Abstract
Background
Gastroenterology has over the past 30 years evolved very rapidly. The societal benefits to which this has led are incompletely determined, yet form a mandate to determine the need for future innovations and further development of the field. A more thorough understanding of societal benefits may help to determine future goals and improve decision making.
Aims
The objective of this article is to determine the societal gains of medical innovations in the field of gastroenterology in the past and future, using peptic ulcer disease as an example of past innovation and the implementation of colorectal cancer screening as an illustration of future gains.
Methods
Literature searches were performed for data on peptic ulcer and colorectal cancer epidemiology, treatment outcomes, and costs. National and governmental databases in the Netherlands were searched to obtain the input for calculations of quality-adjusted life years (QALYs), health-adjusted life expectancy (HALE), and the corresponding societal benefit.
Results
Since 1980 the improvements in peptic ulcer treatment have had a limited impact on life expectancy, rising from 83.6 years to 83.7 years, but have led to a yearly gain of 46,000 QALYs, caused by improved quality of life. These developments in the field of peptic ulcer translated into a yearly gain of 1.8 billion to 7.8 billion euros in 2008 compared with the 1980s.
Mortality due to colorectal cancer is high, with 21.6 deaths per 100,000 per year in the Netherlands (European Standardized Rate (ESR)). The future implementation of a nationwide call-recall colorectal cancer screening by means of biennial fecal immunochemical testing (FIT) is expected to result in a 50%–80% mortality reduction and thus a gain of an estimated 35,000 life years per year, corresponding to 26,000 QALYs per year. The effects of the implementation of FIT screening can be translated to a future societal gain of 1.0 billion to 4.4 billion euro.
Conclusions
The innovations and developments in the field of gastroenterology have led to significant societal gains in the past three decades. This process will continue in the near future as a result of further developments. These calculations provide a template for calculations on the need for specialist training as well as research and implementation of new developments in our field.
Keywords: Peptic ulcer, colon cancer, screening, societal gain, quality of life
Introduction
The field of gastroenterology has in the past 30 years gone through a large number of significant developments that stimulated the specialty to a revolutionary expansion. From being a small field within internal medicine, it has become an unchallenged specialty on its own, closely connected with others such as surgery, medical oncology, intervention radiology, and internal medicine. Recent developments in areas such as treatment for viral hepatitis, gastrointestinal oncology, and therapeutic endoscopy further boost this process. The field of gastroenterology can serve as a clear illustration of past and future innovations in health care and their financial impact.
In recent years, valuation of health outcomes has shifted focus from medical efficacy and safety measures to include patient-centered outcomes, such as health-related quality of life (QoL).1 Understanding the burden of illness and the outcomes of medical treatments has become an important issue. Health-related QoL scales are fit for evaluating the impact of disease and treatment, by measuring physical, psychological, and social functioning and well-being. These outcomes were often poorly reflected in clinical outcomes and symptoms. The field of health care often applies cost-effectiveness studies to demonstrate the applicability of new guidelines, treatments, and other innovations. The societal gain and financial valuation are probably more illustrative of the true costs and gains; however, these concepts are applied less often. Therefore, we designed this study to assess the societal gains and financial valuation of gastroenterology in the past and future using two common diseases.
Peptic ulcer disease (PUD) perfectly exemplifies the developments in gastroenterology over the past three decades. The incidence both of gastric and duodenal ulcers increased throughout the 19th century and reached epidemic proportions during the first half of the 20th century.2 Since then, new insights and medical innovations have resulted in major improvements in the treatment of PUD. These insights and innovations include the introduction of acid suppressive drugs in the late 1970s and 1980s, first antacids and H2-antagonists and later proton pump inhibitors (PPI), the subsequent discovery of H. pylori and eradication treatment, and the introduction of diagnostic and therapeutic endoscopy. Together, this has had a major impact on primary and secondary prevention of PUD, as well as on improved outcome of complications of this disease. As such, PUD underwent a major paradigm shift from a chronic, recurrent, disabling condition related to psychological make-up, to a short-term complication of an infection, treatable with antibiotics, and unrelated to any psychological character type. The exact benefits of this change are incompletely understood, other than that we know for instance that gastric surgery for ulcer disease until the 1970s was more common than appendectomy, yet has nowadays become so rare that surgical residents hardly learn the procedure anymore.3 On a broader societal level, the breakthroughs in PUD may serve as an example for the impact of medical research and knowledge progression.
For future developments the implementation of colorectal cancer (CRC) screening is an appropriate model. CRC is the second most prevalent malignant disease and affects men and women almost equally. Almost one million new incident cases and 250,000 deaths occur worldwide each year. Survival rates have increased throughout the last decades because of earlier diagnosis, improved diagnostic tests, introduction of adjuvant chemotherapy, and advances in the treatment of metastatic disease. Approximately 80% survive the first year after diagnosis and 62% survive five years.3,4 The implementation of population screening in many countries worldwide will have a major impact on morbidity and mortality due to CRC.
