Abstract
The study of gut microbiota is a rapidly moving field of research, and the impact of gut microbial communities on human health is widely perceived as one of the most exciting advancements in biomedicine in recent years. The gut microbiota plays a key role in digestion, metabolism and immune function, and has widespread impact beyond the gastrointestinal tract. Changes in the biodiversity of the gut microbiota are associated with far reaching consequences on host health and development. Further understanding of the importance of developing and maintaining gut microbiota diversity may lead to targeted interventions for health promotion, disease prevention and management. Diet, functional foods and gut microbiota transplantation are areas that have yielded some therapeutic success in modulating the gut microbiota, and warrant further investigation of their effects on various disease states.
Keywords: Microbiota, gut, health, diet, immune system
Introduction
The human gut microbiota is a complex community comprising around 1014 bacteria, increasingly described as another organ of man, hence seen as a super-organism. It plays a role in immunity and defences, digestion and metabolism, inflammation and cell proliferation, and is capable of communication not only with the gut epithelium but also with distant organs and bodily systems. While a healthy gut microbiota may be viewed as a positive attribute, changes and imbalances in gut microbiota are associated with altered health states.
The impact of gut microbial communities on human health is widely perceived as one of the most exciting advancements in biomedicine in recent years. The study of gut microbiota is a rapidly moving field of research, and the finest level of resolution accessible today is that of the microbiome; i.e. the combined genes and genomes of dominant human intestinal microbes. Nutrition-related disorders, inflammatory bowel disease (IBD) and certain allergies may be linked to varying compositions of the intestinal microbiome, and a better understanding of the gut microbiota will provide information essential for efficiently dealing with well-being and diseases such as obesity, the metabolic syndrome, food intolerance, IBD and irritable bowel syndrome (IBS). The central nervous system (CNS) is also affected through gut-brain communication pathways; thus, even mental health and certain brain disorders are likely to benefit from the ongoing research.
This review provides an update of some of the recent research regarding the role of gut microbiota in health and disease. Diet, functional foods (selective probiotics and prebiotics) and gut microbiota transplantation are areas that have yielded some therapeutic success in modulating the gut microbiota; however, as yet there is no clear understanding of the mechanisms involved.
Outcomes from human microbiome projects
A global effort steered by the international human microbiome consortium (IHMC) is seeking to establish and characterise the composition of the human gut microbiome and determine its importance to human health. The Human Microbiome Project (HMP) provided an opportunity to study the structure, function and diversity of the healthy human microbiome from samples of around 300 US adults,1 and the relationships between diet, age, and changes in the microbiome. Similarly, the Metagenomics of the Human Intestinal Tract (MetaHIT) project has studied the metagenomic profile of faecal samples from 600 healthy European adults, overweight/obese individuals, and IBD patients.2,3 Low gene count, or reduced microbial diversity, is found to be associated with an increased risk of inflammatory comorbidities and an increased tendency to overweight/obesity.4
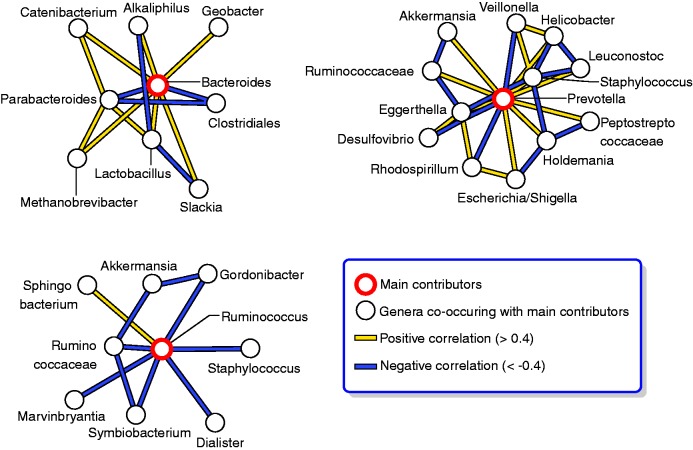
So far, a gene catalogue of 3.3 million non-redundant microbial genes has been established, which is 150-times larger than the human gene complement.2 Over 99% of the genes are bacterial and the entire MetaHIT cohort harbours between 1000–1150 prevalent bacterial species, while each individual hosts at least 160 such species.2 The studies show that at the level of microbiota structure, irrespective of individual differences, all humans share a common core of more prevalent and dominant species. Yet rather than an even distribution around an average human microbiome, gut microbiota distribute into three densely populated zones within the ecological landscape of all possible compositions. Dominated by specific genera, these compositions have been named the Bacteroides-, Prevotella- and Ruminococcus- enterotypes (Figure 1).3 The three-enterotype distribution has also been found in the Chinese population.5 Similarities or differences in enterotypes may allow patient stratification and individualised medicine or preventive nutrition in the future.
