Abstract
Approximately 30–40% of patients taking proton pump inhibitors (PPIs) for presumed gastro-oesophageal reflux (GOR) symptoms do not achieve adequate symptom control, especially when no oesophageal mucosal breaks are present at endoscopy and when extra-oesophageal symptoms are concerned. After failure of optimization of medical therapy, a careful work up is mandatory that aims at determining whether symptoms are related to GOR or not. Most patients with refractory symptoms do not have GOR-related symptoms. Some may have symptoms related to weakly acidic reflux and/or oesophageal hypersensitivity. Baclofen is currently the only antireflux compound available as add-on therapy to PPIs, but its poor tolerability limits its use in clinical practice. There is room for pain modulators in patients with hypersensitive oesophagus and functional heartburn. Antireflux surgery is a suitable option in patients responding to medical therapy who want to avoid taking medication or if persisting symptoms can be clearly attributed to poorly controlled GOR.
Keywords: Asthma, cough, GORD, PPI, surgery
Introduction
Gastro-oesophageal reflux disease (GORD) is a common disorder caused by the reflux of gastric contents into the oesophagus. According to a recent global definition, GORD can cause oesophageal and extra-oesophageal syndromes, which can be associated or not in the same individual.1 The diagnosis of GORD can rely on typical symptoms such as heartburn and regurgitation as well as the presence of oesophageal mucosal breaks at endoscopy. However, many patients present with atypical symptoms (e.g. supra-oesophageal symptoms) and do not have any mucosal abnormalities at endoscopy mainly because most of them have been prescribed proton pump inhibitors (PPIs) before being referred to gastrointestinal specialists. In such situation, it is sometimes difficult to know whether the presenting symptoms are indeed due to reflux. As a consequence, there has been an increasing need for objective tests to confirm abnormal oesophageal reflux. GORD management is primarily based on empiric therapy, with lifestyle modifications and medication, especially in general practice. Acid suppression with PPIs is the mainstay of therapy for GORD. However, although there is no consensus about definition of failure, 30–40% of patients with reflux symptoms do not achieve adequate symptom relief after a 4-week course of a single dose of PPI.2 Although failure of PPIs is one of the most common indications for antireflux surgery, it is generally considered by experts that antireflux surgery in these patients has a less favourable clinical outcome compared to that obtained in patients with adequate symptom control with PPI.2,3 The aim of this article is to summarize the current options for the management of difficult GORD, i.e. the diagnostic work up, the optimization of medical therapy, and indications/complications of surgery.
A patient with refractory reflux symptoms: which diagnostic work up?
The aim of the work up, in the case of refractory symptoms supposed to be related to GORD, is to phenotype the patients: typical vs. atypical symptoms; erosive vs. non-erosive reflux disease (NERD); and acid-sensitive oesophagus vs. functional heartburn (Table 1).4
Table 1.
Definitions of gastro-oesophageal reflux subtypes according to endoscopy and pH (or pH-impedance) monitoring and Rome III definitions4
| Erosive reflux disease: patients with mucosal breaks at endoscopy |
| Non-erosive reflux disease: patients without any mucosal break at endoscopy and abnormal oesophageal acid exposure at 24-h oesophageal pH monitoring |
| Hypersensitive oesophagus: patients without any mucosal break at endoscopy, normal oesophageal acid exposure and positive symptom–reflux association analysis (SI >50%, SAP >95%) |
| Functional heartburn: patients with heartburn refractory to PPIs, without any mucosal break at endoscopy, normal oesophageal acid exposure and negative symptom reflux association analysis (symptom index <50%, symptom association probability <95%) at 24-h oesophageal pH monitoring |
| Weakly acidic reflux: gastro-oesophageal reflux episode detected by oesophageal impedance and associated with an oesophageal pH between 4 and 7 |
| Weakly alkaline reflux: gastro-oesophageal reflux episode detected by oesophageal impedance and associated with an oesophageal pH above 7 |
| Non-acid reflux: usually refers to all reflux episodes detected by oesophageal impedance without any pH drop below 4 (include weakly acidic and weakly alkaline reflux) |
PPI, proton pump inhibitor; SAP, symptom association probability; SI, symptom index.
One critical issue is to determine the presence of typical and/or atypical reflux symptoms. A careful interview is mandatory to determine the nature of symptoms that were initially present (before treatment) and of those that persist on therapy, and which one is considered to be troublesome by the patient. It is important to pay specific attention to the symptom ‘heartburn’ which may in fact correspond to sore throat or epigastric burning. Likewise, many patients report dyspeptic symptoms that were initially present together with heartburn and have been unmasked by the PPI therapy. If atypical reflux symptoms or severe dyspeptic symptoms are diagnosed, a specific work up is mandatory. Physicians should also be aware that a small proportion of patients presenting with regurgitation may have a rumination syndrome that should be ruled out by appropriate tests.
