Abstract
Developmental constraint is indicated when one finds that similar genetic mechanisms are responsible for independent origins of the same derived phenotype. We studied three independent origins of rosette flowering within the mustard family and attempted to evaluate the extent to which the same mechanisms were involved in each transition from the ancestral phenotype, inflorescence flowering. We used transformation to move a candidate gene, LFY, and its cis-regulatory sequences from rosette-flowering species into an inflorescence-flowering recipient, Arabidopsis thaliana, in place of its endogenous LFY gene. The transgenic phenotypes of experimental and control lines (containing an A. thaliana LFY transgene) and the expression driven by the cis-regulatory sequences show that changes at the LFY locus might have contributed to the evolution of rosette flowering in two of the three lineages. In the third case, changes upstream of LFY are implicated. Our data suggest that changes in a single developmental regulatory program were involved in multiple origins of the same derived trait but that the specific genetic changes were different in each case.
Parallelism refers to the independent evolution of the same derived trait via the same developmental changes, whereas convergence refers to superficially similar traits that have a distinct developmental basis (1). The most compelling reason to distinguish these two phenomena is because they serve to document different phenomena. Convergence provides evidence of the efficacy of natural selection, whereas the occurrence of parallelism shows that the path of evolution is constrained to certain channels determined by the structure of developmental programs (2, 3).
Strict parallelism, where the identical mutation occurs repeatedly, is documented in cases of biochemical adaptation to toxins (4–6). When dealing with morphology, however, the concept of parallelism is usually relaxed to include cases of different mutations to the same target locus and even changes to different genes within the same developmental pathway. Under this broader definition, parallelism can be elucidated by using comparative gene expression data (7–10). In such studies, however, there is always the concern that changes in gene expression might be far downstream of the ultimate genetic causes of morphological homoplasy. A few studies have used classical genetic data to study homoplasy (11–14), and some studies have provided strong evidence in favor of parallelism (13, 14). Here we use a transgenic approach that is not limited to cases in which study species are crossable (15). Although interspecies transformation has been used to elucidate male song evolution in Drosophila (16) and the origin of self-compatibility in Arabidopsis thaliana (17), it has not to our knowledge been applied to the problem of parallel evolution.
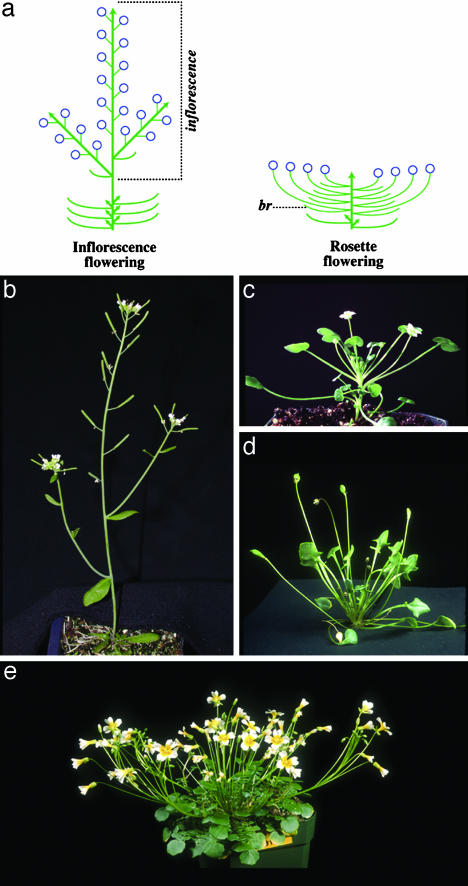
Most species of Brassicaceae, including A. thaliana, bear flowers in an inflorescence, an elongated portion of stem on which the leaves that would otherwise subtend the flowers are suppressed (Fig. 1 a and b). Phylogenies of Brassicaceae (18–20) suggest that inflorescence flowering is ancestral but that a number of independently derived lineages have evolved a striking modification to plant architecture, rosette flowering. Rosette-flowering plants produce solitary flowers in the axils of rosette leaves, and flowers are elevated on elongated pedicels rather than by elongation of internodes in the primary shoot axis (Fig. 1 a and c–e). We investigated three rosette-flowering species: Ionopsidium (Jonopsidium) acaule, Idahoa scapigera, and Leavenworthia crassa. Ionopsidium includes six Mediterranean species, of which two are rosette-flowering. Idahoa contains only one species of diminutive annual from the northwestern United States. Leavenworthia includes eight rosette-flowering, winter annual species from the southeastern United States.