Figure 1.
The human gut microbiota – three main enterotypes. Network analysis of genus abundance across different individuals suggests that the human gut microbiota conforms to well balanced host–microbial symbiotic states driven by groups of co-occurring genera. Multidimensional cluster analysis and principal component analysis of metagenomic sequences from American, European and Japanese individuals, reveal that all individual samples formed three robust clusters, which were designated as ‘enterotypes’. Each of the three enterotypes is identifiable by the variation in the levels of one of three genera: Bacteroides (enterotype 1), Prevotella (enterotype 2) and Ruminococcus (enterotype 3). Networks of genera in the three enterotypes are identified by positive and negative correlations among the dominant genera.
Source: Figure reprinted by permission from Macmillan Publishers Ltd: Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome.3 Nature 2011; 473: 174–180, copyright 2011.
Early life factors greatly affect the make-up and composition of the human gut microbiota, which may have consequences for intestinal development and the risk of diseases. Profound differences in bacterial species assemblages and functional gene repertoires have been noted between individuals residing in the USA compared to those from Venezuela and Malawi. These distinctive features are evident throughout life after the age of three.6
Microbes and immune regulation
From birth, commensal gut bacteria are essential to the development of many intestinal functions and structures including the gut-associated immune system. Reciprocal interactions between the microbiota and the mucosal immune response occur through activation of innate and adaptive immune responses. Microbial composition is determined by host genetic factors and environmental factors including diet. Environmental triggers may alter the gut composition by upsetting the balance between beneficial symbionts and detrimental pathobionts, leading to inflammation.7
Reduced contact of people with natural environmental biodiversity may adversely affect the human commensal microbiota and its immunomodulatory capacity.8 Humans are in a state of evolved dependence on organisms with which we co-evolved as inducers of immunoregulatory circuits: these organisms (‘old friends') are depleted from the modern urban environment and lifestyle (Figure 2).9,10 The recent increase in chronic inflammatory disorders may hence be at least partly attributable to immunodysregulation resulting from lack of exposure to microorganisms. There is evidence that diminished exposure to microbial biodiversity in early life leads to defective immunoregulation, exaggerated cytokine response to social stressors and susceptibility to depression.11,12 An inflammation-associated form of depression identified in rich countries appears to be unusual in developing countries, and might therefore be an appropriate target for novel immunoregulatory treatments or microbiota modulation.13,14
Figure 2.
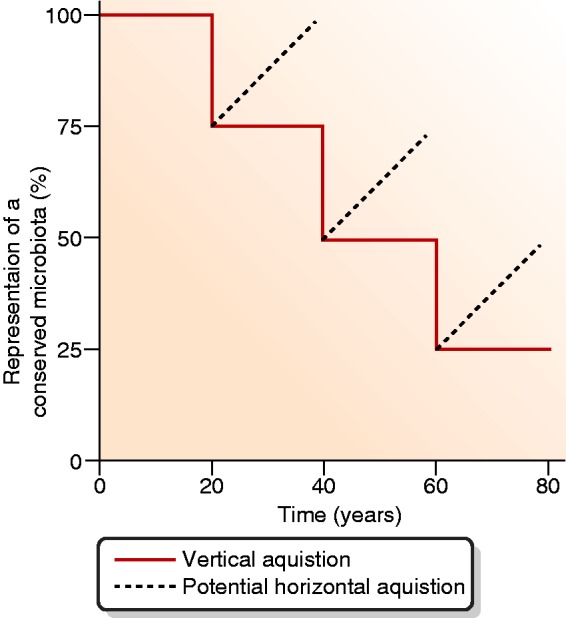
The effect of maternal status on the resident microbiota of the next generation. Microorganisms that have co-evolved with humans as inducers of immunoregulatory circuits are being depleted from the modern urban environment. Blaser and Falkow propose that, since the earliest days of the evolution of mammals, there has been major maternal transmission of microbiota to their offspring (vertical transmission). Modern environmental conditions may affect the transmission and, loss of the conserved microbiota in one generation leads to its loss in the next.