Typical reflux symptoms (heartburn and/or regurgitation)
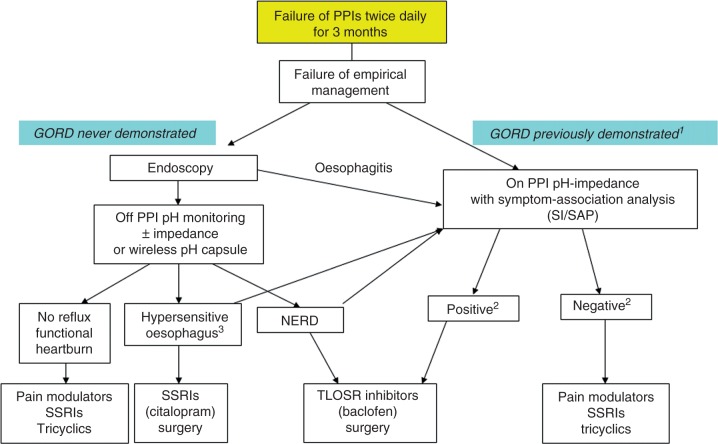
If optimization of PPI therapy fails (see below), patients with persisting reflux symptoms despite 3-month double-dose PPI therapy should be further investigated.5 An upper gastrointestinal endoscopy with oesophageal biopsies should be performed to rule out other oesophageal or gastric disease such as eosinophilic oesophagitis, pill-induced oesophagitis, or skin diseases associated with oesophageal involvement. If erosive oesophagitis or Barrett’s oesophagus is observed in patients on double-dose PPIs for at least 3 months, GORD be considered as being documented.6 Although data to support this proposition are lacking, it may be useful to perform pH-impedance monitoring ‘on’ PPIs in these patients (i) to demonstrate an association between persisting symptoms and reflux if only minimal lesions are present and (ii) to assess the efficacy of gastric acid secretion (when simultaneous gastric and oesophageal pH monitoring is available). Patients in whom GORD has been previously demonstrated with adequate relief of heartburn but persisting regurgitation should be considered for surgery, provided severe oesophageal motility disorders have been ruled out by oesophageal manometry. Achalasia or severe motility disorders can be suspected if oesophageal stasis and/or cardial spasm are present at endoscopy. Oesophageal manometry is routinely performed to position pH or pH-impedance catheters.
In most patients, upper gastrointestinal endoscopy is normal and ambulatory reflux monitoring ‘off’ therapy should be considered if GORD has not been previously documented.5 GORD can be demonstrated by the means of catheter-based 24-hour pH monitoring, but 48–96-hour wireless capsule pH monitoring7 and 24-hour pH-impedance monitoring have been shown to have increased diagnostic yield.8 Recordings ‘off’ PPIs allows to classify the patients as having NERD, acid-sensitive oesophagus, or no reflux (or functional heartburn) based on the symptom association analysis with symptom index (SI) and/or symptom association probability (SAP) (Figure 1). Using pH-impedance monitoring ‘off’ PPIs instead of pH-alone recordings has been shown to reduce the rate of patients with functional heartburn since approximately 10% patients will have a positive symptom–reflux association for weakly acidic reflux.9 Whether the total number of ‘bolus’ reflux events and/or the ‘bolus exposure’ of the oesophageal mucosa could help to classify patients as having NERD remains to be determined.10
Figure 1.
Algorithm of management for patients with typical refractory symptoms. aGORD is demonstrated by history of oesophagitis and/or positive pH monitoring off therapy. bpH-impedance monitoring is considered to be ‘positive’ when both SI and SAP are positive, and ‘negative’ when both SI and SAP are negative; non-concordant SI and SAP should be interpreted with caution before proposing antireflux surgery (adapted from Sifrim and Zerbib5). cMay refer to positive symptom association to acid and/or non-acid reflux if pH-impedance monitoring is performed. NERD, non-erosive reflux disease; PPI, proton pump inhibitor; SAP, symptom association probability; SI, symptom index; SSRI, selective serotonin reuptake inhibitor; TLOSR, transient lower oesophageal relaxation.
Ambulatory reflux monitoring ‘on’ PPIs twice daily can be proposed to patients with documented GORD to establish a correlation between refractory symptoms and reflux events and/or to exclude GORD as the cause of the persisting symptoms. Regarding the low diagnostic yield of pH-alone recordings ‘on’ PPIs twice daily,11 the use of 24-hour pH-impedance monitoring is recommended. Based on the only available outcome data with pH-impedance monitoring ‘on’ therapy, correlation between refractory symptoms and reflux should rely on a SI value above 50%.12 However, whether other parameters (such as SAP, oesophageal bolus exposure, or the total number of reflux events) should be taken into account remains to be further determined. When both SI and SAP are positive, the probability that the residual symptoms are related to GORD is high. When both symptom association indices are negative, GORD is probably not the cause of the remaining symptoms. In the case of non-concordant symptom association tests, one should be very cautious before referring the patient for antireflux surgery.
Presumed extra-oesophageal GORD symptoms
Many patients present with atypical symptoms (e.g. supra-oesophageal symptoms) supposed to be related to GORD. The roles of the gastroenterologist are: (i) to document the presence of abnormal GORD; (ii) to establish a causal relationship between GORD and symptoms; and (iii) to propose an adequate therapeutic option.
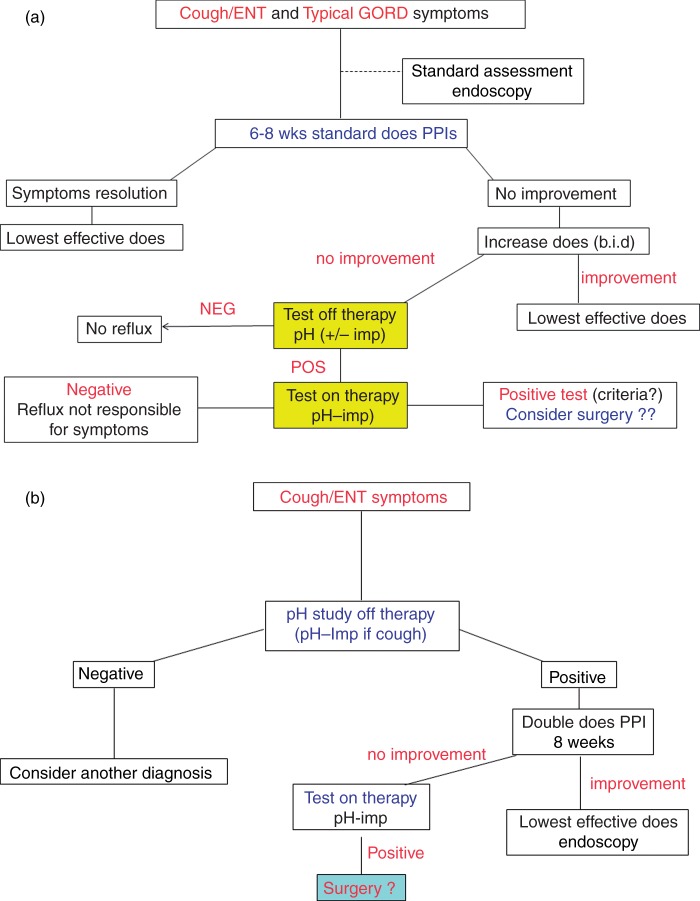
It is crucial to take into account the presence of typical reflux symptoms in the context of presumed extra-oesophageal manifestations of GORD. In this, case, the patient can be managed as if only typical symptoms were present. If no typical symptoms are present, pathological GORD can be demonstrated by endoscopy showing oesophagitis. In case of normal endoscopy, the next step is to perform pH monitoring off therapy which represents the most effective approach.5 Indeed, performing pH-impedance monitoring off therapy adds little value to pH-alone monitoring, except maybe in the specific case of cough (see next section).
