Abstract
Background
Treatment of functional gastrointestinal disorders (FGIDs) is based on symptoms relieve by conventional drugs, but increasingly complementary and alternative medicine (CAM) is used.
Objective
This survey aimed to investigate the current treatments used by FGIDs patients.
Methods
A total of 25 Italian gastroenterologists interviewed outpatients on gastrointestinal symptoms and treatments (pharmacological, CAM, diet/dietary supplements) used during the last year to relieve FGIDs. Consecutive adults with FGIDs according to Rome III were included.
Results
Of the 199 patients, 81% used conventional drugs, 64.3% diet/dietary supplements, and 48.7% CAM. Conventional drugs, diet/dietary supplements, or CAM as exclusive treatment were used by 24.6, 6, and 2.5% of patients, respectively. Two-thirds used more than one treatment: 34.7% conventional drugs, CAM, and diet/dietary supplements, 17.1% conventional drugs and diet/dietary supplements, 10.1% diet and CAM, and 5% conventional drugs and CAM. Benefits and adverse effects were similar for conventional drugs and nonpharmacological treatments. Males (OR 2.4) without lower GI symptoms (OR 5.4) used more frequently exclusive pharmacological treatment of FGIDs.
Conclusions
Conventional drugs are the preferred treatment for FGID. CAM and dietary modifications are more likely used as an adjunct to rather than instead of conventional drugs. Adverse effects occurred in all treatments.
Keywords: Complementary and alternative medicine, functional dyspepsia, functional gastrointestinal disorders, irritable bowel syndrome, treatment
Introduction
Functional gastrointestinal disorders (FGIDs), mainly functional dyspepsia and irritable bowel syndrome are characterized by chronic or recurrent gastrointestinal (GI) symptoms with no identifiable organic pathology.1,2 FGIDs are important for public health because they are highly prevalent, induce major social and economic burdens, and are associated with impaired health-related quality of life.3–5 While the underlying pathophysiological mechanisms still await to be definitively explained,6 criteria for diagnosis of FGIDs, namely Rome III, have been established.2,3,7 Because of their diverse symptomatology and the lack of a single target for drug intervention, FGIDs continue to remain a therapeutic challenge. Current therapies are targeting putative underlying mechanisms, including impaired GI motility and visceral hypersensitivity. Usually, pharmacological treatment of irritable bowel syndrome varies from antidepressants to antispasmodics, agents working on bowel movements and analgaesics.8,9 For functional dyspepsia, drugs usually employed in clinical practice are acid secretion inhibitors, prokinetics, and antidepressants.10,11 However, symptom control is poor for a large proportion of patients. For this reason, the current medical care for FGIDs often employs also nonpharmacological treatments, such as life style changes and dietary advice, psychotherapy, explanation, exercise advice, and reassurance.11,12 The failure of conventional treatment, the poorly understood pathology, and the psychological components of FGIDs have led to the use of complementary and alternative (CAM) therapies targeted at symptom management in FGIDs patients,13–18 ranging from 35–100% of patients in different European and non-European countries.19–23 The efficacy and the role of some CAMs in the treatment of FGIDs, such as acupuncture, hypnotherapy, herbal medicine, and probiotics, have been investigated obtaining conflicting results.24–32 This survey aimed to investigate the current conventional drug and CAM treatments used by patients with FGIDs.
Materials and methods
From September to December 2011, a multicentre cross-sectional survey was conducted in the nonhospital-based outpatient offices of 25 Italian gastroenterologists. Participating gastroenterologists were homogeneous in term of referral as all of them were operating at the specialist primary care level. Consecutive adult patients (aged >18 years) with newly diagnosed or known FGIDs (functional dyspepsia and/or irritable bowel syndrome) according to Rome III criteria were included.3 Accordingly, the presence of organic GI disease was ruled out by noninvasive or invasive investigations in patients under or over 45 years, respectively, and patients with peptic ulcer, coeliac disease, diverticular disease, inflammatory bowel disease, GI tract tumours, or previous gastric or intestinal surgery were excluded. Patients with extra-GI comorbidities, also when on chronic pharmacological treatment, were not excluded from the study.
Institutional approval to conduct the study was obtained from the local ethical committees, and informed consent was obtained from all patients involved in the study.