The societal benefits of these past and future developments are incompletely determined, yet form a mandate to determine the need for future innovations and further development of the field. A more thorough understanding of societal benefits may help to determine future goals and improve decision making. We therefore calculated the societal gain of past innovations and developments in PUD and future implementation of CRC screening in a developed country. We calculated the gain in life years and working years, quality-adjusted life years (QALYs), and health-adjusted life expectancy (HALE) and translated these concepts into a valuation of the societal gain.
Materials
We compiled information on clinical diagnoses, hospital visits, hospitalization, and causes of death from a variety of publicly and privately held databases (see below). From these databases, we extracted data regarding PUD and CRC. Each database provided data in a somewhat different format. For the most important databases the methods are outlined below. Where the data were further aggregated after being retrieved from the original database, descriptions of further calculations are given below.
Literature
Systematic searches of PubMed were performed to collect papers that reported symptoms, health-related quality of life impairment, work loss, and costs associated with PUD and CRC, respectively.
Governmental databases
Within the Dutch medical system, medical care for every patient is delivered by means of diagnosis treatment combinations. This is a combination of codes including predominant symptoms, medical diagnosis, and the treatment provided. Under this specific code, every aspect of care provided is listed. Diagnosis treatment combinations (DBCs) were obtained from the national registry of hospital admissions, which contains information on all admissions in academic and general hospitals throughout the Netherlands.5
Data on mortality of CRC and PUD were extracted from the National Public Health Compass developed by the National Institute for Public Health and Environment.6–8 Other data on costs, cost-effectiveness, life expectancy and epidemiology of PUD and CRC were obtained from the Netherlands Institute for Economic Policy Analysis, the National Institute for Public Health and Environment and the Central Statistics bureau of the Netherlands. From the Dutch Institute for Health Services Research (NIVEL), we acquired data on use of care by general practitioners, coded by the International Classification of Primary Care (ICPC).5
Methods
QoL
QoL is a frequently used term that conveys an overall sense of well-being. In the case of health-related QoL, the measurement used mostly in health care, the term encompasses those aspects that can be clearly shown to affect health.9 QoL is typically measured on a scale from 0 to 1. The Short-Form 36 (SF-36) is one of the most used questionnaires to measure QoL.10 The most relevant aspects of QoL in gastrointestinal disease pertain primarily to symptoms, and to the beneficial effects of symptom relief on general well-being and ability to partake in day-to-day activities.11
QALYs
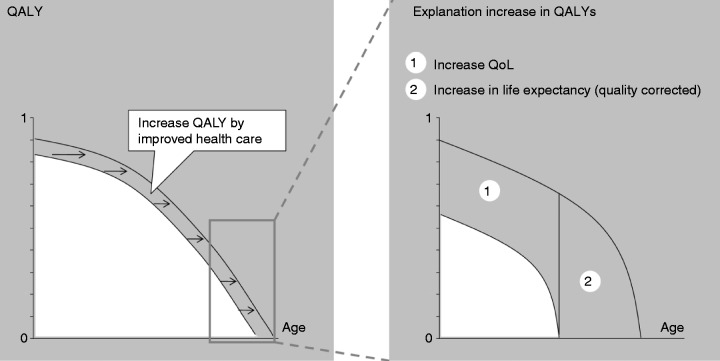
QALYs are estimates of person-years lived at particular levels of health. It is a measurement that takes both the quality and the length of life into account (Figure 1). QALYs are used primarily to correct someone’s life expectancy based on the levels of health-related QoL they are predicted to experience throughout the course of their life.12
Figure 1.
The calculation of quality-adjusted life years (QALYs).
Improvement in QALYs can be attained by either an increase in quality of life or an increase in survival. QALYs are calculated by setting out QoL vs age.
QoL: quality of life.
HALE
HALE is a measurement that builds on the concept of life expectancy. Life expectancy estimates are usually insensitive to the health status of the individual or population. Life expectancy estimates are calculated from data on deaths and population counts. Based on these data, the survivorship of a birth cohort is estimated over time. When graphed, the area under the survivor curve represents the total of life years lived for a cohort (Figure 2). The sum of these years divided by the number of individuals in the population gives their life expectancy. HALE estimates do not treat each of these life years equally but weight the years according to health status.13
Figure 2.
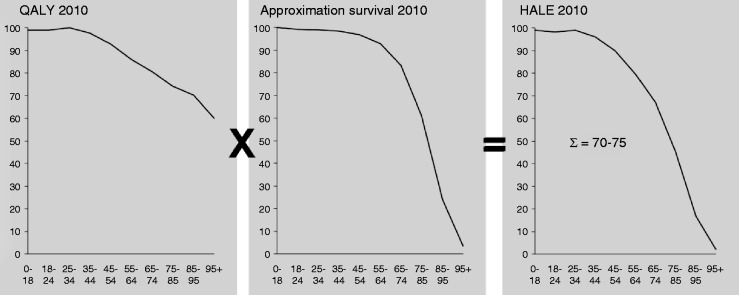
Calculation of the health-adjusted life expectancy (HALE).