Fig. 1.
Comparison of the plant architecture of inflorescence- and rosette-flowering species. (a) Diagram of inflorescence- and rosette-flowering architectures. Leaves (curved lines), shoot meristems (arrows), and flowers (circles) are shown. Bracts (br) are leaves that directly subtend flowers. (b–e) Photographs of inflorescence-flowering A. thaliana (ecotype Landsberg erecta) (b) and the three rosette-flowering members of Brassicaceae studied here, Ionopsidium acaule (c), Idahoa scapigera (d), and L. crassa (e).
The difference between rosette and inflorescence flowering can be attributed at least in part to differences in the fate of axillary meristems in the rosette, which take on a floral identity in rosette-flowering taxa but a shoot identity in inflorescence-flowering taxa. We therefore focused this study on a candidate locus, LEAFY/FLORICAULA (LFY), that plays a role in the regulation of shoot meristem identity and plant architecture in several model species and has been suggested to be a key player in the evolution of inflorescence architecture (21, 22). lfy mutants show proliferation of inflorescence meristems and the formation of shoot–flower intermediates in place of flowers (23). Ectopic expression of LFY in A. thaliana results in the formation of some rosette flowers (22). Furthermore, prior work on the rosette-flowering Ionopsidium acaule showed that LFY is expressed in the shoot apical meristem (SAM) (24). This contrasts with A. thaliana and other plants with racemose inflorescences, which show LFY expression in flowers and young leaves but never in the inflorescence meristem (23, 25).
LFY is orthologous to the Antirrhinum gene FLO (25) and encodes a DNA-binding transcription factor that promotes floral meristem identity by activating floral organ identity genes (23, 26). Vegetative SAMs of A. thaliana are prevented from taking on a floral identity by the activity of TFL1, which indirectly represses LFY (27). Because LFY also represses TFL1, the balance of expression of these two genes is thought to play a key role in determining the floral versus vegetative fate of SAMs (21, 22).
In this study we cloned LFY homologs with intact cis-regulatory regions from the three rosette-flowering species and introduced them into lfy mutant A. thaliana plants. Comparison of the resulting transgenic lines with control lines containing the A. thaliana LFY gene plus its cis-regulatory sequences was made to see whether the exogenous LFY genes can rescue lfy mutants and, if so, whether the transgenic plants show features normally associated with rosette flowering.
Materials and Methods
Plant Materials. Ionopsidium acaule lines were described previously (24). Seeds of Idahoa scapigera were collected by D.A.B. in Pullman, WA (voucher, Baum 365; Gray Herbarium, Cambridge, MA). Seeds of L. crassa were obtained from a cultivated source (voucher, Baum 379; Wisconsin State Herbarium, Madison). Wild-type and lfy-6 A. thaliana seeds were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus).