The survey tool was based on an ad-hoc developed questionnaire which included nine items regarding demographics, life style, family history of GI diseases, comorbidities and drug use for comorbidities, detailed upper and lower GI symptoms according to Rome III criteria, and treatment or treatments used during the last 12 months to relieve FGIDs-related symptoms. In particular, with regard to treatments, the patients were asked whether during the last year (i) they had used conventional drugs such as anti-acids, proton pump inhibitors, H2-antagonists, prokinetics, antispasmodics, analgaesics, antidiarrhoeals, stimulant or osmotic laxatives, antidepressants, or anti-anxiety agents; (ii) they had followed particular diets or had used dietary supplements, such as empirical exclusion diets, IgG/IgG4-based exclusion diets, probiotics, prebiotics, or fibres; and/or (iii) they had used CAM such as herbal products, homeopathy, acupuncture, reflexology, hypnotherapy, relaxation techniques, and cognitive-behavioural psychotherapy. Among nonpharmological treatments (i.e. diet, dietary supplements, and CAM), those previously investigated for FGID were included.12,13,16,19,26–29,31,32,33,35,36,10,11,14,17,24–27,29–31,33,34 The presence of comorbidities was assessed by the physicians on the basis of the medical history, including medical charts and previous hospital admissions. The presence of GI symptoms was investigated by means of a simplified version of the original Italian version of the Rome II questionnaire which has been validated for use in the Italian language.35 Although the Rome II Questionnaire was the only one available at the time the study started, since an Italian version of the Rome III Questionnaire has not yet been validated, its items were applicable to both the Rome II and the Rome III irritable bowel syndrome and functional dyspepsia criteria. The severity of symptoms was assessed by using a 10-point visual analogue scale ranging from 0 to 10 (0 means that the symptom is absent and 10 means that the symptom is the strongest one never felt).
The survey tool was developed on a specific online platform, SurveyMonkey, which the participating gastroenterologists accessed by a link which brought them directly to the first question of the questionnaire. The physicians interviewed their patients face to face following the online questionnaire, which was structured in the way that the following question could be visualized only when the current question has been answered (required fields).
FGIDs were defined as the presence of functional symptoms of the upper and/or lower GI tract during the last 6 months for a least once a week of the symptoms included for the diagnosis of functional dyspepsia or irritable bowel syndrome according to Rome III, given that organic GI disease had been ruled out by noninvasive (<45 years) or invasive (>45 years) investigations.3
Data analysis and statistics
When the study period run out, the provider of the online platform provided the files of the complete electronic data set. The primary outcome of interest were the prevalence of use of the different forms of treatments for symptoms related to FGIDs. A descriptive statistical analysis was performed on all available input variables. Data were expressed as number and percentage (%) of total, mean ± SD, or median (range). Subgroups of continuous variables were compared by Student’s t-test or Mann–Whitney test, as appropriate. Subgroups of categorical variables were compared by chi-squared test. Multiple regression analysis was used to assess features associated with use of pharmacological treatment of FGIDs-related symptoms. Two-tailed p-values <0.05 were considered statistically significant. Statistics were performed on a dedicated software package, MedCalc Software version 12.2.1 (Mariakerke, Belgium).
Results
Of the 222 elegible patients with FGID, 204 (91.9%) gave their consent to participate in the study and the remaining 18 (72.2% female, median age 49.9 years) refused to participate because they were not interested in clinical investigations (n = 12), or they did not remember the treatments used during the last year for FGID (n = 6). Of the 204 records, a completed questionnaire was obtained from 199 patients (97.5%) with FGIDs. Five records were excluded due to incomplete data.
Table 1 summarizes demographics and clinical features of the 199 patients. The median age was 50 years, and patients aged 20–39, 40–59, 60–79, and older than 80 years of age were as frequent as 26.6, 48.7, 21.6, and 3%, respectively. The female gender was predominant (75.9%) and the median (range) body mass index was 24.2 (17.1–41.6) kg/m2, with 39.2% of patients having a BMI higher than 25 kg/m2. More than half of patients (56.3%) had a higher education level, 41.5% completed a secondary school, and 15.1% a high school, and only 7.5% left at the primary school level. With regard to working status, 30% were employees, 19.6% were retired, 18.6% were housewives, and 10% were workers. Only 27.6% never consumed coffee, while amongst the 144 (72.4%) coffee-drinkers, more than half (55.8%) had 1–2 cups daily, 15.1% had 3–4 cups daily, and only 3% had more than 4 cups daily. Of the 86 (43.2%) smokers, 31 (15.6%) smoked less than 20 and five (2.5%) more than 20 cigarettes daily, while 50 (25.1%) were past smokers. Amongst the 71 (35.7%) alcohol consumers, only four (2%) drank more than three units daily, while the others (n = 67, 33.7%) drank less than three units daily. Half of the patients (49.7%) had comorbidities, which were mainly anxiety or depression (50.5%) and cardiovascular diseases (48.5%), followed by dyslipidaemia (16.2%), thyroid diseases (9.1%), bone diseases (7.1%), diabetes (6.1%), and respiratory disorders (5.0%). These comorbidities were pharmacologically treated in 81 (40.7%) patients and the mean number of drugs taken per patient was 1.8 ± 1.5. In particular, 40 (80%) out of the 50 patients with anxiety or depression were on pharmacological treatment.
