Abstract
Background:
Several studies have reported that faecal calprotectin and lactoferrin showed a close correlation with endoscopic inflammation in patients with inflammatory bowel disease. However, the clinical significance of faecal calprotectin or lactoferrin in postoperative Crohn’s disease (CD) is not fully evaluated. This prospective study was to investigate the relationship between endoscopic activity, and faecal calprotectin and lactoferrin, and assess the predictive value of these markers for future recurrence.
Methods:
Twenty patients who remained in remission during 6–12 months after ileocolonic resection for CD were included. All patients underwent ileocolonoscopy for assessing endoscopic activity (Rutgeerts score) in the neo-terminal ileum. A stool sample was collected for measurement of calprotectin and lactoferrin. All patients were then followed up for 12 months, and clinical recurrence was defined as a CDAI >150 with an increase of ≥70 points.
Results:
The mean time between surgery and the endoscopic examination at entry was 7.2 months. The endoscopic scores were i0 or i1 in 10 patients, i2 in six patients, i3 in three patients, and i4 in one patient. Both calprotectin and lactoferrin positively correlated with the endoscopic scores (p = 0.0001 and p = 0.038, respectively). Six patients developed clinical recurrence during the 12-month follow-up. Both calprotectin and lactoferrin levels were significantly higher in patients with clinical recurrence than those in remission (p = 0.0007 and p = 0.025, respectively). A cutoff value of 170 µg/g for calprotectin had a sensitivity of 83% and a specificity of 93% to predict a risk of clinical recurrence, while a cutoff value of 140 µg/g for lactoferrin had a sensitivity of 67% and a specificity of 71%.
Conclusions:
Both calprotectin and lactoferrin levels correlate well with endoscopic activity after ileocolonic resection for CD. Calprotectin and lactoferrin could be clinically relevant biomarkers for predicting postoperative recurrence. Further well-designed large trials should strengthen the findings of the present investigation.
Keywords: Calprotectin, Crohn’s disease, faecal marker, lactoferrin. postoperative recurrence
Introduction
The assessment of intestinal inflammation in patients with inflammatory bowel disease (IBD) remains a challenge. Endoscopy is the most reliable and the gold standard method for assessing intestinal inflammation, but it is invasive and time-consuming.1 To overcome these limitations, a non-invasive marker to monitor inflammatory activity would be desirable. In clinical settings, the use of non-invasive markers for the diagnosis and management of IBD has been increasing.
Calprotectin and lactoferrin are specific neutrophil-derived proteins, which can be detected in small stool samples because they are released by cells in inflammatory conditions.2–9 Faecal calprotectin and lactoferrin showed a close correlation with endoscopic inflammation in patients with Crohn’s disease (CD) and ulcerative colitis (UC).10–13 Accordingly, these faecal markers appear to be useful for the assessment of endoscopic disease activity. Additionally, the value of faecal calprotectin or lactoferrin in predicting disease relapse has been investigated in patients with CD and UC.14–19 Elevated faecal calprotectin or lactoferrin level was associated with subsequent clinical relapse. Therefore, faecal calprotectin and lactoferrin may be useful to predict relapse in patients with quiescent IBD.
Postoperative recurrence is common in patients with CD, and currently, there is no convenient method to monitor and predict disease progression after surgery.20 Ileocolonoscopy is considered to be the gold standard for the assessment of recurrence after ileocolonic resection.21 Our hypothesis is that faecal calprotectin and lactoferrin measurement may correlate with early endoscopic lesions and thus predict risk of clinical recurrence after surgery for CD. However, the use of these markers in the postoperative setting is limited.22,23 With this in mind, the present prospective study was undertaken to investigate the relationship between faecal calprotectin or lactoferrin and endoscopic findings, and the value of these markers for predicting future clinical recurrence after ileocolonic resection for CD.
Patients and methods
Study design
This was a prospective, single-centre study undertaken at the Yokkaichi Social Insurance Hospital, a referral centre treating a large number of patients with IBD in the Mie Prefecture of Japan. The study was conducted in accordance with the principle of good clinical practice and the Declaration of Helsinki. Our study protocol was reviewed and approved by the Institutional Review Board at our hospital.