Source: Figure reprinted by permission from Macmillan Publishers Ltd: Blaser MJ and Falkow S. What are the consequences of the disappearing human microbiota?10 Nature Rev Microbiol 2009; 7: 887–894, copyright 2009.
Gut microbiota and brain function
There are multiple pathways of communication between the gut and the brain, including immune, humoral and neural mechanisms, and alterations in bidirectional gut-brain interactions are implicated in both gastrointestinal (GI) disorders (functional GI disorders, IBD, obesity) and CNS disorders (autism, anxiety and depression).15 The nervous system plays a prominent role in modulating immune and bacterial function in the gut through both direct and indirect effects. Indirect effects mediated through stress-induced modulation of the microbial environment include GI motility and changes to intestinal and mucosal secretions.16 Direct effects on luminal bacterial behaviour occur via release of various signalling molecules from epithelial cells into the gut lumen, including noradrenaline.17
Moreover, there are also studies suggesting that alterations in gut microbiota-brain may play a role in non-GI related human brain disorders. A body of evidence largely gained from preclinical studies suggests that microbiota can affect brain development, brain signalling systems and affective behaviour in rodents.18–20 A role of intestinal microbiota on the development of stress, emotion and pain modulation systems has been identified involving neuroplastic changes in emotion regulation regions and signalling systems (Figure 3).11 Differences in motor activity and anxiety-like behaviour observed between germ-free mice and specific pathogen-free (SPF) mice suggest that the microbial colonisation process that occurs during perinatal life initiates signalling mechanisms that affect neuronal circuits involved in motor control and anxiety behaviour.11 The administration of antimicrobials to SPF mice transiently alters the composition of the microbiota, and leads to an increase in exploratory behaviour and an increase in levels of circulating neuroactive substances in the brain.20 The changes observed in central signalling systems and anxiety-like behaviour may be due to specific strains of gut bacteria.21 Moreover, a recent study in healthy women demonstrated that a four-week intake of a fermented milk product with probiotic affected activity of brain regions that control central processing of emotion and sensation, which is the first study showing a clear effect of manipulation of gut microbiota composition on brain function in humans.22 The role of the gut serotonin system in mediating microbiota to brain communication is also under investigation. Studies in animal models have demonstrated that CNS neurotransmission can be disturbed by the absence of a normal gut microbiota and the aberrant serotonin profile is resistant to restoration of a normal gut microbiota in later life.23 In humans, a putative link between altered serotonin biology along the gut-brain axis and autistic spectrum disorder (ASD) is proposed.24 Evidence for altered gut microbiota in ASD is accumulating and includes: higher levels of Bacteriodetes and lower levels of Firmicutes, and an increase in lactobacilli and reduction in bifidobacteria.25,26
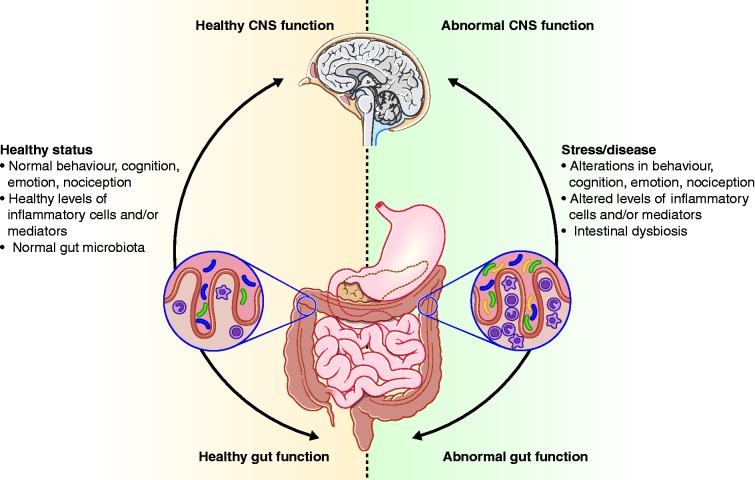
Figure 3.