This figure demonstrates the aspects taken into account when calculating the HALE. In the left figure QALY are calculated by the self-reported QoL (index 100 is the highest score in the age group 25–34) vs age; in the middle figure the approximation of survival is represented by the percentage of survival per age group; the right figure shows the health-corrected life expectancy at birth vs age.
QALY: quality-adjusted life years; QoL: quality of life.
HALE increases when the amount of life years increases or the QoL improves. To calculate the HALE it is necessary to identify the QoL per age and disease. In general the QoL decreases with increasing age.
Euro valuation
To translate the above-mentioned concepts into a financial (euro) valuation, different methods were applied.14
-
–
A euro valuation of a life year based on the gross domestic product (GDP). Calculations assume a GDP of 37,000 euros per inhabitant in 2008 and an annual 1.25% growth of GDP since then. Taking this growth into account with a life expectancy of 80.59 years results in a euro valuation for the current population ranging between 56,962 and 66,154 based on the number of years in which the GDP will increase with 1,25% per year.15–17
-
–
A euro valuation based on the public preparedness to pay for potential life-saving measures. The example taken is the preparedness to pay 300 euros for a car airbag. An airbag saves on average one in 10,000 lives per year. Based on an average remaining life expectancy of 40 years, the buyer of an airbag thus values his life at at least 75,000 euros per year (10,000 × (300/40)).14
-
–
A euro valuation based on a GDP growth of 2.5% per year and an increase in life expectancy between 1950 and 2008. The lower limit is based on a 20-year-old in 1950 and the upper limit is based on a 50-year-old in 1950. This method assumes that economic growth and an increase in life years are just as valuable.16–18 This method takes into account an average increase in life expectancy of 0.24% per year and a growth of GDP from 8812 euros in 1950 to 36,260 euros in 2008 (average increase of 2.5% per year). Dividing the total increase in GDP per person by the amount of life years gained results in a euro valuation per life year gained. Applying this method results in a valuation of 40,000 for a 50-year-old in 1950 to 85,000 euros for a 20-year-old in 1950 per life year.
-
–
The fourth and final method applied is based on the same method as the above. It is based on a GDP growth of 1.25% and the predicted increase in life expectancy after 2008.16–18 The lower limit is based on a 20-year-old in 2008 and the upper limit is based on a 50-year-old in 2008. This method also assumes that economic growth and an increase in life years are just as valuable. It takes into account an average increase in life expectancy of 0.18% per year and a growth of GDP from 36,260 euros in 2008 to 36,713 euros in 2009, 37172 in 2010 and so on (average increase of 1.25% per year). Dividing the total increase in GDP per person by the amount of life years gained results in a euro valuation per life year. Applying this method results in a valuation of 85,000 to 170,000 euros per life year.
All together, these methods demonstrate that the euro valuation of a life year is considerable and that depending on the method used the valuation varies greatly.
Results
PUD
The incidence and mortality of PUD has declined rapidly in the past 30 years (Figure 3). The incidence of PUD has in the Netherlands decreased by 55%, from 130/100,000/year in 1980 to 55/100,000/year in 2010 (standardized morbidity ratio).7 The mortality decreased even more by 75% from 900 to 230 deaths per year in 2010.7
Figure 3.
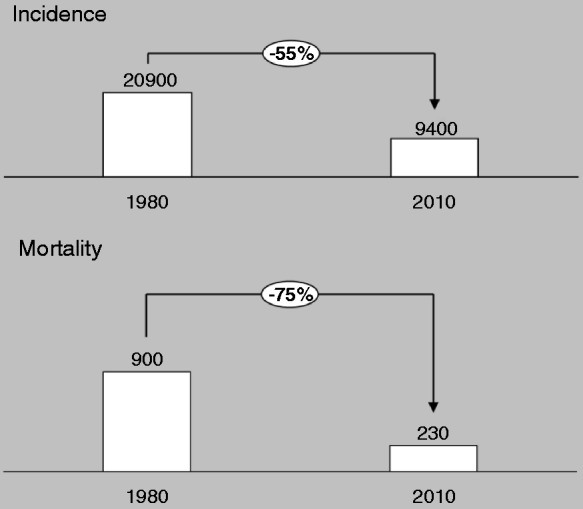
Changes in incidence and mortality in the past three decades.2,18
The corrected incidence is based on standard morbidity rates; the mortality on the number of deaths per year.
PUD: peptic ulcer disease.