Table 1.
Demographics and clinical features of 199 patients with functional GI disorders
| Characteristic | Study population |
|---|---|
| Age (years) | 50 (21–85) |
| Female gender | 151 (75.9) |
| Body mass index (>25 kg/m2) | 78 (39.2) |
| Secondary school or higher | 112 (56.3) |
| Occupation/working status | |
| Employee | 60 (30.2) |
| Retired | 39 (19.6) |
| Housewife | 37 (18.6) |
| Self-employed | 21 (10.6) |
| Worker | 19 (9.5) |
| Unemployed | 11 (5.5) |
| Student | 4 (2.0) |
| Other occupation | 8 (4.0) |
| Coffee | 144 (72.4) |
| Actual or past smoker | 86 (43.2) |
| Alcohol intake | 71 (35.7) |
| Comorbidity | 99 (50) |
| Drugs for comorbidities | 81 (40.7) |
| Anxiety/depression | 50 (25.1) |
| Drugs for anxiety/depression | 40 (20.1) |
Data are median (range) or n (%).
Gastrointestinal symptoms
Figure 1 shows the presence of functional symptoms in the upper and lower GI tract as reported by the 199 patients. With regard to the upper GI tract, at least one symptom was present in 84.4% of patients, represented by postprandial fullness, epigastric burning, epigastric pain, early satiation, belching, and nausea in 55.8, 45.7, 41.7, 39.7, 33.7, and 10.6% of patients, respectively. The mean number of upper GI symptoms per patient was 2.2 ± 1.5, with 22.6, 25.6, and 18.1% having two, three or four, and up to six upper GI symptoms. The mean severity score of upper GI symptoms was 5.8 ± 1.8.
Figure 1.
Prevalence of GI symptoms in 199 patients: upper and lower GI symptoms (a) and functional dyspepsia and irritable bowel syndrome (b).
In (a), white = upper GI symptoms, black = lower GI symptoms, and grey = both upper and lower GI symptoms. In (b), white = functional dyspepsia, black = irritable bowel syndrome, and grey = both functional dyspepsia and irritable bowel syndrome.
With regard to the lower GI tract, at least one symptom was present in 79.9% of patients, represented among others by abdominal bloating or distension, abdominal pain or discomfort, sensation of incomplete defecation, more frequent bowel habits, looser stools, less frequent bowel habits, and harder stools in 69.3, 66.8, 24.1, 20.6, 16.1, 15.1, and 20.6% of patients, respectively. The mean number of lower GI symptoms per patient was 2.9 ± 2.1, with 9.5, 19.1, 21.6, 11.1, 6.0, and 4% having two, three, four, five, six, or up to nine lower GI symptoms. The mean severity score of lower GI symptoms was 6.1 ± 1.8. Symptoms in both the upper and lower GI tract were present in 130 (65.3%).
According to Rome III, the diagnosis of functional dyspepsia was present in 164 (82.4%) patients and the diagnosis of irritable bowel syndrome was present in 93 (43.7%) patients, which was diarrhoea-predominant in 23 (28%), constipation-predominant in 50 (53.8%), and of the mixed subtype in 17 (18.3%). Both functional dyspepsia and irritable bowel syndrome were present in 71 (35.7%) patients.
Treatment of FGIDs
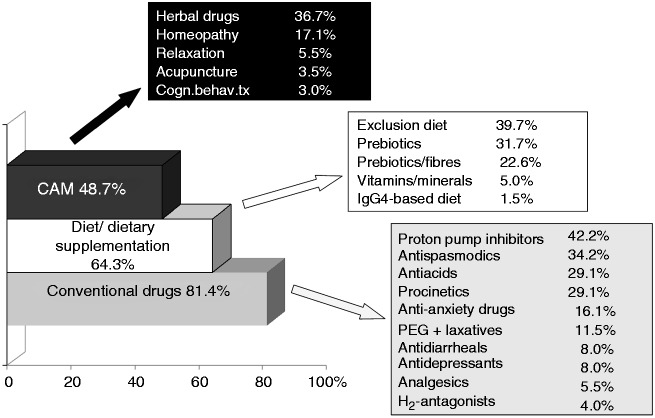
As shown in Figure 2, 81.4% of patients used conventional drugs to relieve their FGIDs symptoms. The class of drugs mainly used were proton pump inhibitors (42.2%), antispasmodics (34.2%), anti-acids (29.1%), and prokinetics (29.1%). A dietary approach was used by 64.3% of patients, mainly empirical exlusion diets (39.7%), probiotics (31.7%), and prebiotics (22.6%). Nearly half of the patients (48.7%) used CAM such as herbal medicine (36.7%) or homeopathy (17.1%).