Patients
Inclusion criteria were: 1) patients who were between 20 and 70 years of age; 2) patients who had undergone ileocolonic resection for active CD (including previous anastomosis); 3) patients who had remained in clinical remission with CD activity index (CDAI)24 <150 during 6–12 months after surgery; 4) patient agreed to undergo endoscopic examination. Exclusion criteria were: 1) patients in whom a defunctioning stoma was created for protection of ileocolonic anastomosis; 2) patients who had gastroduodenal, jejunal, or proximal ileal disease at the time of surgery; 3) patient with active colonic or anorectal disease at entry. A total of 20 patients who met the inclusion criteria were included in this study. The baseline clinical features of the 20 eligible patients are presented in Table 1.
Table 1.
Baseline characteristics of the 20 patients included in this study
| Age (mean ± SE) | 32 ± 1.6 years |
| Male:female | 12:8 |
| Duration of CD before entry (mean ± SE) | 32 ± 3.7 months |
| Smoker (n) | 4 |
| Previous surgery (n) | 9 |
| Indication for surgery (n) | |
| Stricture | 14 |
| Abscess and/or fistula | 6 |
| Preoperative medications (n) | |
| Mesalazine | 17 |
| Steroids | 15 |
| Azathioprine | 10 |
| Biologics (infliximab:adalimumab) | 6:2 |
CD: Crohn’s disease.
Endoscopic assessment
At entry, ileocolonoscopy was done in all patients. The endoscopic severity of inflammation in the neo-terminal ileum was graded according to Rutgeerts et al.21 Rutgeerts score is a well-established endoscopic scoring system based on examination of the ileal segment proximal to ileocolonic anastomosis: i0, no lesions; i1, ≤5 aphthous lesions; i2, >5 aphthous lesions with normal mucosa between lesions, or skip areas of larger lesions or lesions confined to the ileocolonic anastomosis; i3, diffuse aphthous ileitis with diffusely inflamed mucosa; i4, diffuse inflammation with larger ulcers, nodules, and/or narrowing. Endoscopic findings were recorded on colour pictures, and evaluated by two specialist physicians who were blinded to the patient’s clinical details. Endoscopic recurrence was defined as a Rutgeerts score ≥i2.21
Treatment and follow-up
After surgery, all patients were treated with standard medications (mesalazine, immunosuppressants, or biologics) to suppress recurrence. However, this study was not designed to evaluate the efficacy of any medication for the prevention of postoperative recurrence. Therefore, patients were not randomly assigned to medications. In patients at a high risk of postoperative recurrence like smokers, those with perforating disease, or with multiple surgeries,20,25 patients who had received azathioprine, biologics (infliximab or adalimumab) immediately before surgery, treatment with azathioprine and/or biologic agent had to have started within 4 weeks after surgery. In patients at a low risk of recurrence (non-smokers, those with non-perforating disease, or those without previous surgery), mesalazine treatment was started soon after surgery. Based on the endoscopic score in this study, the prophylactic medication could be changed or continue unchanged. In patients with an endoscopic score of i2–i4, stepping up treatment was considered. In contrast, in patients with an endoscopic score of i0 or i1, the ongoing medication could continue.
All patients were regularly reviewed in our clinic for 12 months after the endoscopic examination. Patients were required to record their symptoms in a diary every day. At clinic visits, body weight, general well-being, body temperature, stool frequency and consistency, presence or absence of abdominal discomfort, tenderness, tenesmus and haematochezia were recorded. Clinical recurrence was defined as a CDAI >150 with an increase of ≥70 points. Peripheral blood samples were taken for the measurement of white blood cell count (WBC), platelet count, C-reactive protein (CRP), and albumin.