QALYs
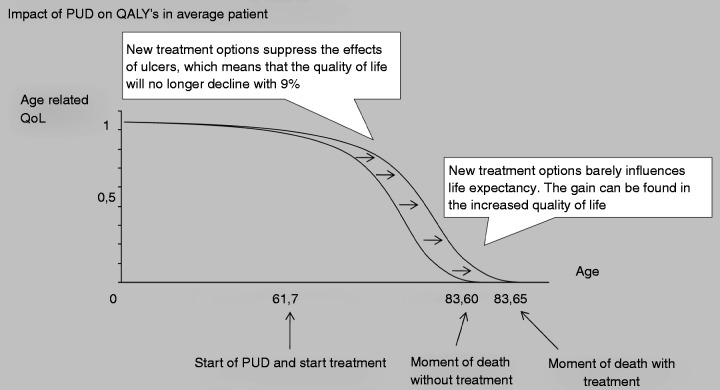
PUD decreases QoL, while the life expectancy is only marginally affected. Mortality due to peptic ulcer in particular results from bleed-related mortality, which predominantly affects the elderly with significant co-morbidity and limited life-expectancy.18 Figure 4 demonstrates the impact of PUD in QALYs for an average PUD patient. It shows that the gain for patients is mostly explained by an increase in the QoL, while the life expectancy showed little change. Improved ulcer therapy has led to a yearly gain of 43,950 QALYs. This gain can be explained by two factors. First, the incidence of PUD over the past three decades has declined by 55%–60%, causing a decline in the annual incidence of 11,492 patients. This group of subjects who will not develop PUD will have an improved QoL of 2.06 QALYs and a small increase in life expectancy equal to 0.04 QALYs, leading to a gain in QALYs of 24.166 per year (11.492*2.06). Second, the 9408 patients a year who will still suffer from PUD will have an improved QoL (2.06 QALYs) and a small increase in life expectancy (0.04 QALYs) as well, since the treatment of PUD has improved. The QoL in PUD patients without treatment is 9% lower than in the general population. The improved treatment prevents this loss in QoL. This loss in QoL is prevented from the development of PUD, on average at the age of 61.7 years, until time of death at the average age of 83, resulting in a gain of 19,784 QALYs per year (9408*2.06).
Figure 4.
Impact of PUD in QALYs on the average patient.1,3,7
This figure demonstrates that the changes in PUD result mostly in an increased quality of life and less in an increase in life years.
QALYs: quality-adjusted life years; QoL: quality of life; PUD: peptic ulcer disease.
Valuation
The gain of 43,950 QALYs per year can be translated into a financial valuation by applying the previously mentioned methods of valuating a life year, which results in a gain of 1.8 billion to 7.8 billion euros per year in a population of 16.8 million people (Table 1).
Table 1.
Financial valuation of the gain in quality-adjusted life years (QALYs) in patients with PUD over the past three decades in a population of ∼16.8 million
| Method | Low estimate | High estimate |
|---|---|---|
| GDP of 37,000 (2008) 1.25% growth of GDP | €2,576,000,000 | €3,036,000,000 |
| Valuation of an airbag €300 | €3,450,000,000 | €3,450,000,000 |
| GDP ↑ 2.5%/year ↑ life expectancy 1950–2008 | €1,840,000,000 | €3,910,000,000 |
| GDP ↑ 1.25%/year ↑ life expectancy >2008 | €3,910,000,000 | € 7,820,000,000 |
PUD: peptic ulcer disease; GDP: gross domestic product.
CRC screening
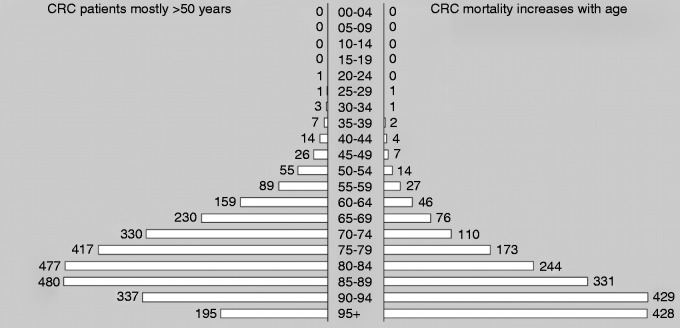
CRC comprises 13.2% of new cancer cases in women and 14.2% of new cancer cases in men. Most cases of CRC are diagnosed in patients over 50 years of age and the incidence and mortality increase rapidly with age (Figure 5). In 2008, 4500 patients died of CRC in the Netherlands, which resulted in a loss of 52,000 life years (Figure 6). Because of the changing composition of the population, CRC mortality is in the absence of a screening program estimated to increase to 9000 deaths per year until 2040.
Figure 5.
Colorectal cancer incidence and mortality per age group.8
Numbers from 2008; the numbers in the figure are the number of cases per 100,000, on the left is incidence and on the right side is mortality.
CRC: colorectal cancer.
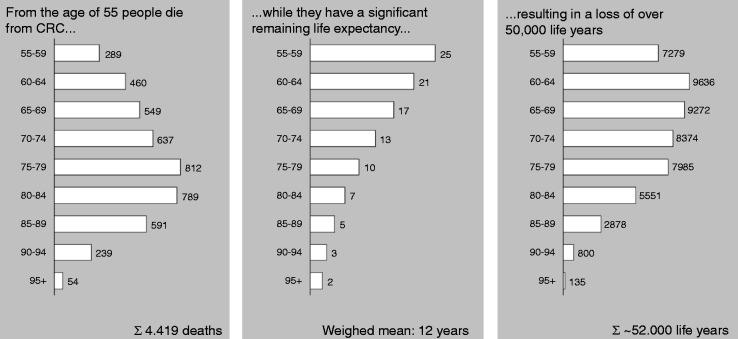
Figure 6.