Figure 2.
Treatments used in the last year by 199 patients to relieve symptoms of functional GI disorders.
Categories are not mutually exclusive. CAM, complementary and alternative medicine; Cogn.behav.tx, cognitive behavioural psychotherapy.
As shown in Figure 3, conventional drugs, diet and dietary supplements, or CAM as exclusive treatment were used by 24.6, 6, and 2.5% of patients, respectively. In contrast, two-thirds of patients used more than one treatment: slightly more than one-third (34.7%) used conventional drugs together with CAM and dietary approach, 17.1% used conventional drugs and diet or dietary supplements, 10.1% used diet and CAM, and 5% used conventional drugs and CAM.
Figure 3.
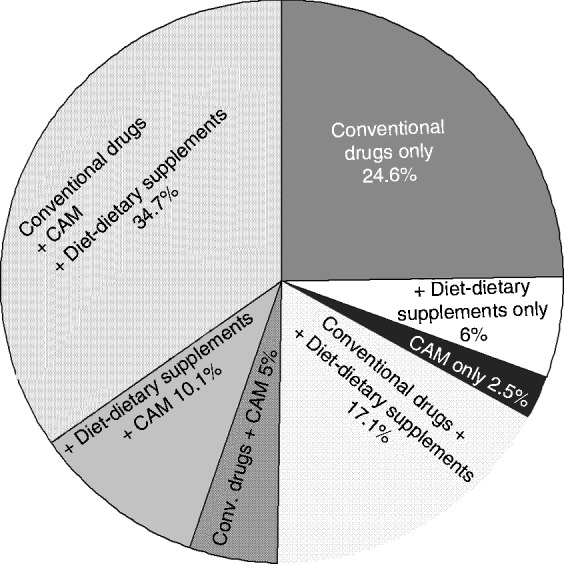
Combinations of treatments in the last year by 199 patients to relieve symptoms of functional GI disorders.
CAM, complementary and alternative medicine.
Patients with comorbidities used less frequently conventional drugs alone (16.2 vs. 33%, p < 0.009), but used more frequently all treatment options, conventional drugs, diet or dietary supplements and CAM (49.5 vs. 20%, p < 0.0001) to relieve FGIDs-related symptoms when compared to patients without comorbidities. In contrast, when compared to patients with only lower GI symptoms or both upper and lower GI symptoms, patients with only upper GI symptoms used more frequently conventional drugs alone (56.4 vs. 23.3% and 15.4%, p < 0.01 and <0.0001), but less frequently all three treatment options (conventional drugs, diet or dietary supplements, and CAM; 5.1 vs. 30% and 44.6%, p < 0.01 and < 0.0001).
Table 2 shows the subjectively reported symptomatic benefits and adverse effects of the single treatments. Benefit, partial benefit, or no benefit from conventional drugs was reported by 41.9, 52.1, and 5.5% of patients compared to 31.8, 54.3, and 13.9% of nonpharmacological treatments (p > 0.05). Adverse effects were reported by 10.5 and 13.3% of patients using pharmacological and nonpharmacological treatments, respectively (p = 0.3). As shown by logistic regression, male gender (OR 2.4, 95% CI 1.1–5.8) and absence of lower GI tract symptoms (OR 5.4, 95% CI 2.3–12.5) were the features significantly associated with the use of exclusive pharmacological treatment of FGIDs, while age over 45 years, body mass index over 25 kg/m2, consuming coffee or alcohol, smoking, education level, comorbidities, presence of anxiety or depression, or presence of upper GI symptoms showed no significant association.
Table 2.