Cough
GORD is one of the most frequent aetiologies of chronic cough, probably accounting for approximately 20% of chronic cough cases.13 However, only a minority of patients with chronic cough and GORD have typical digestive symptoms and/or clear evidence of oesophagitis. A temporal relationship between cough and acid or non-acid reflux can be demonstrated in approximately 45% of patients by pH-impedance monitoring especially by using oesophago-gastric manometry14 or an acoustic cough recording device15 instead of events marker activation by the patient. However, in these patients, the role of cough reflex hypersensitivity is crucial and may explain antireflux therapy failures.15 Whether oesophageal testing can predict response to therapy is still unknown since outcome studies are scarce. Laparoscopic Nissen fundoplication led to disappearance of symptoms in six patients with cough associated with nonacid reflux (positive SI) during pH-impedance monitoring on therapy, with a median follow up of 17 months.16 Since most placebo-controlled studies showed no benefit of PPI as first-line therapy in unexplained chronic cough, the best option in patients without typical reflux symptoms appears to perform pH-impedance monitoring ‘off’ therapy with careful analysis of the symptom–reflux temporal relationship in order to select the patients who can truly benefit from GORD treatment.13 However, this approach requires further validation by appropriate controlled outcome studies.
Ear, nose, and throat symptoms
The so-called laryngopharyngeal reflux is defined by the association of laryngeal symptoms with laryngeal inflammation at laryngoscopy.17 However, the relationship between reflux and laryngeal symptoms is frequently difficult to establish with a high degree of certainty and cannot rely on laryngoscopic signs that have a very poor specificity.18 The presence of oesophagitis and/or abnormal distal oesophageal acid exposure on pH-alone or pH-impedance monitoring can confirm the presence of pathological GORD in less than 40% of patients19 but cannot provide any information on causality. Indeed, the link between reflux and laryngeal symptoms cannot rely on symptom–reflux association analysis since these symptoms are usually long lasting (or permanent) and do not have a sudden onset that could be easily perceived by the patient. Moreover, the presence of abnormal proximal and/or distal acid reflux on pH monitoring does not predict response to therapy.20 In view of these difficulties, it is currently recommended to start with an empirical 3-month therapy with high doses of PPIs,21 but most placebo-controlled trials have failed to demonstrate any benefit of PPIs in patients with suspected reflux-related laryngeal symptoms.22,23 Promising preliminary results have been reported with pharyngeal impedance24–26 but whether this technique will be further developed and prove to be helpful for the management of these difficult patients remains to be determined, since this technique appears to be poorly reproducible.27
In clinical practice, it is recommended to take into account the presence of typical symptoms associated with ear, nose, and throat (ENT) symptoms (Figure 2). If heartburn and/or regurgitation are present, an empirical treatment with PPI can be proposed. In case of refractory symptoms, oesophageal testing should be proposed ‘on' therapy, ideally with pH-impedance monitoring. If ENT symptoms are not associated with typical symptoms, it seems that performing pH monitoring ‘off’ therapy could be proposed, although its usefulness is questionable. Nevertheless, it may help the physicians, especially the gastroenterologist to whom the patient is referred, to rule out the diagnosis of GORD in these patients with chronic difficult-to-treat symptoms but considered by a majority of ENT physicians as having GORD-related symptoms.28
Figure 2.
Algorithm of management for patients with cough and/or ear, nose, and throat (ENT) symptoms according the presence (a) or not (b) of typical GORD symptoms. Regarding the potential role of weakly acidic reflux in this situation, patients with unexplained chronic cough may have pH-impedance monitoring off therapy instead of pH-alone reflux monitoring.
PPI, proton pump inhibitor.
Asthma
In patients with asthma, the average prevalence of abnormal oesophageal pH monitoring, oesophagitis, and hiatus hernia was 50.9, 37.3, and 51.2%, respectively. Theoretically, oesophageal pH studies may help to identify patients with pathological reflux as well as to evaluate the temporal association between reflux and respiratory symptoms. Although uncontrolled data have suggested that abnormal proximal acid reflux was associated with worse asthma, two controlled studies have shown that the results of pH monitoring could not predict the response to PPI therapy, either on asthma symptoms or on pulmonary function.29,30
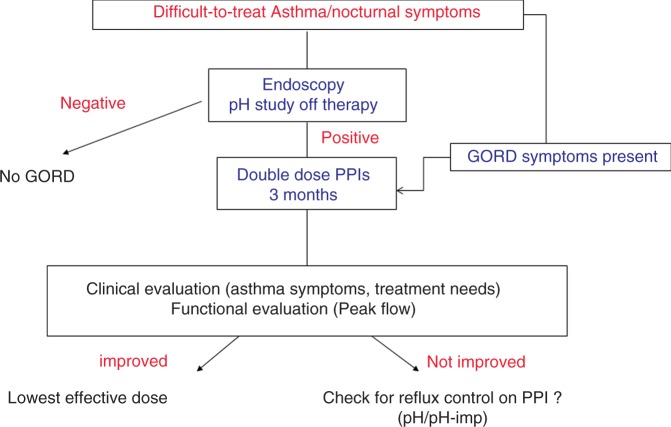
In clinical practice, considering the results of the most important randomized trial with PPIs,30 patients with difficult-to-treat asthma and/or nocturnal symptoms without GORD symptoms should be offered an oesophageal pH study ‘off' therapy to detect ‘silent GORD’ (Figure 3). Patients presenting with typical GORD symptoms and/or abnormal pH study should be treated with 3-month double-dose PPI therapy. Treatment efficacy should be assessed according to clinical (asthma symptoms, treatment needs) and functional (peak flow) endpoints. In patients who do not respond to this first therapeutic approach, pH-impedance monitoring performed on therapy may be useful to detect those with persistent reflux who need better therapeutic control.13 Considering the results of the few controlled studies reported to date, one should be very cautious before embarking on surgical antireflux procedures to improve asthma outcome. This approach could also be proposed in patients with documented acid reflux and clear temporal correlations between respiratory symptoms and reflux episodes during 24-hour pH monitoring.