Assays of faecal calprotectin and lactoferrin
Patients provided a stool sample for the measurements of calprotectin and lactoferrin. The collection of stool was performed before the start of bowel cleansing for ileocolonoscopy. Faecal calprotectin was measured by a quantitative enzyme immunoassay (Human Calprotectin ELISA Kit, Cell Sciences Inc., Massachusetts, USA). Lactoferrin was measured by a colloidal gold agglutination reagent (Auto Lf-Plus, Alfresa Pharma Corp., Osaka, Japan) using a high-throughput discrete clinical chemistry analyser (Hemo Techt NS-Plus C, Alfresa Pharma Corp., Osaka, Japan).
Statistical analysis
Numerical data are presented as mean ± SE values or as indicated otherwise. Comparisons of frequencies were done by using the chi-square test with Yates’ correction. Continuous data are presented as the mean ± SE values. The average values between two groups are compared by unpaired t-test. For comparisons involving more than two groups, the one-way analysis of variance (ANOVA) was applied. Changes in data with time were evaluated by paired t-test. Correlations were calculated using the Spearman’s ‘r’ test. The cumulative recurrence rate was calculated by the Kaplan–Meier method, and was compared between the groups by using the log-rank test; p < 0.05 was considered statistically significant.
To determine an optimal cutoff value for prediction of recurrence, a receiver operating characteristic (ROC) curve was constructed. The ROC curve is a plot of the true positive rate (sensitivity) against the false positive rate (1 – specificity) for the different possible cutoffs of a diagnostic test. The closer the curve follows the left hand-border and then the top border of the ROC space, the more accurate the test. We defined the most optimal cutoff point by looking at the sensitivity and specificity for various cutoff values.
Results
Endoscopic score
The mean ± SE time between surgery and the endoscopic examination at entry was 7.2 ± 0.4 months, range 6–12 months. The endoscopic scores were i0 or i1 in 10 patients, i2 in six patients, i3 in three patients, and i4 in one patient. There was no significant correlation between the CDAI and endoscopic scores (p = 0.74). There was also no significant correlation between endoscopic score and the time from surgery to the endoscopic examination (p = 0.73). Demographic variables including age (≤30/>30 years; p = 0.26), gender (p = 0.64), duration of CD (≤30/>30 months; p = 0.53), smoking habit (p = 0.44), previous surgery (p = 0.11), and concomitant abscess or fistula at laparotomy (p = 0.14) were not significantly associated with the endoscopic scores. Similarly, laboratory measurements including WBC (p = 0.93), platelet count (p = 0.71), albumin (p = 0.75), and CRP (p = 0.95) at the time of endoscopy did not significantly correlate with the endoscopic scores.
Calprotectin and lactoferrin levels
There was a significant correlation between calprotectin and lactoferrin levels (r = 0.62, p = 0.003). Both calprotectin and lactoferrin levels positively correlated with the endoscopic scores in the neo-terminal ileum (p = 0.0001 for calprotectin and p = 0.038 for lactoferrin; Figure 1). The mean ± SE values of faecal calprotectin and lactoferrin were significantly higher in patients with endoscopic recurrence (Rutgeerts score ≥i2) than in those without endoscopic recurrence (i0 or i1) (calprotectin, 229.5 ± 34.8 µg/g vs. 102.3 ± 19.0 µg/g, p = 0.005; lactoferrin, 161.4 ± 19.1 µg/g vs. 83.7 ± 23.2 µg/g, p = 0.02). A cutoff value of 140 µg/g for calprotectin had a sensitivity of 70% (95% confidence interval [CI], 42–98%), a specificity of 70% (95% CI, 42–98%), a positive predict value of 70% (95% CI, 42–98%), a negative predictive value of 70% (95% CI, 42–98%) and a diagnostic accuracy of 70% (95% CI, 50–90%) to detect endoscopic recurrence, while a cutoff value of 125 µg/g for lactoferrin had a sensitivity of 70% (95% CI, 42–98%), a specificity of 60% (95% CI, 30–90%), a positive predict value of 64% (95% CI, 35–92%), a negative predictive value of 67% (95% CI, 36–97%) and a diagnostic accuracy of 65% (95% CI, 44–86%) to detect endoscopic recurrence. There was no significant correlation between the CDAI and the levels of faecal markers (r = 0.26, p = 0.28 for calprotectin; r = 0.18, p = 0.46 for lactoferrin). Laboratory measurements including WBC, platelet count, albumin, and CRP at the time of endoscopy did not correlate with faecal markers (calprotectin vs. WBC, r = 0.30, p = 0.21; platelet count, r = 0.37, p = 0.11; albumin, r = −0.30, p = 0.21; CRP, r = 0.31, p = 0.19; lactoferrin vs. WBC, r = 0.30, p = 0.20; platelet count, r = 0.25, p = 0.29; albumin, r = −0.25, p = 0.28; CRP, r = 0.40, p = 0.08).