Subjectively reported symptomatic benefits and adverse effects of treatments by patients with functional GI disorders
| No. of patients | Benefit | Partial benefit | No benefit | Adverse effectsa | |
|---|---|---|---|---|---|
| Conventional drugs (n = 162) | |||||
| Antiacids | 58 | 25.9 | 60.3 | 13.8 | 1 (1.7) |
| Proton pump inhibitors | 84 | 50.6 | 38.5 | 10.8 | 1 (1.2) |
| H2-antagonists | 8 | 60.0 | 40.0 | 0 | 0 |
| Prokinetics | 58 | 16.4 | 67.2 | 16.4 | 3 (5.2) |
| Antispasmodics | 68 | 39.4 | 56.1 | 4.5 | 0 |
| Analgaesics | 11 | 38.5 | 53.8 | 7.7 | 2 (18.2) |
| Antidiarrhoeals | 16 | 37.5 | 56.2 | 1.0 | 1 (6.2) |
| Laxatives | 23 | 53.5 | 46.4 | 0 | 2 (8.7) |
| Antidepressants | 16 | 40.0 | 53.3 | 6.7 | 4 (25) |
| Anti-anxiety agents | 32 | 45.4 | 54.5 | 0 | 3 (9.4) |
| Mean ± SD | 41.9 ± 12.9 | 52.1 ± 8.7 | 5.5 ± 1.8 | 17 (10.5)b | |
| Nonpharmacological treatments (n = 143) | |||||
| Exclusion diets | 79 | 17.7 | 64.6 | 17.7 | 3 (3.8) |
| Probiotics | 63 | 32.8 | 59.4 | 7.8 | 0 |
| Prebiotics/fibres | 45 | 19.6 | 76.1 | 4.3 | 2 (4.4) |
| Vitamin supplements | 10 | 42.8 | 57.2 | 0 | 0 |
| Herbal products | 73 | 23.6 | 52.8 | 23.6 | 11 (15.1) |
| Homeopathy | 34 | 30.3 | 49.8 | 19.9 | 1 (2.9) |
| Acupuncture | 7 | 50.0 | 16.7 | 33.3 | 0 |
| Relaxation | 11 | 36.4 | 45.4 | 18.2 | 2 (18.2) |
| Cognitive-behavioural psychotherapy | 6 | 33.3 | 66.7 | 0 | 0 |
| Media ± SD | 31.8 ± 3.5 | 54.3 ± 5.6 | 13.9 ± 3.8 | 19 (13.3)c |
Values are % unless otherwise stated.
Values are n (%).
With respect to the total number of patients using conventional drugs.
With respect to the total number of patients using nonpharmacological treatments.
Discussion
Despite the proposed standardized treatments for irritable bowel syndrome36,37 and functional dyspepsia,11,38 FGIDs remain a therapeutic challenge, and little is known about which treatments FGIDs patients actually use. This Italian multicentre survey focused on a wide range of treatment options for FGIDs, including pharmacological and nonpharmacological treatments, and showed that in the last year the majority of patients (81%) used conventional drugs to relieve FGIDs-related symptoms, followed by dietary modifications (64%) and CAM (49%). To our knowledge, no previous studies have assessed detailed pharmacological and and nonpharmacological treatment options in FGIDs patients. Data on functional dyspepsia are scarce, because previous surveys mainly focused on treatments in irritable bowel syndrome.12,19 A US survey on the usual medical care for irritable bowel syndrome showed that patients were more likely to be treated with education, reassurance, and advice about lifestyle than with drugs.12 The results of the present survey are apparently in contrast, which probably may be explained by methodological differences and different settings. For example, the US survey investigated irritable bowel syndrome patients only, while this survey included patients with FGIDs of the upper and lower GI tract. While for irritable bowel syndrome patients, drug therapy is not recommended for the routine treatment,36 for functional dyspepsia as first-line treatment, proton pump inhibitors followed by tricyclic antidepressants have been proposed.37 In this survey, patients with only upper GI symptoms, indeed, preferred more frequently exclusive pharmacological treatment compared to patients with only lower or both upper and lower GI symptoms, suggesting that preferred treatment options may be different with regard to the topography of FGIDs. The potential reasons for this difference are not clear, but it is possible that the commonly prescribed drugs for functional dyspepsia, in particular antisecretory agents, are perceived as acceptably efficacious to relieve functional dyspepsia-related symptoms. Our data showed that proton pump inhibitors were the most frequently used drugs, more than half of functional dyspepsia patients were treated with proton pump inhibitors or H2 blockers, and in turn, more of half of these patients perceived symptomatic benefits and about 40% a partial benefit from these drugs, thus supporting this idea. Another potential reason may be that, at least in the Italian National Health System, antisecretory agents and some antiacids are released free of charge on medical prescription, while for nonconventional treatments and dietary supplements patients need to pay from their own pockets. This economic advantage may in part contribute to the patient’s preference of pharmacological treatment for functional upper GI symptoms.
This survey showed that in an Italian nonhospital-based specialty setting, the preferred treatment option for FGIDs-related symptoms was conventional drug therapy; however, only 25% of patients used it exclusively. In fact, more commonly more than one treatment option was used; most frequently (35%) patients used all three treatment options (conventional drugs, CAM, and dietary modifications), followed by 17% of patients who used conventional drugs and dietary approach. Similar results have been reported recently by Weizman et al.,39 who showed in patients with inflammatory bowel disease a relatively high rate of use of nonconventional treatments (56%), which, however, had no impact on adherence to conventional medications, indicating that patients with organic bowel disease also often use CAM in adjunct to conventional therapy.