Figure 3.
Algorithm of management for patients with difficult-to-treat asthma according to presence of GORD symptoms.
pH-imp, pH-impedance monitoring; PPI, proton pump inhibitor.
Optimizing medical therapy: what works in clinical practice?
Persistent symptoms on PPI therapy can be due to insufficient response to PPIs (i.e. persistent acid reflux) or persistent volume reflux (i.e. non-acid or weakly acid reflux) or can be independent of reflux (i.e. due to other conditions or functional).31–33 The most important step in optimizing therapy for patients with persistent symptoms on PPI therapy is establishing the relationship between symptoms and gastro-oesophageal reflux episodes on acid-suppressive therapy. To date, the best method to assess this relationship is ambulatory 24-h combined impedance-pH monitoring.
If typical reflux symptoms persist (i.e. heartburn and regurgitation), compliance and dosing time should be checked, since taking PPIs before meals provides a better gastric acid secretion control. Though not supported by strong clinical data, switching to another PPI brand may be an option before doubling the dose. Twice-daily PPIs (single dose taken before breakfast and before dinner) is not an approved dose by health authorities but could achieve adequate symptom control after 6–8 weeks of therapy in approximately 20–30% of patients with inadequate symptom control on once daily PPI.34,35 If clinical remission is achieved, titration can be proposed after 2–3 months of double-dose therapy. In case of failure, adding alginates or H2RAs at bedtime may be tested, but one has to keep in mind that H2RAs should better be taken on demand or intermittently to avoid tolerance.
Patients with persistent volume reflux could benefit from a reflux-reducing agent such as GABA-B receptor agonists or glutamate receptors antagonists. These agents reduce the frequency of transient lower oesophageal relaxations and consecutively both acid and non-acid reflux episodes. Two placebo-controlled studies demonstrated that monotherapy for 2–4 weeks with baclofen, a GABA-B receptor agonist, decreased acidic reflux occurrence, oesophageal acid exposure, and improved reflux-related symptoms.36,37 An uncontrolled study demonstrated the efficacy of baclofen as add-on therapy to PPIs in patients with persisting pathological ‘bile reflux’ demonstrated by Bilitec monitoring.38 However, although prescribed by many physicians, baclofen has a poor tolerability profile that limits its use in clinical practice since many patients report CNS symptoms like dizziness, drowsiness, nausea, and vomiting despite a progressively increased dosage. The results obtained with baclofen opened the quest for developing more-efficient and better-tolerated compounds. Lesogaberan (AZD3355) 65 mg, a GABA-B agonist added to PPI therapy, reduced the number of gastro-oesophageal reflux episodes by 35% by reducing the number of transient lower oesophageal relaxations and increasing the lower oesophageal basal pressure.39 With regards to symptom control over a 4-week period, lesogaberan 65 mg b.i.d. was superior to placebo (16 vs. 8%; p = 0.026) in improving heartburn and regurgitation, an effect considered to be too small to continue further development of this compound. ADX10059, a metabotropic glutamate receptor-5 (mGluR5) negative allosteric modulator was shown to reduce post-prandial distal oesophageal acid exposure and number of non-acid reflux episodes in healthy volunteers.40 This compound was found to be effective also in GORD patients responsive to PPI therapy but liver side-effects stopped further development of this compound.41 Arbaclofen 60 mg, the active R-isomer of baclofen, was found to reduce the total number of GOR episodes,42 but a trial evaluating the efficacy and safety of arbaclofen in subjects with symptomatic GORD over 4 weeks found that the active compound was no superior to placebo in reducing the number of weekly heartburn episodes decreasing the enthusiasm to further develop this compound.43 In summary, several pharmaceutical companies tried developing better tolerable reflux-reducing agents but stopped development due to limited efficacy or unwanted adverse events.
Another approach in patients with persistent non-acid reflux is decreasing the perception of reflux episodes using visceral analgesics. Visceral analgesics (tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors) increase oesophageal perception threshold, thus reducing sensitivity due to reflux episodes. To date, the most compelling evidence for this approach is the results of a double-blind randomized, placebo-controlled study:44 patients with typical reflux symptoms (heartburn, regurgitation, chest pain), normal endoscopy, normal distal oesophageal acid exposure, and a positive SI were randomized to receive citalopram 20 mg or placebo once daily for 6 months. At the end of the follow-up period, 15/39 patients receiving citalopram (38.5%) and 24/36 patients receiving placebo (66.7%) continued to report reflux symptoms (p = 0.021). Based on these results, it can be concluded that selective serotonin reuptake inhibitors (citalopram) are effective in patients with hypersensitive oesophagus.