Figure 1.
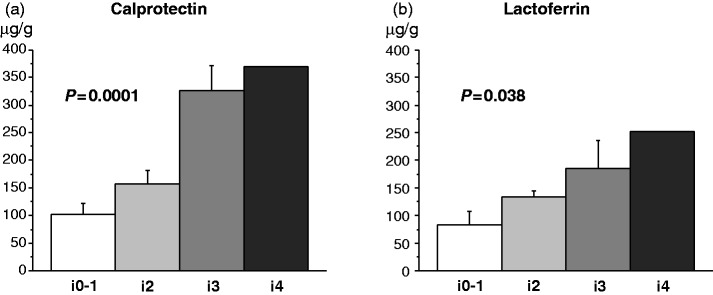
Both calprotectin (a) and lactoferrin (b) levels significantly positively correlated with the endoscopic scores in the neo-terminal ileum. The mean ± SE values are presented.
Medications
After surgery, 10 patients were given mesalazine (3 g/day), four were given azathioprine (50–100 mg/day), and six were given biologics, either infliximab 5 mg/kg every 8 weeks, n = 4 or adalimumab 40 mg every 2 weeks, n = 2 for the prevention of recurrence. Based on the endoscopic findings at entry, in six of the 10 patients on mesalazine, azathioprine and/or infliximab therapy was started (azathioprine 50 mg/day, n = 1; infliximab 5 mg/kg at weeks 0, 2, and 6, then every 8 weeks, n = 4; both azathioprine and infliximab, n = 1). In two of the four patients treated with azathioprine, infliximab treatment (5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks) was started. In the remaining 12 patients, the patients’ medications at entry were allowed to continue.
Follow-up data
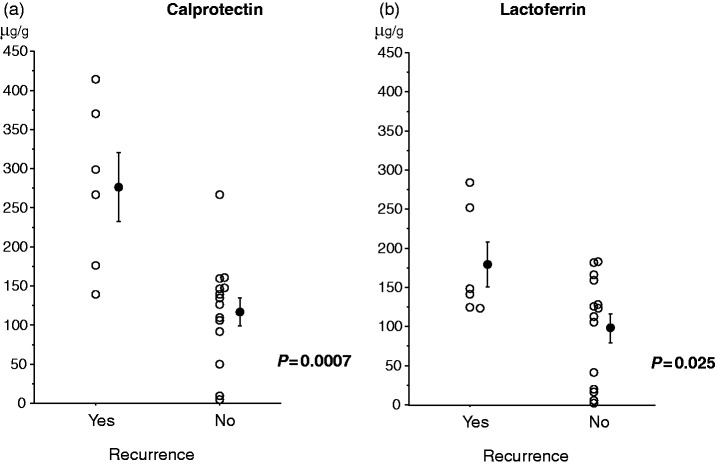
Six patients developed clinical recurrence during the 12-month follow-up. The mean ± SE CDAI score at the time of recurrence was 246 ± 16, range 201–297. Both calprotectin and lactoferrin levels were significantly higher in patients with recurrence as compared with patients who maintained remission (p = 0.0007 for calprotectin and p = 0.025 for lactoferrin; Figure 2). Sensitivity and specificity of faecal markers for prediction of future recurrence at different cutoff values are presented in Table 2. The most reliable cutoff values were 170 µg/g for calprotectin and 140 µg/g for lactoferrin. A cutoff value of 170 µg/g for calprotectin had a sensitivity of 83% (95% CI, 54–113%), a specificity of 93% (95% CI, 79–106%), a positive predict value of 83% (95% CI, 54–113%), a negative predictive value of 93% (95% CI, 79–106%) and a diagnostic accuracy of 90% (95% CI, 77–103%) to predict a risk of clinical recurrence, while a cutoff value of 140 µg/g for lactoferrin had a sensitivity of 67% (95% CI, 29–104%), a specificity of 71% (95% CI, 48–95%), a positive predict value of 50% (95% CI, 15–85%), a negative predictive value of 83% (95% CI, 62–104%) and a diagnostic accuracy of 70% (95% CI, 50–90%) to predict a risk of clinical recurrence. The cumulative recurrence rate was significantly higher in patients with elevated calprotectin (≥170 µg/g) than in those with low calprotectin level (<170 µg/g) (p = 0.0001; Figure 3(a)). The cumulative recurrence rate was higher in patients with elevated lactoferrin level (≥140 µg/g) than in those with low lactoferrin level (<140 µg/g), but the difference did not reach statistical significance (p = 0.077; Figure 3(b)).