In the present survey, an exclusive use of nonpharmacological and CAM treatment was less common (18.6%) with 6% using exclusively a dietary approach, 2.5% using exclusively CAM, and 10.1% using both. An increasing use of CAM in FGIDs patients has been reported, ranging from 35 to 100% in different European and non-European countries, notwithstanding the conflicting results with regard to efficacy,24–32 and the failure of conventional treatment is reported as one of the main reasons for CAM use.19–23 Thus, our data do not support the view that patients with FGIDs are increasingly using CAM as an exclusive and alternative treatment to conventional drugs, but suggest that CAM use cannot be attributed primarily to perceived dissatisfaction with conventional medical care or caregivers, but rather because these healthcare alternatives are felt to be more congruent with values and orientations toward health and life.40–42
Half of the investigated patients had comorbidities and many (82%) were on continuous pharmacological treatment, taking nearly two drugs daily. Patients on continuous drug treatment for comorbidities could have been expected to prefer pharmacological treatment also to relieve FGIDs-related symptoms. In contrast, it emerged that, compared to patients without comorbidities, those with comorbidities preferred less frequently exclusive pharmacological treatment for FGIDs, but used more frequently all treatment options. This may have at least two reasons: (i) patients on continuous drug treatment for organic diseases like cardiovascular diseases, from which withdrawal is not possible, are likely to use also nonpharmacological or CAM treatments at least for functional disorders; or (ii) FGIDs may in part be interpreted as possible adverse effects of the chronic pharmacological treatment for comorbidities. Drugs are frequently implicated as a possible cause in dyspeptic and other FGIDs-related symptoms, with few drugs being free of this suspicion, but it is challenging to discern between FGIDs and true drug-related FGIDs.43 Although representing a potential bias for our results, we feel that this specific setting represents real life, raising the question about polypharmacy and its role in FGIDs.
The results of this survey show also that the subjectively perceived symptomatic benefits of conventional and nonconventional therapies were similar, but suboptimal for all treatments, suggesting that none of the treatments really works in an optimal manner; thus the patient suffering from FGIDs-related symptoms likely goes on to seek optimal care to find a better solution. Anyway, in FGIDs the placebo response is a significant confounder of the drug efficacy assessment ranging in clinical trials on conventional drugs from 3 to 84% and in CAM trials from 15 to 84%.44 Thus, it is possible that placebo response may have played a role in a subjective estimation of symptomatic benefit of FGIDs treatments.
This survey showed that a relatively low proportion of patients experienced adverse effects, which was similar in conventional drug therapy and nonpharmacological treatments, CAM, and dietary changes. Generally, nonpharmacological and natural treatments are believed safe and harmless, but these results showed that the occurrence of adverse effects overlaps that of drug therapy. Previous studies have reported the potential harmfulness of nonconventional treatments.28,45–47 Thus, the popularity of CAM among FGIDs patients challenges the physicians, who should enhance their awareness and knowledge about this common practice to better advise their patients. However, a significant lack of data on the safety and tolerability of the current pharmacological agents used to treat irritable bowel syndrome in Europe has been shown,48 thus raising the question on the need of post-marketing surveillance and post-marketing studies for pharmacological and nonpharmacological treatments used in FGIDs.
There are limitations to our study. The time sequence of the different treatments used was not recorded. So, we are not able to know whether treatments were used together or consecutively, and which treatment option was used before or after another one, thus limiting the interpretation of the results. The sample number of investigated patients was relatively low, making the findings of this survey not necessarily representative of the nationwide population with FGIDs, albeit primary care gastroenterlogists from many Italian regions participated in the study. This was in part due to the relatively short study period of 4 months and the rigorous inclusion and exclusion criteria. Finally, it is possible that cultural differences as well as the differences between national health systems, which may guarantee or not the free release of some drugs or CAM treatments, may limit the extrapolation of the findings to other European and Western countries.
In conclusion, the present survey shows that the patients’ preferred treatment for FGIDs is conventional drugs, especially by males and when lower GI symptoms are not present. CAM and dietary modifications are more likely used as an adjunct to rather than instead of conventional drugs. While subjectively perceived benefits are suboptimal for all treatments, the occurrence of adverse effects is relatively low, but possible in all treatments.
Acknowledgements
We thank the members of the Study Group ‘Primary Care in Gastroenterology’ who actively participated in the study by interviewing the patients and collecting data: Gizela Beccari, Milano; Edoardo Benedetto, Rende (Cosenza); Luciano Bertolusso, Cuneo; Stefano Bellentani, Carpi (Modena); Alberto Bozzani, Monza; Carla Bruschelli, Roma; Carlo Casamassima, Bari; Alberto Chiaritti, Roma; Angela Ciaccia, Monopoli (Bari); Giovanni Coltraro, Catania; Carmelo Cottone, Palermo; Rudi De Bastiani, Feltre (Belluno); Manuela De Polo, Venezia; Giuseppe Disclafani, Palermo; Wilma Fornoni, Milano; Pierluigi Fracasso, Roma; Ignazio Grattagliano, Monopoli (Bari); Luigi Napoli, Napoli; Cristina Nebiocolombo, Genova; Guido Sanna, Oristano; Anna Scorpiniti, Albano Sant'Alessandro (Bergamo); Cesare Tosetti, Porretta Terme (Bologna); Enzo Ubaldi, San Benedetto del Tronto (Ancona); Salvatore Vetrano, Palermo; Maria Zamparella, Bari.