Impact of the gut microbiota on the gut-brain axis in health and disease. A stable gut microbiota is essential for normal gut physiology and contributes to appropriate signalling along the gut-brain axis and, thereby, to the healthy status of the individual, as shown on the left-hand side of the figure. As shown on the right-hand side of the figure, intestinal dysbiosis can adversely influence gut physiology, leading to inappropriate gut-brain axis signalling and associated consequences for central nervous system (CNS) functions and resulting in disease states. Conversely, stress at the level of the CNS can affect gut function and lead to perturbations of the microbiota.
Source: Figure reprinted by permission from Macmillan Publishers Ltd: Cryan JF and Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour.19 Nature Rev Neuroscience 2012; 13: 701–712, copyright 2012.
Metabolic disorders
Obesity is characterised by a cluster of metabolic disorders, related to the glucose homeostasis and it is associated with multiple co-morbidities including cardiovascular diseases. The development of such pathologies has been associated with a low-grade inflammatory state.27 The gut microbiota can play at least two roles in obesity. It may extract energy from the indigestible part of the diet, thereby contributing up to 10% of the energy influx derived from food. It may also interact with host physiology, via crosstalk mechanisms impacting the inflammatory tone. One of the mechanisms for the interaction of gut microbiota is dependent on the bacterially-derived lipopolysaccharide. Changes in gut microbiota composition are hence associated with weight gain and may play a critical role in the development of obesity-related inflammation. Gut microbes are, therefore, a potential nutritional and pharmacological target in the management of obesity and obesity-related disorders,28 and low microbial diversity appears to be a predictor of poor outcome for the dietary management of obesity.4
By profiling the gut microbiota, a catalogue of putative bacterial targets that may affect host metabolism in obesity and diabetes may be identified (such as Akkermansia muciniphila).5,29 Prebiotic feeding in mice decreased Firmicutes and increased Bacteroidetes phyla, but also changed 102 distinct taxa, 16 of which displayed a >10-fold change in abundance.29 In addition, specific gut microbiota modulation with prebiotics improves glucose homeostasis, leptin sensitivity, and targets enteroendocrine cell activity in obese and diabetic mice.29
Dietary interventions
There are many determinants of gut microbiota composition of which diet is only one: the impact of diet on microbiota composition is demonstrated by comparative studies of children in Europe versus Rural Africa, and the USA versus Bangladesh.30,31 Moreover, long-term dietary habits may be the major determinant of enterotypes.32 There is also a strong link between diet, the gut microbiota and the development of IBD, which requires further investigation.33
The small intestine microbiota is comparatively less studied than that of the large intestine due to poor accessibility; nonetheless this is the first site of interaction between the diet and the microbiota. The gut epithelium is less protected by mucus in the small intestine compared with the colon, and microbiota and the mucosal barrier may be amenable to dietary modulation. The small intestine microbiota is of relatively low density and complexity, dominated by species belonging to Bacteroides, Escherichia and Clostridium clusters. The addition of lactobacilli by dietary intervention transiently swamps the ecosystem, leading to differential mucosal responses to probiotics which support clinical outcomes.34 Findings of a systematic review demonstrate that some probiotics are efficacious in IBS, although the magnitude of benefit is moderate, and it is as yet unclear which species and strains are the most effective in the various IBS sub-types.35