Figure 2.
The bar represents mean value with SE. Both calprotectin (a) and lactoferrin (b) levels were significantly higher in patients with clinical recurrence than in patients in remission.
Table 2.
Sensitivity and specificity of faecal markers for prediction of recurrence at different cutoff values
| Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|
| Calprotectin | ||
| 50 µg/g | 100% | 21% (0–43%) |
| 100 µg/g | 100% | 29% (5–52%) |
| 150 µg/g | 83% (54–113%) | 79% (57–100%) |
| 170 µg/g | 83% (54–113%) | 93% (79–106%) |
| 200 µg/g | 67% (29–104%) | 93% (79–106%) |
| Lactoferrin | ||
| 50 µg/g | 100% | 36% (11–61%) |
| 100 µg/g | 100% | 36% (11–61%) |
| 140 µg/g | 67% (29–104%) | 71% (48–95%) |
| 150 µg/g | 33% (−4–71%) | 71% (48–95%) |
| 200 µg/g | 33% (−4–71%) | 100% |
CI: confidence interval.
Figure 3.
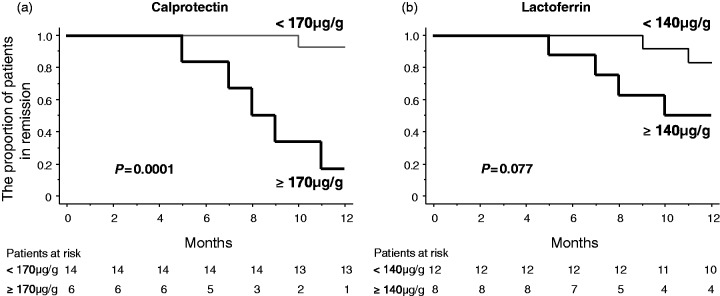
The cumulative recurrence rate was significantly higher in patients with high calprotectin levels (≥170 µg/g) than in those with low calprotectin levels (<170 µg/g) (a). The cumulative recurrence rate was higher in patients with high lactoferrin levels (≥140 µg/g) than in those with low lactoferrin levels (<140 µg/g), but the difference did not reach statistical significance (b).
Demographic factors including age (≤30/>30 years; p = 0.63), gender (p = 0.81), duration of CD (≤30/>30 months; p = 0.16), smoking habit (p = 0.80), previous surgery (p = 0.14), and concomitant abscess or fistula at laparotomy (p = 0.83) did not significantly affect the cumulative clinical recurrence rate. Laboratory measurements including WBC (p = 0.37), platelet count (p = 0.79), albumin (p = 0.69), and CRP (p = 0.26) at the time of endoscopy were not significantly different between patients with clinical recurrence and those in remission.
Discussion
Up to now, the use of faecal markers for the assessment of disease activity in the postoperative setting has been limited. To our knowledge, this is the first study which has rigorously investigated the relationship between endoscopic activity and faecal markers in patients with quiescent CD after surgery. We included only patients who had maintained clinical remission during 6–12 months following ileocolonic resection. All patients underwent ileocolonoscopy to evaluate the degree of inflammation in the neo-terminal ileum.