Patients with symptoms that are independent of acid or non-acid reflux (i.e. functional symptoms) should be counselled to avoid unnecessary increase of acid-suppressive therapy or reflux-reducing therapies (in particular antireflux surgery) and offered visceral analgesics. To date, the best evidence to support the use of visceral analgesics in patients with functional chest pain are data from a 4-week randomized, double-blind, placebo-controlled study comparing venlafaxine 75 mg vs. placebo in patients with normal endoscopy, normal manometry, and normal reflux monitoring.45 The authors randomized patients to either venlafaxine or placebo for 4 weeks and defined the primary end-point as the percentage of patients with at least 50% improvement of symptoms score. The primary end-point was achieved in 50% of patients receiving venlafaxine compared to 4% of patients receiving placebo indicating an odds ratio of 26.0 (95% CI 5.7–118.8, p < 0.001) in the intention-to-treat analysis. Of note is that the depression scores (Becks Depression Inventory) were neither elevated at the beginning of therapy nor changed by venlafaxine or placebo, underscoring that functional oesophageal symptoms occur independently of psychiatric comorbidities. A shortcoming of this study, however, is the relatively short treatment period (4 weeks), which leaves open the question as to how long this therapy should be continued in those who respond.
In summary, patients with difficult-to-treat GORD should be carefully evaluated for the relationship between symptoms and gastro-oesophageal reflux episodes. Patients with symptoms independent of reflux should be treated as functional patients. Patients with symptoms related to reflux episodes should receive optimal acid-suppressive therapy, trial of reflux-reducing agents and visceral analgesics, and, if medical therapy is unsuccessful, should be offered antireflux surgery.
Surgery for refractory reflux disease: risks and outcomes
Antireflux surgery is defined for this discussion as an operative intervention by the combination of repair of hiatus hernia and fundoplication of one kind or another. Laparoscopy has revolutionized the recovery rates from antireflux surgery, and it also facilitates a very high standard of surgery.
Who is antireflux surgery suitable for?
Antireflux surgery is suitable for a range of patients and for a variety of reasons. An operation can be considered if a patient is fit to have a general anaesthetic and has a desire to stop their medication or to relieve symptoms not treated by their medication. An operation is suitable when symptoms are poorly controlled despite medication, especially for patients who suffer large volume regurgitation and those who wake at night time with coughing and choking and who have acid-tasting fluid or food regurgitated into their throat and airway. Regurgitation into the throat when stooping or exercise can limit patients’ ability to work, play sports, or even do simple housework. A few patients cannot tolerate medical treatment. Acid-suppressing drugs are sometimes associated with significant side effects, the commonest of which is diarrhoea, headache, and, more rarely, pins and needles feelings in the head, face, and neck. Also hypomagnesaemia can be a problem. If these limit the quality of life for someone who suffers heartburn, then having an operation may allow them to avoid medication and its side effects. For some patients who are well controlled on acid suppression but do not want to stay on pills for the rest of their life, an operation is a way of avoiding the need to be on prescription medication in the long term. The decision on the need for an operation to control reflux symptoms should come from the patient’s desire for symptom control or quality of life issues. A major reason for considering surgery is for those patients who have persistent symptoms while on a PPI. Some 10% of patients on full-dose PPI still suffer persistent heartburn and even more suffer regurgitation. This group are sometimes described as having refractory reflux. They are not a uniform group of patients and they fall into a number of clinical patterns.5 Some patients report refractory symptoms that are different to their initial symptoms. PPIs often resolve severe heartburn but may fail to reduce volume reflux and regurgitation while stooping or straining. Occasionally, weakly acidic or non-acidic reflux is the cause of persistent symptoms while on a PPI and can be detected with pH-impedance monitoring.33 Laparoscopic fundoplication has been shown to reduce weakly acidic reflux,46 and Mainie et al.32 reported that for patients with such symptoms a successful outcome from surgery could be achieved. However, this study was uncontrolled and included a small and heterogeneous group of patients. A group of patients comprises those whose refractory symptoms are due to an altered sensitivity of perception of a normal amount of acid exposure in the oesophagus. These are sometimes described as acid hypersensitivity, or even functional heartburn. When this can be confidently measured through 24-hour pH testing with good symptom association, then this may predict a successful outcome of antireflux surgery.47 Extra-oesophageal respiratory symptoms of cough (and occasionally asthma) may also be effectively treated by antireflux surgery when the association with reflux can be confirmed, although long-term outcome data are lacking.16
Morbid obesity
Although antireflux surgery may improve symptoms of GORD, the holistic improvement achieved with Bariatric techniques of gastro-jejunal bypass, excluding the majority of the stomach from the oesophagus provides a complete reflux control in the context of the other benefits of marked weight reduction. For patients with moderate obesity (body mass index 35–40 kg/m2), Anvari and Bamehriz48 showed good outcomes for Nissen fundoplication with both symptom control and a moderate degree of weight loss. For patients with body mass index >40 kg/m2, gastrojejunal bypass is the preferable route but requires great patient cooperation.49 On the other hand, some bariatric surgery such as balloons or bands actually can provoke or aggravate reflux and should be avoided in overweight patients where reflux symptoms are already a problem.
Preoperative assessment
Before surgery, manometry and 24-hour pH test should be performed. One advantage of this is that the patient’s persistent symptoms may be due to another disease. It is essential to confirm reflux before subjecting a patient to an operation and this should avoid the errors in management of achalasia or eosinophilic oesophagitis. Additionally, the tests may exclude reflux as the cause of symptoms and direct treatment
The benefits of surgery
The benefits of antireflux surgery have been the subject of a large European multicentre randomized control trial: the Lotus trial, which included >350 patients randomized to escalating doses of esomeprazole for symptom relief and compared to a standardized laparoscopic Nissen fundoplication, with hiatal repair. The reliability of this in the long term has been identified in the 5-year follow up.50 This has also shown a better quality of heartburn control and less regurgitation. The strict follow-up protocol highlighted an overall better quality of life after antireflux surgery, although the differences in this study were not marked, because the definition of entry into the study was satisfactory control of acid reflux by PPI. In many patients, there is a response to PPI but the symptoms are still present as defined above in the term ‘refractory reflux’.