As stated above, a recent study shows that intake of fermented milk product with a specific probiotic modulates the responsiveness of an extensive brain network in humans.22 These findings are consistent with preclinical studies showing a modulatory effect of intake of some probiotics on a range of brain regions in rodents involved in central signalling systems, anxiety-like behaviour, emotional behaviour and brain neurochemistry.36 The widespread changes observed suggest the possible involvement of a probiotic-induced change in metabolite modulation of brain systems, which alter the responsiveness of regions involved in viscerosensory perception, which might be beneficial for patients with functional GI disorders, such as IBS.37
Gut microbiota transplantation
Gut microbiota transplantation has gained in popularity in recent years for the treatment of recurrent Clostridium difficile infection (CDI), and there is evidence supporting gut microbiota transplantation for its cure. Recurrent CDI occurs in around 15–30% of CDI patients and repeated relapses can continue for many years. Recurrent CDI infection may occur as a result of impaired host-response and an alteration in the intestinal microbiome.38,39 The hypothesis for gut microbiota transplantation is to re-establish a normal gut microbiota to act as a barrier against proliferation of C. difficile. Recent guidelines from the American College of Gastroenterology (ACG) recommend consideration of faecal microbiota transplantation as an option for patients after a third recurrence of CDI following failure of vancomycin treatment.40
Evidence for gut microbiota transplantation efficacy in recurrent CDI has accumulated from 317 patients across 27 case series in eight countries.41 A systematic review of the data showed that disease resolution was achieved in 92% of patients, 89% after a single treatment, and relapse occurred in only 4% of patients.41 In a small randomised controlled trial, the infusion of donor faeces was found to be significantly more effective for the treatment of recurrent CDI than the use of vancomycin, resulting in resolution of C. difficile-associated diarrhoea after the first infusion in 81% of cases compared with resolution in 31% of patients receiving vancomycin alone and in 23% receiving vancomycin with bowel lavage (p < 0.001).42 Adverse events were limited to mild diarrhoea and abdominal cramping in the infusion group on the infusion day. A three-month follow-up survey has demonstrated that sustained outcomes are obtained with resolution of symptoms and no recurrence after single gut microbiota transplantation in 91% of patients.43
Further randomised controlled trials are warranted to better determine the efficacy and safety of gut microbiota transplantation, long-term outcomes, and the potential role of gut microbiota transplantation in other disease areas. There is evidence from one study for improved peripheral insulin sensitivity and increased microbial diversity after lean donor faecal infusion in males with metabolic syndrome.44
Detailed studies on gut microbiota composition before and after transplantation may help to understand the intricacies of ecological manipulation and identify novel therapeutic strategies that can replace gut microbiota transplantation in the long-term. A preclinical study has identified a mixture of six phylogenetically diverse intestinal bacteria (Staphylococcus warneri, Enterococcus hirae, Lactobacillus reuteri, and three novel species from Anaerostipes, Bacteroidetes and Enterorhabdus) that re-establish a healthy microbiota and clear CDI from mice.45 Also, in humans changes in faecal microbiota after gut microbiota transplantation for recurrent CDI have been recorded (Figure 4).42,46 The provision of defined cocktails of intestinal bacteria may be the way of the future and has so far been investigated using a purified intestinal bacterial culture of 33 strains isolated from a healthy stool donor.47
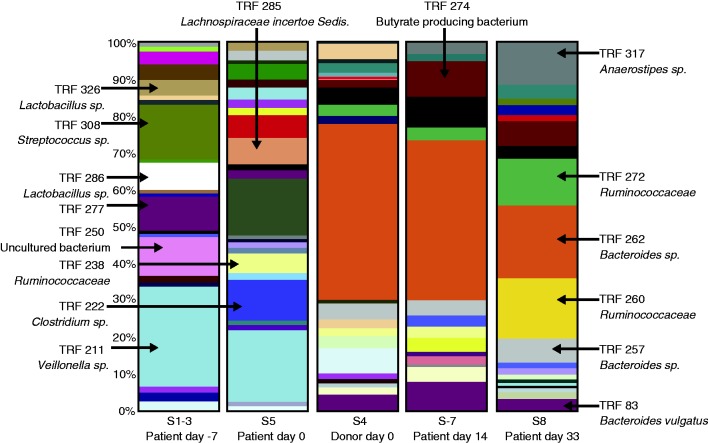
Figure 4.