In earlier studies,10–13 faecal calprotectin and lactoferrin levels significantly correlated with endoscopic disease activity in patients with colonic CD and UC. However, such correlation has not been reported for patients with small bowel CD due to inadequate data, because the small bowel is not accessible to routine endoscopic techniques. In contrast, the neo-terminal ileum after ileocolonic resection is accessible to conventional colonoscopy. Therefore, the present data are relevant to the assessment of intestinal inflammation in patients with ileal CD.
The levels of faecal calprotectin and lactoferrin correlated closely, which might suggest a concordance in their expression patterns. Both calprotectin and lactoferrin levels significantly and positively correlated with the severity of endoscopic findings in the neo-terminal ileum. In contrast, demographic factors and laboratory measurements including WBC, platelet count, and CRP did not correlate with the endoscopic score. Further, CDAI did not show correlation with either endoscopic score or with faecal markers. These results suggest that it is not appropriate to predict endoscopic activity by using patients’ clinical features or conventional blood markers in the perioperative period. Ileocolonoscopy is still considered to be the gold standard for assessing postoperative disease activity. Our findings in this study suggest that faecal calprotectin or lactoferrin testing might be an alternative and low-cost non-invasive method to avoid complicated invasive techniques.
A number of studies suggest that faecal calprotectin and lactoferrin are predictive markers of relapse in patients with UC and CD.14–19 A recent meta-analysis assessed the predictive value of faecal calprotectin in IBD relapse.26 A total of 672 IBD patients (318 UC and 354 CD) were analysed. The pooled sensitivity and specificity of faecal calprotectin to predict relapse of quiescent IBD was 78% and 73%, respectively. The sensitivity of faecal calprotectin to predict relapse was comparable between UC and CD. In CD patients the predictive value of faecal calprotectin in isolated small bowel CD was not assessed due to inadequate data. However, faecal calprotectin appeared to be a more sensitive biomarker in ileocolonic and colonic CD.
In this study, both calprotectin and lactoferrin levels significantly increased in patients who developed clinical recurrence. The sensitivity and specificity of calprotectin to predict recurrence were an 83% and 93%, respectively. In contrast, the sensitivity and specificity of lactoferrin were 67% and 71%, respectively, suggesting that calprotectin provides more sensitive than lactoferrin. Faecal markers, especially calprotectin, are useful to predict future recurrence in patients with quiescent CD after ileocolonic resection.
The main limitation of our data could be the relatively small sample size. With this in mind, we believe that the findings of this investigation should be strengthened by a future study involving a large cohort of patients and with a longer follow-up time to fully evaluate the clinical significance of faecal calprotectin or lactoferrin measurement in this clinical setting. Further, in this study, prophylactic medication was determined based on the risk of postoperative recurrence on an individual basis. Patients at a high risk of recurrence (smokers, those with perforating disease, with multiple surgeries) or patients who had developed severe endoscopic lesions were treated with immunosuppressants or biologics. However, the effects of prophylactic medications on clinical course and laboratory measurements were not evaluated in this study. To rigorously assess the predictive value of faecal markers, their measurement should be done once patients reach a stable dose of these medications. We are now conducting another study to investigate the relationship between these markers and disease relapse during maintenance therapy with a stable dose of a biological agent.