Disadvantages of antireflux surgery
Dysphagia early after the operation is common but usually resolves within 3 months. Late dysphagia (5%) may require a dilatation or rarely revisional surgery. Early satiety, weight loss, and discomfort with large meals may be a benefit or a problem. Associated weight loss after a Nissen fundoplication is often reversed after 6 months, but may facilitate improved lifestyle and activity. Hiccup, difficult burping, or vomiting are usually transient but occasionally troublesome. Feelings of trapped wind, bloatedness, and passing increased rectal flatus are frequently reported in both medically and surgically treated patients, although more so after surgery.50 Recurrence of heartburn and regurgitation can occur in 10% of patients at 5–10 years post operation. This relatively high rate may be improved by following a standardized procedure such as that achieved by 40 surgeons across Europe who contributed to the LOTUS trial. The quality of surgery is very important when considering an operation and this should be restricted to units with experience and high volume throughput.51 Revisional surgery may be variably needed (2–5%) but is often effective when indicated.52
Quality assurance in surgery for refractory reflux disease
For patients where surgery is an option, one must ensure the right surgeon performs a standardized operation for the right indications in the right patient and provides good preoperative education and testing and post operative support. When a patient is refractory to medical therapy, it is mandatory to reconsider the diagnosis of GORD before switching to surgery. One must always discuss the responsibility of GORD, especially if extra-digestive manifestations are concerned.
Management of persistent severe symptoms and complications after reflux surgery
After surgery, some patients develop recurrent symptoms because of wrap disruption or migration or hiatus disruption. Para-oesophageal herniation with mediastinal migration of the wrap or stomach, disruption of the wrap, and slipping of the wrap may all cause symptoms or recurrent reflux. A tight wrap is an uncommon cause behind obstructive complains (about 5%) but stricture formation is rare (<2%).53,54
The concept of tailoring the design of the repair based on the motor function of the oesophagus is not based on solid scientific evidence. Provided that we are dealing with well-established and diagnosed chronic GORD, it is clear that either a short and floppy total wrap or a partial fundoplication can safely be carried out irrespective of the motor characteristics of the distal oesophagus.
Exactly what constitutes a failed antireflux procedure is a matter of debate and controversy. Surgeons typically consider recurrence of preoperative reflux symptoms or the postoperative development of new problems such as dysphagia as failure and representing significant complications.53,54 Revisional antireflux surgery can be performed safely but at the cost of high morbidity and even mortality, substantially higher than after a primary operation.52,55,56 Therefore, careful patient selection is important. It has frequently been stated that the primary therapeutic endpoint for patients with GORD is complete relief of symptoms and normalization of quality of life. Patients opting for surgery often expect complete resolution of symptoms in contrast to the expectations of patients under PPI. Medically treated patients do not all expect complete relief as it has been clearly shown that approximately only 60% achieve a complete relief from disease-related symptoms despite good satisfaction scores. Therefore, a moderate deviation from a high rate of patient satisfaction, which is regularly seen after high-quality antireflux surgery, should not be regarded as a failure.
It is important to take patient characteristics into account when evaluating the outcome. It has been shown that suboptimal improvement in quality of life and accumulation of side effects without any objective evidence of failure is seen more frequently in patients with preoperative psychiatric comorbidities. Illness behaviour categories can and shall probably be addressed, by use of structured questionnaires such as general hypochondriasis, disease conviction, and psychological vs. somatic illness perception, affective inhibition, affective disturbance, denial irritability, index of hypochondriasis, affective state, and disease affirmation. Until now, studies have shown that there seems to be no apparent differences in illness behaviour categories between patients who experience postoperative problems compared to those who are scored as successful with regard to the control of reflux disease by the surgical intervention.57
Gastroenterologists may be called upon to manage patients who have had antireflux surgery that obviously has failed. The available literature on this topic contains predominantly reports of retrospective nature and studies written by surgeons, who often have focused on how alleged technical deficiencies in performing the operation led to the failure. Such reports are of limited value to the gastroenterologist seeking guidance on a patient’s management. Furthermore, conclusions in the reports are confounded by the lack of standardized definition of failure after this kind of surgery. Symptoms that may be addressed by gastroenterologists after antireflux surgery are: symptoms incompletely relieved or reappearing early or late after surgery, symptoms due to persistent gastro-oesophageal reflux, and symptoms caused by the surgery in the form of dysphagia, gas bloat syndrome, and diarrhoea. Many of these complaints can be extremely difficult to treat and the scientific evidence for the efficacy of different regimens is virtually absent.