Distribution of bacterial species before and after gut microbiota transplantation. The distribution of bacterial species in the faeces of donor and patient before and after transplantation. The bacterial species represented by terminal restriction fragments (TRFs) are colour-coded and are valid across columns. A purgative wash-out occurred on day 0 prior to gut microbiota transplantation.
Source: Figure reprinted with permission from Lippincott Williams & Wilkins: Khoruts A, Dicksved J, Jansson JK, et al. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea.46 J Clin Gastroenterol 2010; 44: 354–360, copyright 2010.
Microbiota in gastrointestinal issues − future directions
Future studies building on the gene and organism catalogues established by the human microbiome projects will help us to more fully understand the links between the human microbiome, health and disease states, and to identify common core functions, and functions that are subject-specific. Longitudinal studies and interventions will allow us to move from associations to the identification of predictors, and microbiome-based diagnostics will find their place in targeted interventions for health promotion, disease prevention and management, via the identification of taxa critical for microbe-host crosstalk and immune homeostasis.
There is growing evidence that microbial exposure in the early years of life impacts upon immunoregulation, stress resilience and susceptibility to depression. Future studies will address differences in microbiota to brain signalling based on development stage, i.e. during infancy, teenage and adulthood. Whether there is a role of the gut microbiota in the development of human brain disorders is now under investigation, with interesting results in the field of autism, where a putative role of altered serotonin biology is proposed. The relationship between gut microbial ecology and brain structural networks is also an emerging area of interest, and future studies may identify whether microbial enterotypes correspond to structural and functional brain endophenotypes.
The gut microbiota plays a pivotal role in energy homeostasis and is a potential target for nutritional and pharmacological intervention in the management of obesity. Mechanisms of action concern both energy extraction and promotion of low grade inflammation. Modulation of specific gut microbe with certain prebiotics is shown to improve glucose homeostasis and leptin sensitivity in obese and diabetic mice, with upcoming evidence from human studies. Some probiotics are demonstrated to transiently alter the gut microbiota, and appear to be efficacious in symptoms alleviation in IBS. Emerging evidence suggests that some probiotics may modulate the responsiveness of an extensive brain network in humans. Further confirmatory studies are needed and also to identify which probiotics affect the brain and if different probiotics mediate effects via different pathways.
Bacteriotherapy shows promising results in the treatment of recurrent CDI, and there are data emerging for a potential role of gut microbiota transplantation in other chronic diseases including metabolic syndrome. Gut microbiota transplantation appears to be safe in short-term studies but there is a need for studies to assess long-term safety and efficacy. Standard operating procedures for screening of donor and transplantation per se would be most useful. A sound ecological understanding is fully warranted to identify interventions that specifically suppress, eliminate or enhance the presence of key microbes by manipulating the enteric environment of the host. Targeted pharmacotherapy that acts synergistically with dietary manipulations or the provision of defined cocktails of intestinal microbes may well be the way of the future.
Funding
This Review Article received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
JD is involved in research funded by Danone, Pfizer, PiLeJe; is member of an advisory board for Danone Research; and has given presentations for Tillots Pharma, PiLeJe, Yoplait, Yakult, Milumel.
FG is a member of the scientific board of Instituto Danone (Barcelona, Spain).
MS has received unrestricted research grants from Danone and AstraZeneca, and served as a Consultant/Advisory Board member for Danone, Novartis, Boehringer-Ingelheim, and Shire/Movetis. LB has received funding from Danone.