Funding
This work was supported in part by the Italian Foundation ‘Aldo Torsoli’ for Digestive, Liver and Pancreatic Diseases, Rome, Italy.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Mayer EA. Irritable bowel syndrome. N Engl J Med 2008; 358: 1692–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006; 130: 1466–1479 [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006; 130: 1377–1390 [DOI] [PubMed] [Google Scholar]
- 4.Lea R, Whorwell PJ. Quality of life in irritable bowel syndrome. Pharmacoeconomics 2001; 19: 643–653 [DOI] [PubMed] [Google Scholar]
- 5.Spiegel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep 2009; 11: 265–269 [DOI] [PubMed] [Google Scholar]
- 6.Öhman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol 2010; 7: 163–173 [DOI] [PubMed] [Google Scholar]
- 7.Longstreth GF, Thomspon WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491 [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M, Andresen V. Current and novel therapeutic options for irritable bowel syndrome management. Dig Liver Dis 2009; 41: 854–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JY, Talley NJ. Current and emerging therapies in irritable bowel syndrome: from pathophysiology to treatment. Trends Pharmacol Sci 2010; 31: 326–334 [DOI] [PubMed] [Google Scholar]
- 10.Brun R, Kuo B. Functional dyspepsia. Therap Adv Gastroenterol 2010; 3: 145–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacy BE, Talley NJ, Locke GR, 3rd, et al. Review article: current treatment options and management of functional dyspepsia. Aliment Pharmacol Ther 2012; 36: 3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitehead WE, Levy RL, Von Korff M, et al. The usual medical care for irritable bowel syndrome. Aliment Pharmacol Ther 2004; 20: 1305–1315 [DOI] [PubMed] [Google Scholar]
- 13.Nahin RL, Pontzer CH, Chesney MA. Racing toward the integration of complementary and alternative medicine: a marathon or a sprint? Health Aff 2005; 24: 991–993 [DOI] [PubMed] [Google Scholar]
- 14.Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in the irritable bowel syndrome. Arch Intern Med 2003; 163: 265–274 [DOI] [PubMed] [Google Scholar]
- 15.Kessler RC, Davis RB, Foster DF, et al. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med 2001; 135: 262–268 [DOI] [PubMed] [Google Scholar]
- 16.Langmead L, Rampton DS. Herbal treatment in gastrointestinal and liver disease – benefits and dangers. Aliment Pharmacol Ther 2001; 15: 1239–1252 [DOI] [PubMed] [Google Scholar]
- 17.Hussain Z, Quigley EMM. Systematic review: complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther 2006; 23: 465–471 [DOI] [PubMed] [Google Scholar]
- 18.Ernst E. The role of complementary and alternative medicine. BMJ 2000; 321: 1133–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong SC, Hurlstone DP, Pocock CY, et al. The incidence of self-prescribed oral complementary and alternative medicine use by patients with gastrointestinal diseases. J Clin Gastroenterol 2005; 39: 138–141 [PubMed] [Google Scholar]
- 20.Stake-Nilsson K, Hultcrantz R, Unge P, et al. Complementary and alternative medicine used by persons with functional gastrointestinal disorders to alleviate symptom distress. J Clin Nurs 2012; 21: 800–808 [DOI] [PubMed] [Google Scholar]
- 21.Kav T. Use of complementary and alternative medicine: a survey in Turkish gastroenterology patients. BMC Complement Altern Med 2009; 9: 41–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Tilburg MA, Palsson OS, Levy RL, et al. Complementary and alternative medicine use and cost in functional bowel disorders: a six month prospective study in a large HMO. BMC Complement Altern Med 2008; 8: 46–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmona-Sánchez R, Tostado-Fernández FA. Prevalence of use of alternative and complementary medicine in patients with irritable bowel syndrome, functional dyspepsia and gastroesophageal reflux disease. Rev Gastroenterol Mex 2005; 70: 393–398 [PubMed] [Google Scholar]
- 24.Lim B, Manheimer E, Lao I, et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2006. (5): CD005111 [DOI] [PubMed] [Google Scholar]
- 25.Webb AN, Kukuruzovic R, Catto-Smith AG, et al. Hypnotherapy for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2007. (4): CD005110 [DOI] [PubMed] [Google Scholar]