In conclusion, based on the findings of this study, both calprotectin and lactoferrin levels correlate well with endoscopic activity after ileocolonic resection for CD. Calprotectin and lactoferrin could be clinically relevant biomarkers for predicting postoperative recurrence. Further well-designed large trials should strengthen the findings of the present investigation. Assays of faecal calprotectin and lactoferrin should serve as low-cost and non-invasive biomarkers to monitor disease activity and predict recurrence after surgery for CD. This should spare the patients from going through complicated colonoscopy procedures. We believe that patients with quiescent CD may provide a stool sample for determining their disease activity level, and in case of an impending clinical relapse, medication can be given to suppress relapse. This should spare medical cost.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis 2010; 4: 7–27 [DOI] [PubMed] [Google Scholar]
- 2.Poullis A, Foster R, Mendall MA, et al. Emerging role of calprotectin in gastroenterology. J Gastroenterol Hepatol 2003; 18: 756–762 [DOI] [PubMed] [Google Scholar]
- 3.Voganatsi A, Panyutich A, Miyasaki KT, et al. Mechanism of extracellular release of human neutrophil calprotectin complex. J Leukoc Biol 2001; 70: 130–134 [PubMed] [Google Scholar]
- 4.Roseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol 1992; 27: 793–798 [DOI] [PubMed] [Google Scholar]
- 5.Johne B, Fagerhol MK, Lyberg T, et al. Functional and clinical aspects of the myelomonocytic protein calprotectin. J Clin Pathol Mol Pathol 1997; 50: 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baveye S, Elass E, Mazurier J, et al. Lactoferrin: A multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med 1999; 37: 281–286 [DOI] [PubMed] [Google Scholar]
- 7.Baynes RD, Bezwoda WR. Lactoferrin and the inflammatory response. Adv Exp Med Biol 1994; 357: 133–141 [DOI] [PubMed] [Google Scholar]
- 8.Kane SV, Sandborn WJ, Rufo PA, et al. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol 2003; 98: 1309–1314 [DOI] [PubMed] [Google Scholar]
- 9.Sugi K, Saitoh O, Hirata I, et al. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: Comparison with other neutrophil-derived proteins. Am J Gastroenterol 1996; 91: 927–934 [PubMed] [Google Scholar]
- 10.Sipponen T, Kärkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther 2008; 28: 1221–1229 [DOI] [PubMed] [Google Scholar]
- 11.Jones J, Loftus EV, Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2008; 6: 1218–1224 [DOI] [PubMed] [Google Scholar]
- 12.Sipponen T, Björkesten CG, Färkkilä M, et al. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn’s disease treatment. Scand J Gastroenterol 2010; 45: 325–331 [DOI] [PubMed] [Google Scholar]
- 13.D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 2218–2224 [DOI] [PubMed] [Google Scholar]
- 14.Tibble JA, Sigthorsson G, Bridger S, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 2000; 119: 15–22 [DOI] [PubMed] [Google Scholar]
- 15.Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 2005; 54: 364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Inca R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol 2008; 103: 2007–2014 [DOI] [PubMed] [Google Scholar]
- 17.Walkiewicz D, Werlin SL, Fish D, et al. Fecal calprotectin is useful in predicting disease relapse in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2008; 14: 669–673 [DOI] [PubMed] [Google Scholar]
- 18.Gisbert JP, Bermejo F, Perez-Calle JL, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis 2009; 15: 1190–1198 [DOI] [PubMed] [Google Scholar]
- 19.García-Sánchez V, Iglesias-Flores E, González R, et al. Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J Crohns Colitis 2010; 4: 144–152 [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T. Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol 2005; 11: 3971–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990; 99: 956–963 [DOI] [PubMed] [Google Scholar]
- 22.Scarpa M, D’Incà R, Basso D, et al. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn’s disease. Dis Colon Rectum 2007; 50: 861–869 [DOI] [PubMed] [Google Scholar]
- 23.Lamb CA, Mohiuddin MK, Gicquel J, et al. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn’s disease. Br J Surg 2009; 96: 663–674 [DOI] [PubMed] [Google Scholar]
- 24.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index: National Cooperative Crohn’s Disease Study. Gastroenterology 1976; 70: 439–444 [PubMed] [Google Scholar]
- 25.De Cruz P, Kamm MA, Prideaux L, et al. Postoperative recurrent luminal Crohn’s disease: A systematic review. Inflamm Bowel Dis 2012; 18: 758–777 [DOI] [PubMed] [Google Scholar]
- 26.Mao R, Xiao YL, Gao X, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: A meta-analysis of prospective studies. Inflamm Bowel Dis 2012; 18: 1894–1899 [DOI] [PubMed] [Google Scholar]