Amount of life years lost to colorectal cancer every year.6,8
Based on the numbers of 2008. The left figure demonstrates the number of deaths by CRC per age group; the middle figure the average remaining life expectancy per age group in years, and the right figure shows the amount of life years lost per age group.
CRC: colorectal cancer.
As of 2013, a nationwide, call-recall CRC screening program will be implemented based on biennial fecal immunochemical testing (FIT) of 55- to 75-year-old subjects. This is expected to lead to a significant CRC-related mortality reduction, which is estimated between 50% and 80% depending on participation.6,19–21
QALYs
QoL is barely affected by CRC; one year after diagnosis the average difference in QoL between the general population and CRC patients, adjusted for age and sex, is 1 on a scale of 100.4,22 The life expectancy, however, is significantly affected. In 2008, 4500 patients died because of CRC, mostly above the age of 55, but with a reasonable remaining life expectancy. This life expectancy is on average 12 years. Consequently the amount of life years lost in 2008 was 54,000 life years. With an increase in CRC mortality in the coming years and the implementation of FIT screening, and thus a mortality reduction of 50%–80%, we can gain 26,000 QALYs per year until 2040 (Figure 7).
Figure 7.
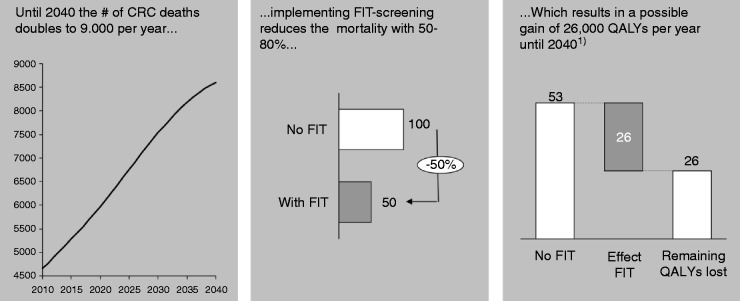
QALYs gained by implementing FIT screening.
The left figure shows the amount of deaths set out against the years 2010–2040, the middle figure shows the result of implementing FIT screening, and the right figure shows the amount of QALYs gained in thousands.
CRC: colorectal cancer; FIT: fecal immunochemical test; QALY: quality-adjusted life years.
HALE
Implementing CRC screening results in improved HALE. Figure 8 demonstrates the HALE for individuals aged 55–95 with and without the implementation of CRC screening. To calculate age-related HALE, we first took the annual survival per age group into account (ranging from 94% for subjects aged 55–59 to 15% in subjects aged 90–94 years). Secondly, we determined the reduction of mortality by implementing CRC screening per age group, and thirdly the remaining QALYs per age group. This resulted in the effect on the HALE for the entire population between 55 and 95. This then allowed calculation of the improvement in HALE per individual above the age of 55 as a result of introduction of CRC screening, which resulted in a gain of 0.025 years on average per subject in the population.
Figure 8.
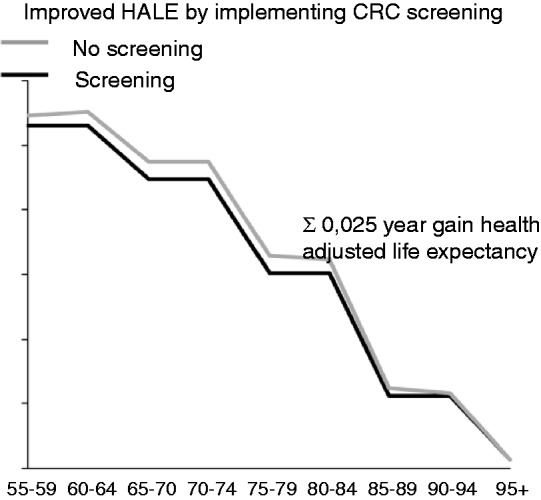
Improved health-adjusted life expectancy with and without colorectal cancer screening.
The average gain per person is 0.025 years gain between screening and not implementing screening for persons aged 55–95+.
HALE: health-adjusted life expectancy; CRC: colorectal cancer.
Valuation
The gain of 26,000 QALYs per year could be translated into a financial valuation by applying the previously mentioned methods of valuating a life year, which resulted in a gain of 1.0 billion to 4.4 billion euros per year in a population of 16.8 million people (Table 2).
Table 2.
Financial valuation of the gain in quality-adjusted life years (QALYs) by implementing colorectal cancer screening in a population of 16.8 million
| Method | Low estimate | High estimate |
|---|---|---|
| GDP of 37,000 (2008) 1.25% growth of GDP | €1,456,000,000 | €1,716,000,000 |
| Valuation of an airbag €300 | €1,950,000,000 | €1,950,000,000 |
| GDP ↑ 2.5%/year ↑ life expectancy 1950–2008 | €1,040,000,000 | €2,210,000,000 |
| GDP ↑ 1.25%/year ↑ life expectancy >2008 | €2,210,000,000 | €4,420,000,000 |
GDP: gross domestic product.