Patients presenting with severe complaints after antireflux surgery should be referred to a specialized unit with a multidisciplinary investigational approach, followed by therapeutic interventions that are discussed between gastroenterologists and specialized surgeons in the field.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101: 1900–1920 [DOI] [PubMed] [Google Scholar]
- 2.Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut 2009; 58: 295–309 [DOI] [PubMed] [Google Scholar]
- 3.Lundell L. Surgical therapy of gastro-oesophageal reflux disease. Best Pract Res Clin Gastroenterol 2010; 24: 947–959 [DOI] [PubMed] [Google Scholar]
- 4.Galmiche JP, Clouse RE, Balint A, et al. Functional esophageal disorders. Gastroenterology 2006; 130: 1459–1465 [DOI] [PubMed] [Google Scholar]
- 5.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut 2012; 61: 1340–1354 [DOI] [PubMed] [Google Scholar]
- 6.Frazzoni M, Conigliaro R, Melotti G. Weakly acidic refluxes have a major role in the pathogenesis of proton pump inhibitor-resistant reflux oesophagitis. Aliment Pharmacol Ther 2011; 33: 601–601 [DOI] [PubMed] [Google Scholar]
- 7.Prakash C, Clouse RE. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2005; 3: 329–334 [DOI] [PubMed] [Google Scholar]
- 8.Savarino E, Tutuian R, Zentilin P, et al. Characteristics of reflux episodes and symptom association in patients with erosive esophagitis and nonerosive reflux disease: study using combined impedance-pH off therapy. Am J Gastroenterol 2010; 105: 1053–1061 [DOI] [PubMed] [Google Scholar]
- 9.Savarino E, Marabotto E, Zentilin P, et al. The added value of impedance-pH monitoring to Rome III criteria in distinguishing functional heartburn from non-erosive reflux disease. Dig Liver Dis 2011; 43: 542–547 [DOI] [PubMed] [Google Scholar]
- 10.Frazzoni M, Conigliaro R, Mirante VG, et al. The added value of quantitative analysis of on-therapy impedance-pH parameters in distinguishing refractory non-erosive reflux disease from functional heartburn. Neurogastroenterol Motil 2012; 24: 141–146 [DOI] [PubMed] [Google Scholar]
- 11.Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100: 283–289 [DOI] [PubMed] [Google Scholar]
- 12.Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55: 1398–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galmiche JP, Zerbib F, Bruley des Varannes S. Review article: respiratory manifestations of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2008; 27: 449–464 [DOI] [PubMed] [Google Scholar]
- 14.Blondeau K, Dupont LJ, Mertens V, et al. Improved diagnosis of gastro-oesophageal reflux in patients with unexplained chronic cough. Aliment Pharmacol Ther 2007; 25: 723–732 [DOI] [PubMed] [Google Scholar]
- 15.Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology 2010; 139: 754–762 [DOI] [PubMed] [Google Scholar]
- 16.Tutuian R, Mainie I, Agrawal A, et al. Nonacid reflux in patients with chronic cough on acid-suppressive therapy. Chest 2006; 130: 386–391 [DOI] [PubMed] [Google Scholar]
- 17.Koufman JA, Aviv JE, Casiano RR, et al. Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg 2002; 127: 32–35 [DOI] [PubMed] [Google Scholar]
- 18.Zerbib F, Stoll D. Management of laryngopharyngeal reflux: an unmet medical need. Neurogastroenterol Motil 2010; 22: 109–112 [DOI] [PubMed] [Google Scholar]
- 19.de Bortoli N, Nacci A, Savarino E, et al. How many cases of laryngopharyngeal reflux suspected by laryngoscopy are gastroesophageal reflux disease-related? World J Gastroenterol 2012; 18: 4363–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116: 254–260 [DOI] [PubMed] [Google Scholar]
- 21.Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA 2005; 294: 1534–1540 [DOI] [PubMed] [Google Scholar]
- 22.Gatta L, Vaira D, Sorrenti G, et al. Meta-analysis: the efficacy of proton pump inhibitors for laryngeal symptoms attributed to gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2007; 25: 385–392 [DOI] [PubMed] [Google Scholar]
- 23.Qadeer MA, Phillips CO, Lopez AR, et al. Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials. Am J Gastroenterol 2006; 101: 2646–2654 [DOI] [PubMed] [Google Scholar]
- 24.Oelschlager BK, Quiroga E, Isch JA, et al. Gastroesophageal and pharyngeal reflux detection using impedance and 24-hour pH monitoring in asymptomatic subjects: defining the normal environment. J Gastrointest Surg 2006; 10: 54–62 [DOI] [PubMed] [Google Scholar]
- 25.Kawamura O, Aslam M, Rittmann T, et al. Physical and pH properties of gastroesophagopharyngeal refluxate: a 24-hour simultaneous ambulatory impedance and pH monitoring study. Am J Gastroenterol 2004; 99: 1000–1010 [DOI] [PubMed] [Google Scholar]
- 26.Hoppo T, Sanz AF, Nason KS, et al. How much pharyngeal exposure is ‘normal’? Normative data for laryngopharyngeal reflux events using hypopharyngeal multichannel intraluminal impedance (HMII). J Gastrointest Surg 2012; 16: 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zerbib F, Roman S, Bruley des Varannes S, et al. Normal values of pharyngeal and esophageal 24-hour pH-impedance on and off therapy and inter-observer reproducibility. Clin Gastroenterol Hepatol 2012 (Epub ahead of print) [DOI] [PubMed]
- 28.Ahmed TF, Khandwala F, Abelson TI, et al. Chronic laryngitis associated with gastroesophageal reflux: prospective assessment of differences in practice patterns between gastroenterologists and ENT physicians. Am J Gastroenterol 2006; 101: 470–478 [DOI] [PubMed] [Google Scholar]
- 29.Mastronarde JG, Anthonisen NR, Castro M, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 2009; 360: 1487–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiljander TO, Harding SM, Field SK, et al. Effects of esomeprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2006; 173: 1091–1097 [DOI] [PubMed] [Google Scholar]