- 26.Hussain Z, Quigley EMM. Systematic review: complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther 2006; 23: 465–471 [DOI] [PubMed] [Google Scholar]
- 27.Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in the irritable bowel syndrome. Arch Intern Med 2003; 163: 265–274 [DOI] [PubMed] [Google Scholar]
- 28.Langmead L, Rampton DS. Herbal treatment in gastrointestinal and liver disease – benefits and dangers. Aliment Pharmacol Ther 2001; 15: 1239–1252 [DOI] [PubMed] [Google Scholar]
- 29.Hoveyda N, Heneghan C, Mahtani KR, et al. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel sindrome. BMC Gastroenterology 2009; 9: 15–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simrén M, Abrahamsson H, Bosaeus I, et al. Nutritional aspects in patients with functional gastrointestinal disorders and motor dysfunction in the gut. Working team report of the Swedish Motility Group (SMog). Digest Liver Dis 2007; 39: 495–504 [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Yang M, Liu Y, et al. Herbal medicines for treatment of irritable bowel syndrom. Cochrane Database Syst Rev 2006. (1): CD004116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madisch A, Holtmann G, Plein K, et al. Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled multi-centre trial. Aliment Pharmacol Ther 2004; 19: 271–279 [DOI] [PubMed] [Google Scholar]
- 33.Soo S, Moayyedi P, Deeks JJ, et al. Psychological intervention for non-ulcer dyspepsia. Cochrane Database Syst Rev 2005. (2): CD002301 [DOI] [PubMed] [Google Scholar]
- 34.Atkinson W, Sheldon TA, Shaath N, et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut 2004; 53: 1459–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drossman DA, Talley NJ, Whitehead WE, et al. The Rome II Modular Questionnaire: investigator and respondent forms. In: Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE. (eds). Rome II. The functional gastrointestinal disorders, Vol. 2, McLean, VA, USA: Degnon Associates, 2000, pp. 669–678 [Google Scholar]
- 36.Paterson WG, Thompson WG, Vanner SJ, et al. Recommendations for the management of irritable bowel syndrome in family practice. IBS Consensus Conference Participants. CMAJ 1999; 161: 154–160 [PMC free article] [PubMed] [Google Scholar]
- 37.Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002; 123: 2108–2131 [DOI] [PubMed] [Google Scholar]
- 38.Camilleri M, Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013; 10: 187–194 [DOI] [PubMed] [Google Scholar]
- 39.Weizman AV, Ahn E, Thanabalan R, et al. Characterisation of complementary and alternative medicine use and its impact on medication adherence in inflammatory bowel disease. Aliment Pharmacol Ther 2012; 35: 342–349 [DOI] [PubMed] [Google Scholar]
- 40.Eisenberg DM, Kessler RC, Van Rompay MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med 2001; 135: 344–351 [DOI] [PubMed] [Google Scholar]
- 41.Astin JA. Why patients use alternative medicine: results of a national study. JAMA 1998; 279: 1548–1553 [DOI] [PubMed] [Google Scholar]
- 42.Druss BG, Rosenheck RA. Association between use of unconventional therapies and conventional medical services. JAMA 1999; 282: 651–656 [DOI] [PubMed] [Google Scholar]
- 43.Bytzer P. Dyspepsia as an adverse effect of drugs. Best Pract Res Clin Gastroenterol 2010; 24: 109–120 [DOI] [PubMed] [Google Scholar]
- 44.Enck P, Horing B, Weimer K, et al. Placebo responses and placebo effects in functional bowel disorders. Eur J Gastroenterol Hepatol 2012; 24: 1–8 [DOI] [PubMed] [Google Scholar]
- 45.Licata A, Macaluso FS, Craxì A. Herbal hepatotoxicity: a hidden epidemic. Intern Emerg Med 2013; 8: 13–22 [DOI] [PubMed] [Google Scholar]
- 46.Angell M, Kassirer JP. Alternative medicine: the risks of untested und unregulated remedies (editorial). N Engl J Med 1998; 339: 839–841 [DOI] [PubMed] [Google Scholar]
- 47.Posadzki P, Alotaibi A, Ernst E. Adverse effects of homeopathy: a systematic review of published case reports and case series. Int J Clin Pract 2012; 66: 1178–1188 [DOI] [PubMed] [Google Scholar]
- 48.Heading R, Bardhan K, Hollerbach S, et al. Systematic review: the safety and tolerability of pharmacological agents for treatment of irrititable bowel syndrome – a European perspective. Aliment Pharmacol Ther 2006; 24: 207–236 [DOI] [PubMed] [Google Scholar]