Discussion
This study demonstrates the impact from a patient-centered and societal perspective of two major developments in gastroenterology, i.e. the past changes in the management of PUD and the future implementation of CRC screening. Improved care for patients with peptic ulcer led to major gains in working years and quality of life, while having little effect on life expectancy and mortality. The amount of QALYs lost as a result of PUD was considerable in the past and the evolution of peptic ulcer treatment has led to a gain of 46,000 QALYs per year in the Dutch population of 16.7 million people. In contrast, the future implementation of CRC screening will have a predominant effect on life expectancy and not so much on QoL. However, the amount of QALYs gained will again be substantial.
Past
Since the 1930s the mortality of PUD has decreased 15-fold with in particular a 75% decline since the 1980s.2,23 The decline has been steady, especially in men and patients under the age of 65.24 From a patient-centered point of view, PUD leads to a significant decline of the QoL in almost every domain of the SF-36 scores.10 This is in line with a further observation that H. pylori eradication leads to an increase in QoL scores in PUD patients.25
Several studies have addressed these topics from a societal viewpoint and demonstrated that the innovations in the initial treatment of peptic ulcer were cost effective. Empiric antibiotic/antisecretory therapy has a cost-utility ratio of €2100 to €9000 per QALY gained. These numbers are lower than the €35,000 per QALY, which is generally used as a cut-off for cost efficacy.26 Studies from the 1970s and 1980s showed high rates of absenteeism due to PUD and furthermore showed a trend for reduction in absenteeism over time.10 This effect of PUD on loss of productivity was described as well in a study by Henke et al., who demonstrated that about 40% of PUD patients experienced some income loss; the average quarterly income decreased from $10,941 to $10,335, leading to a 6% productivity loss in three months caused by PUD.3,27 These studies confirm the impact that PUD has on society and the societal gain that was attained by all innovations and developments.
All the above-mentioned studies confirm the results of our study; the approach used, however, in evaluating the societal gains provides a new mode in the field of societal impact studies. Of course the discovery of H. pylori and the development of eradication therapy have had an even wider effect on society than the effects on PUD alone. The effects on the incidence of gastric cancer cannot be neglected and will have led to a drop in mortality with according effects on societal gain.
Future
Preventive medicine progressively becomes a field of interest. It has already been demonstrated that implementing CRC screening in the general population between 50 and 75 years of age reduces mortality by allowing physicians to detect CRC at an earlier, more treatable stage, as well as by identification and removal of adenomatous polyps.20 Implementing preventive measures touches on several dilemmas in the field of cost effectiveness, HALEs gained, and other economic and societal issues, especially for policymakers and insurance companies. Various studies demonstrated that lifetime-related CRC costs are substantial and vary by stage at diagnosis and treatment phase.19,28 Annual treatment costs are nine times higher per year of survival for patients diagnosed at stage IV than patients diagnosed at stage 0.29,30 We previously showed that CRC screening as a result of relatively low, stable costs of screening, and high, rapidly increasing costs of treatment for advanced cancer in fact led to net savings, with the exception of primary screening with colonoscopy.19 Implementing FIT screening will lead to an average savings of 1222 euros vs an average cost of 1090 euros per individual in the population.19 Several cost-effectiveness studies in the field of CRC suffered from heterogeneous data based on unrealistic assumptions such as 100% participation in screening.31–35 Participation in CRC screening depends on the screening method used,36,37 and is never 100%. Those studies that did not take the participation rate into account demonstrated that FIT screening resulted in a decrease in direct CRC-related treatment costs.20,34,38 Furthermore, cost-effectiveness studies estimate the average cost per life year gained by implementing screening as between €4000 and €12,300, an amount well below the cost-effectiveness threshold of €35,000.6,19,20,30,39,40
A more patient-centered approach was applied in several other studies and confirmed our findings that the QoL is good to excellent in disease-free CRC survivors and appears comparable to the general population.4,22
In CRC, as was the case in PUD, our study confirms the results published previously and provides an illustrative addition to the previously performed studies.
Several limitations of this study need to be mentioned. The data on incidence of peptic ulcer are based on hospital visits and endoscopy results. Some peptic ulcer patients are being treated by their general practitioner without undergoing diagnostic endoscopy; these patients were missed in our analysis. The databases used to retrieve data may have some limitations as well such as possible administrative errors.
This study is furthermore limited in the amount of diseases studied and the assessment of data from one country. PUD and CRC screening serve well as examples but can of course not be used as a general model for other diseases. The data obtained from one country could be a limitation; nevertheless, it seems that they can easily be translated to other developed countries. These countries indeed show the same trends in incidence of CRC and drop in PUD incidence and have largely the same increase in the population with similar trends in aging, etc. Finally, as was described in the results we focused in the calculations for CRC screening on the FIT test as a screening method and did not take any other screening methods into account. However, as was previously published, similar effects can be reached with other methods, at different cost efficacy.19,40,41
Despite these limitations, our results provide an illustration of the societal gain in the field of gastroenterology.