- 31.Iwakiri K, Kawami N, Sano H, et al. Acid and non-acid reflux in Japanese patients with non-erosive reflux disease with persistent reflux symptoms, despite taking a double-dose of proton pump inhibitor: a study using combined pH-impedance monitoring. Journal of Gastroenterology 2009; 44: 708–712 [DOI] [PubMed] [Google Scholar]
- 32.Mainie I, Tutuian R, Agrawal A, et al. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg 2006; 93: 1483–1487 [DOI] [PubMed] [Google Scholar]
- 33.Zerbib F, Roman S, Ropert A, et al. Esophageal pH-impedance monitoring and symptom analysis in GERD: a study in patients off and on therapy. Am J Gastroenterol 2006; 101: 1956–1963 [DOI] [PubMed] [Google Scholar]
- 34.Fass R, Murthy U, Hayden CW, et al. Omeprazole 40 mg once a day is equally effective as lansoprazole 30 mg twice a day in symptom control of patients with gastro-oesophageal reflux disease (GERD) who are resistant to conventional-dose lansoprazole therapy-a prospective, randomized, multi-centre study. Aliment Pharmacol Ther 2000; 14: 1595–1603 [DOI] [PubMed] [Google Scholar]
- 35.Fass R, Sontag SJ, Traxler B, et al. Treatment of patients with persistent heartburn symptoms: a double-blind, randomized trial. Clin Gastroenterol Hepatol 2006; 4: 50–56 [DOI] [PubMed] [Google Scholar]
- 36.Ciccaglione AF, Marzio L. Effect of acute and chronic administration of the GABA B agonist baclofen on 24 hour pH metry and symptoms in control subjects and in patients with gastro-oesophageal reflux disease. Gut 2003; 52: 464–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cossentino MJ, Mann K, Armbruster SP, et al. Randomised clinical trial: the effect of baclofen in patients with gastro-oesophageal reflux – a randomised prospective study. Aliment Pharmacol Ther 2012 (Epub ahead of print) [DOI] [PubMed]
- 38.Koek GH, Sifrim D, Lerut T, et al. Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut 2003; 52: 1397–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boeckxstaens GE, Beaumont H, Mertens V, et al. Effects of lesogaberan on reflux and lower esophageal sphincter function in patients with gastroesophageal reflux disease. Gastroenterology 2010; 139: 409–417 [DOI] [PubMed] [Google Scholar]
- 40.Zerbib F, Keywood C, Strabach G. Efficacy, tolerability and pharmacokinetics of a modified release formulation of ADX10059, a negative allosteric modulator of metabotropic glutamate receptor 5: an esophageal pH-impedance study in healthy subjects. Neurogastroenterol Motil 2010; 22: 859–865 [DOI] [PubMed] [Google Scholar]
- 41.Zerbib F, Bruley des Varannes S, Roman S, et al. Randomised clinical trial: effects of monotherapy with ADX10059, a mGluR5 inhibitor, on symptoms and reflux events in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2011; 33: 911–921 [DOI] [PubMed] [Google Scholar]
- 42.Gerson LB, Huff FJ, Hila A, et al. Arbaclofen placarbil decreases postprandial reflux in patients with gastroesophageal reflux disease. Am J Gastroenterol 2010; 105: 1266–1275 [DOI] [PubMed] [Google Scholar]
- 43.Vakil NB, Huff FJ, Bian A, et al. Arbaclofen placarbil in GERD: a randomized, double-blind, placebo-controlled study. Am J Gastroenterol 2011; 106: 1427–1438 [DOI] [PubMed] [Google Scholar]
- 44.Viazis N, Keyoglou A, Kanellopoulos AK, et al. Selective serotonin reuptake inhibitors for the treatment of hypersensitive esophagus: a randomized, double-blind, placebo-controlled study. Am J Gastroenterol 2012; 107: 1662–1667 [DOI] [PubMed] [Google Scholar]
- 45.Lee H, Kim JH, Min BH, et al. Efficacy of venlafaxine for symptomatic relief in young adult patients with functional chest pain: a randomized, double-blind, placebo-controlled, crossover trial. Am J Gastroenterol 2010; 105: 1504–1512 [DOI] [PubMed] [Google Scholar]
- 46.Broeders JA, Bredenoord AJ, Hazebroek EJ, et al. Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut 2011; 60: 435–441 [DOI] [PubMed] [Google Scholar]
- 47.Broeders JA, Draaisma WA, Bredenoord AJ, et al. Oesophageal acid hypersensitivity is not a contraindication to Nissen fundoplication. Br J Surg 2009; 96: 1023–1030 [DOI] [PubMed] [Google Scholar]
- 48.Anvari M, Bamehriz F. Outcome of laparoscopic Nissen fundoplication in patients with body mass index >or=35. Surg Endosc 2006; 20: 230–234 [DOI] [PubMed] [Google Scholar]
- 49.Patterson EJ, Davis DG, Khajanchee Y, et al. Comparison of objective outcomes following laparoscopic Nissen fundoplication versus laparoscopic gastric bypass in the morbidly obese with heartburn. Surg Endosc 2003; 17: 1561–1565 [DOI] [PubMed] [Google Scholar]
- 50.Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011; 305: 1969–1977 [DOI] [PubMed] [Google Scholar]
- 51.Attwood SE, Lundell L, Ell C, et al. Standardization of surgical technique in antireflux surgery: the LOTUS Trial experience. World J Surg 2008; 32: 995–998 [DOI] [PubMed] [Google Scholar]
- 52.Furnee EJ, Draaisma WA, Broeders IA, et al. Surgical reintervention after failed antireflux surgery: a systematic review of the literature. J Gastrointest Surg 2009; 13: 1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamolz T, Granderath FA, Pointer R. The management of patients who have ‘failed’ antireflux surgery. Am J Gastroenterol 2004; 99: 2070–2071 [DOI] [PubMed] [Google Scholar]
- 54.Spechler SJ. The management of patients who have ‘failed’ antireflux surgery. Am J Gastroenterol 2004; 99: 552–561 [DOI] [PubMed] [Google Scholar]
- 55.Safranek PM, Gifford CJ, Booth MI, et al. Results of laparoscopic reoperation for failed antireflux surgery: does the indication for redo surgery affect the outcome? Dis Esophagus 2007; 20: 341–345 [DOI] [PubMed] [Google Scholar]
- 56.Symons NR, Purkayastha S, Dillemans B, et al. Laparoscopic revision of failed antireflux surgery: a systematic review. Am J Surg 2011; 202: 336–343 [DOI] [PubMed] [Google Scholar]
- 57.Hayden JD, Myers JC, Jamieson GG. Analysis of illness behavior in patients after ‘failed’ antireflux surgery. Arch Surg 2006; 141: 243–246 [DOI] [PubMed] [Google Scholar]