]
- 51.Wilt TJ, Shaukat A, Shamliyan T, et al. Lactose intolerance and health. Evid Rep Technol Assess (Full Rep) 2010. (192): 1–410 [PMC free article] [PubMed] [Google Scholar]
- 52. Food and Nutrition Information Center. Dietary reference intakes. Beltsville, MD: USDA. Available at: http://fnic.nal.usda.gov/ (consulted December 2012)
- 53.Nicklas TA, Qu H, Hughes SO, et al. Self-perceived lactose intolerance results in lower intakes of calcium and dairy foods and is associated with hypertension and diabetes in adults. Am J Clin Nutr 2011; 94: 191–198 [DOI] [PubMed] [Google Scholar]
- 54.Kanis JA, Johansson H, Oden A, et al. A meta-analysis of milk intake and fracture risk: low utility for case finding. Osteoporos Int 2005; 16: 799–804 [DOI] [PubMed] [Google Scholar]
- 55.Obermayer-Pietsch BM, Bonelli CM, Walter DE, et al. Genetic predisposition for adult lactose intolerance and relation to diet, bone density, and bone fractures. J Bone Miner Res 2004; 19: 42–47 [DOI] [PubMed] [Google Scholar]
- 56.Enattah NS, Sulkava R, Halonen P, et al. Genetic variant of lactase-persistent C/T–13910 is associated with bone fractures in very old age. J Am Geriatr Soc 2005; 53: 79–82 [DOI] [PubMed] [Google Scholar]
- 57.Agueda L, Urreizti R, Bustamante M, et al. Analysis of three functional polymorphisms in relation to osteoporosis phenotypes: replication in a Spanish cohort. Calcif Tissue Int 2010; 87: 14–24 [DOI] [PubMed] [Google Scholar]
- 58. GastroNet for medical education and research in gastroenterology. Available at: http://www.gastro.net.au (accessed December 2012)