It is important to keep in mind that societal gain is more than merely a financial gain for society. We have chosen this approach since it is a demonstration of the impact of PUD and CRC screening that appeals to policymakers as well as patients and physicians. Most concepts used in medical research are difficult to grasp and by translating them into a concept that is more comprehensible (e.g. money) it will demonstrate its impact even more.
Policymakers and insurance companies worldwide are confronted with developments and innovations in medicine. The field of gastroenterology has over the past 30 years constantly been at the forefront as one of the medical fields with major changes, both diagnostic and therapeutic, accompanied by major changes in workforce. The most recent example is the implementation of CRC screening in most Western countries. These kinds of decisions ask for specific measurements in the field of health care. Not only the success rate and the numbers of adverse events, but also more patient-centered outcomes and cost-utility and cost-effectiveness analyses need to be taken into account.41 The subject of societal gain, whether in money or more theoretical measures, is often overlooked even though these measurements can give a clear illustration of whether new health care measures will be worthwhile.
Our study demonstrates, by taking PUD as an example of past and CRC screening as an example of pending developments, that the societal gain and the financial valuation of major changes in the field of gastroenterology are significant. Our study has illustrated that the field of gastroenterology has brought a large societal gain in PUD, with a decline in the loss of working years and an increase in QoL and QALYs. Implementing CRC screening will yield major gains in life years, life expectancy, and QALYs.
In conclusion, the innovations and developments in the field of gastroenterology have led to a significant societal gain in the past three decades and will lead to a societal gain in the future. By using PUD and the future implementation of CRC screening as models we have demonstrated the amount of life years, QALYs, and HALE gained by developments in our field of work. This supports the need for expansion of the workforce to allow for implementation of such health care interventions.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Revicki DA, Wood M, Maton PN, et al. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med 1998; 104: 252–258 [DOI] [PubMed] [Google Scholar]
- 2.Sonnenberg A. Time trends of ulcer mortality in Europe. Gastroenterology 2007; 132: 2320–2327 [DOI] [PubMed] [Google Scholar]
- 3.Henke CJ, Levin TR, Henning JM, et al. Work loss costs due to peptic ulcer disease and gastroesophageal reflux disease in a health maintenance organization. Am J Gastroenterol 2000; 95: 788–792 [DOI] [PubMed] [Google Scholar]
- 4.Arndt V, Merx H, Stegmaier C, et al. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: A population-based study. J Clin Oncol 2004; 22: 4829–4836 [DOI] [PubMed] [Google Scholar]
- 5. van der Linden MW, Westert GP, de Bakker DH, et al. 2nd National study on diseases and operations in the general practitioners' office. In: Complaints and Diseases in population and in the General Practitioners' office. Utrecht: Netherlands Institute for Health Services Research (NIVEL), 2004.
- 6. Health Council of the Netherlands. Population screening for colorectal cancer. ISBN 978-90-5549-772-0. The Hague: 2009.
- 7. Gommers AM and Poos MJJC. Numbers of colorectal cancer (prevalence, incidence and mortality). Data from Volksgezondheid Toekomst Verkenning. Bilthoven: National Institute for Public Health and the Environment (RIVM), 2010.
- 8. Gommers AM and Poos MJJC. Numbers of colorectal cancer (prevalence, incidence and mortality). In: Health NCP (ed.) VTV 2010. Bilthoven: RIVM, 2010.
- 9.Centers for Disease Control and Prevention. Measuring healthy days: Population assessment of health-related quality of life, Atlanta, GA: CDC, 2000 [Google Scholar]
- 10.Barkun A, Leontiadis G. Systematic review of the symptom burden, quality of life impairment and costs associated with peptic ulcer disease. Am J Med 2010; 123: 358–366.e2 [DOI] [PubMed] [Google Scholar]
- 11.Mokrowiecka A, Jurek K, Pinkowski D, et al. The comparison of Health-Related Quality of Life (HRQL) in patients with GERD, peptic ulcer disease and ulcerative colitis. Adv Med Sci 2006; 51: 142–147 [PubMed] [Google Scholar]
- 12.Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan 2006; 21: 402–408 [DOI] [PubMed] [Google Scholar]
- 13. Wolfson MC. Health-adjusted life expectancy. Health Rep 1996; 8: 41–46 (Eng); 43--49 (Fre) [PubMed]
- 14.Cutler DM. Your money or your life: Strong medicine for America's health care system, New York: Oxford University Press, 2004 [Google Scholar]
- 15. CBS. Data on 2008. Central Bureau for Statistics, the Netherlands, 2009.
- 16. CPB. Data on 2008. Central Planning Bureau, the Netherlands, 2009.