References
- 1.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486: 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464: 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature 2011; 473: 174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013; Epub ahead of print, 28 August 2013. DOI: 10.1038/nature12480. [DOI] [PubMed]
- 5.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012; 490: 55–60 [DOI] [PubMed] [Google Scholar]
- 6.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486: 222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology 2010; 139: 1816–1819 [DOI] [PubMed] [Google Scholar]
- 8.Hanski I, von Hertzen L, Fyhrquist N, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. P Natl Acad Sci USA 2012; 109: 8334–8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allerg Immun 2012; 42: 5–15 [DOI] [PubMed] [Google Scholar]
- 10.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nature Rev Microbiol 2009; 7: 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. P Natl Acad Sci USA 2011; 108: 3047–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey MT, Dowd SE, Galley JD, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav Immun 2011; 25: 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDade TW. Early environments and the ecology of inflammation. P Natl Acad Sci USA 2012; 109: S17281–S17288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rook GAW, Raison CL, Lowry CA. Childhood microbial experience, immunoregulation, inflammation and adult susceptibility to psychosocial stressors and depression in rich and poor countries. Evolution, Medicine and Public Health 2013; 1: 14–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Ann Rev Med 2011; 62: 381–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nature Rev Gastroenterol & Hepatol 2009; 6: 306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walters M, Sperandio V. Autoinducer 3 and epinephrine signaling in the kinetics of locus of enterocyte effacement gene expression in enterohemorrhagic Escherichia coli. Infect Immun 2006; 74: 5445–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol Motility 2011; 23: 187–192 [DOI] [PubMed] [Google Scholar]
- 19.Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Rev Neuroscience 2012; 13: 701–712 [DOI] [PubMed] [Google Scholar]
- 20.Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011; 141: 599–609, e1–e3 [DOI] [PubMed] [Google Scholar]
- 21.Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motility 2011; 23: 1132–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013; 144: 1394–1401; e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry 2013; 18: 666–673 [DOI] [PubMed] [Google Scholar]
- 24.Prasad HC, Steiner JA, Sutcliffe JS, et al. Enhanced activity of human serotonin transporter variants associated with autism. Philos T Roy Soc B 2009; 364: 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finegold SM, Dowd SE, Gontcharova V, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010; 16: 444–453 [DOI] [PubMed] [Google Scholar]
- 26.Adams JB, Johansen LJ, Powell LD, et al. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol 2011; 11: 22–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharma Des 2009; 15: 1546–1558 [DOI] [PubMed] [Google Scholar]
- 28.Delzenne NM, Neyrinck AM, Backhed F, et al. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nature Rev Endocrinol 2011; 7: 639–646 [DOI] [PubMed] [Google Scholar]
- 29.Everard A, Lazarevic V, Derrien M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011; 60: 2775–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. P Natl Acad Sci USA 2010; 107: 14691–14696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin A, Bik EM, Costello EK, et al. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PloS One 2013; 8: e53838–e53838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011; 334: 105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albenberg LG, Lewis JD, Wu GD. Food and the gut microbiota in inflammatory bowel diseases: A critical connection. Curr Opin Gastroenterol 2012; 28: 314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Baarlen P, Troost FJ, van Hemert S, et al. Differential NF-kappa B pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. P Natl Acad Sci USA 2009; 106: 2371–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: A systematic review. Gut 2010; 59: 325–332 [DOI] [PubMed] [Google Scholar]
- 36.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. P Natl Acad Sci USA 2011; 108: 16050–16055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013; 62: 159–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyne L, Warny M, Qamar A, et al. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 2001; 357: 189–193 [DOI] [PubMed] [Google Scholar]
- 39.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis 2008; 197: 435–438 [DOI] [PubMed] [Google Scholar]
- 40.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108: 478–498 [DOI] [PubMed] [Google Scholar]
- 41.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53: 994–1002 [DOI] [PubMed] [Google Scholar]
- 42.Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. NEJM 2013; 368: 407–415 [DOI] [PubMed] [Google Scholar]
- 43.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107: 1079–1087 [DOI] [PubMed] [Google Scholar]
- 44.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913–916; e7 [DOI] [PubMed] [Google Scholar]
- 45.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathogens 2012; 8: e1002995–e1002995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khoruts A, Dicksved J, Jansson JK, et al. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2010; 44: 354–360 [DOI] [PubMed] [Google Scholar]
- 47. Petrof EO, Claud EC, Gloor GB, Allen-Vercoe E. Microbial ecosystems therapeutics: a new paradigm in medicine? Benef Microbes 2013; 4: 53–65. [DOI] [PubMed]