- 17.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011; 128: e9–e17 [DOI] [PubMed] [Google Scholar]
- 18.Post PN, Kuipers EJ, Meijer GA. Declining incidence of peptic ulcer but not of its complications: A nation-wide study in the Netherlands. Aliment Pharmacol Ther 2006; 23: 1587–1593 [DOI] [PubMed] [Google Scholar]
- 19.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, et al. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst 2009; 101: 1412–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, et al. Evaluating test strategies for colorectal cancer screening: A decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2008; 149: 659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hol L, van Leerdam ME, van Ballegooijen M, et al. Screening for colorectal cancer: Randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 2010; 59: 62–68 [DOI] [PubMed] [Google Scholar]
- 22.Jansen L, Koch L, Brenner H, et al. Quality of life among long-term (≥5 years) colorectal cancer survivors—systematic review. Eur J Cancer 2010; 46: 2879–2888 [DOI] [PubMed] [Google Scholar]
- 23.Svanes C. Trends in perforated peptic ulcer: Incidence, etiology, treatment, and prognosis. World J Surg 2000; 24: 277–283 [DOI] [PubMed] [Google Scholar]
- 24.Schwesinger WH, Page CP, Sirinek KR, et al. Operations for peptic ulcer disease: Paradigm lost. J Gastrointest Surg 2001; 5: 438–443 [DOI] [PubMed] [Google Scholar]
- 25.Glise H, Hallerback B, Johansson B. Quality of Life assessments in the evaluation of gastroesophageal reflux and peptic ulcer disease before, during and after treatment. Scand J Gastroenterol Suppl 1995; 208: 133–135 [DOI] [PubMed] [Google Scholar]
- 26.Groeneveld PW, Lieu TA, Fendrick AM, et al. Quality of life measurement clarifies the cost-effectiveness of Helicobacter pylori eradication in peptic ulcer disease and uninvestigated dyspepsia. Am J Gastroenterol 2001; 96: 338–347 [DOI] [PubMed] [Google Scholar]
- 27.Sonnenberg A, Everhart JE. Health impact of peptic ulcer in the United States. Am J Gastroenterol 1997; 92: 614–620 [PubMed] [Google Scholar]
- 28.Lang K, Lines LM, Lee DW, et al. Lifetime and treatment-phase costs associated with colorectal cancer: Evidence from SEER-Medicare data. Clin Gastroenterol Hepatol 2009; 7: 198–204 [DOI] [PubMed] [Google Scholar]
- 29.Brown ML, Riley GF, Schussler N, et al. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care 2002; 40(8 Suppl): IV-104–IV-117 [DOI] [PubMed] [Google Scholar]
- 30.Etzioni R, Ramsey SD, Berry K, et al. The impact of including future medical care costs when estimating the costs attributable to a disease: A colorectal cancer case study. Health Econ 2001; 10: 245–256 [DOI] [PubMed] [Google Scholar]
- 31.Blom J, Yin L, Liden A, et al. Toward understanding nonparticipation in sigmoidoscopy screening for colorectal cancer. Int J Cancer 2008; 122: 1618–1623 [DOI] [PubMed] [Google Scholar]
- 32.Goulard H, Boussac-Zarebska M, Ancelle-Park R, et al. French colorectal cancer screening pilot programme: Results of the first round. J Med Screen 2008; 15: 143–148 [DOI] [PubMed] [Google Scholar]
- 33.Segnan N, Senore C, Andreoni B, et al. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 2007; 132: 2304–2312 [DOI] [PubMed] [Google Scholar]
- 34.van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008; 135: 82–90 [DOI] [PubMed] [Google Scholar]
- 35.Vernon SW. Participation in colorectal cancer screening: A review. J Natl Cancer Inst 1997; 89: 1406–1422 [DOI] [PubMed] [Google Scholar]
- 36.Hol L, Kuipers EJ, van Ballegooijen M, et al. Uptake of faecal immunochemical test screening among non-participants in a flexible sigmoidoscopy screening programme. Int J Cancer 2012; 130: 2096–2102 [DOI] [PubMed] [Google Scholar]
- 37.van Roon AH, Hol L, Wilschut JA, et al. Advance notification letters increase adherence in colorectal cancer screening: A population-based randomized trial. Prev Med 2011; 52: 448–451 [DOI] [PubMed] [Google Scholar]
- 38.van Rossum LG, van Rijn AF, Verbeek AL, et al. Colorectal cancer screening comparing no screening, immunochemical and guaiac fecal occult blood tests: A cost-effectiveness analysis. Int J Cancer 2011; 128: 1908–1917 [DOI] [PubMed] [Google Scholar]
- 39. Hamberg-van Reenen HH, Bovendeur I, Feenstra TL, et al. Cost-effectiveness of prevention and care. In: National Institute for Public Health and the Environment (RIVM) (ed.) Bilthoven: RIVM, 2009.
- 40.Sonnenberg A, Delco F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med 2000; 133: 573–584 [DOI] [PubMed] [Google Scholar]
- 41.Porter ME. What is value in health care? N Engl J Med 2010; 363: 2477–2481 [DOI] [PubMed] [Google Scholar]