Cloning, Transformation, and Phenotype Scoring. We amplified partial LFY homologs from genomic DNA using a series of degenerate primers situated in highly conserved regions of the gene. This strategy has been used successfully to isolate LFY homologs from a diversity of other Brassicaceae (H.-S.Y., R. Oldham, and D.A.B., unpublished data), suggesting that this approach should allow one to identify all functional LFY paralogs. Amplified products were cloned and sequenced, and the data were used to design primers for genome walking (Clontech), allowing us to obtain flanking genomic sequences both 5′ and 3′ of the coding region. Based on this extended region of sequence, primers were designed to amplify an ≈7-kb DNA fragment of each gene containing ≈3 kb upstream of the LFY ORF and ≈1 kb downstream. These fragments likely contain a majority of the important cis-regulatory elements because 2.3 kb of 5′ noncoding DNA has been reported to contain all elements necessary for proper expression of LFY in A. thaliana (28). Using high-fidelity PCR (PCR using Pfu DNA polymerase, Stratagene) and UV-free gel extraction (Invitrogen), we cloned AthLFY, IacLFY (GenBank accession no. AY219226), IscLFY1 (GenBank accession no. AY219228), and LcrLFY (GenBank accession no. AY219227) in the pCR-Blunt II-TOPO vector (Invitrogen). Six independent LFY clones from each rosette-flowering species (three in each orientation) and four clones from A. thaliana (two in each orientation) were then moved into the binary vector pCAMBIA3300, which includes the BAR selectable marker conferring Basta resistance.
A. thaliana Landsberg erecta heterozygous lfy-6/LFY plants were transformed with the 22 clones by using the floral dip method (29). An empty vector pCAMBIA3300 was used as an additional control. T1 seeds were selected by spraying 0.2 mg/liter Basta. Resistant plants were genotyped at the endogenous LFY locus by using cleaved amplified polymorphic sequences (CAPS) markers developed by D. Weigel (www.salk.edu/LABS/pbio-w/CAPS.html). The primers used did not amplify the exogenous genes (IacLFY, IscLFY1, or LcrLFY). To genotype the endogenous LFY locus for AthLFY transgenic lines, we developed a new primer set [forward, 5′-GGT TCC TCC CTA AAA ACT CTT CAA AAT CCC-3′, and reverse, 5′-GTC CCT CTA AAC CAC CAA GTC GCA TCC C-3′). The forward primer is situated outside the cloned region, thus ensuring that only the endogenous LFY is amplified, allowing CAPS analysis to be conducted by means of a second PCR. Wild-type and lfy-6 plants were distinguished by using the restriction enzyme BstAPI, which generates 345- and 25-bp fragments for the wild type and a single 370-bp product for lfy-6. We selected resistant T1 plants that were determined to be lfy-6/lfy-6 at the endogenous locus and used them for analysis in the T2 generation. We characterized T2 populations in long-day conditions (16 h light/8 h dark). The segregation ratio of Basta-sensitive/Basta-resistant in T2 and T3 plants was used to estimate the number of transgene loci (in most cases, one or two).
GUS Fusion Constructs and Staining. The 5′ fragment of the genes was amplified by high-fidelity PCR from genomic DNA using the same forward primers used to clone the 7-kb LFY fragments and reverse primers that anneal to the middle of the first exon of each LFY homolog. The resulting 3.3-kb fragments were cloned into pCR-Blunt II-TOPO vector (Invitrogen) and subsequently moved to pBI101 vector (Clontech) in frame. Wild-type A. thaliana plants were transformed by the floral dip method, and transformants were selected on agar plates in Murashige and Skoog medium (ICN) containing 50 μg/ml kanamycin. GUS expression was determined in whole-mount shoot apices. Tissue was incubated in 0.5 mg/ml X-Gluc staining solution (50 mM potassium phosphate buffer, pH 7.2/0.1% Triton X-100/0.1% 2-mercaptoethanol/4 mM potassium ferricyanide/4 mM potassium ferrocyanide), first on ice under vacuum for 15 min, then at 37°C for 12 h. Then tissues were transferred and incubated in 70% (vol/vol) ethanol at 4°C until the chlorophyll was completely extracted. Stained whole-mount specimens were examined and photographed with an Olympus SZX12 dissecting microscope.
Analysis of Cis-Regulatory and Intron Sequences. Alignments of the cis-regulatory regions were conducted manually in macclade version 4.05 (30) with the help of the motif-searching algorithms implemented in meme (http://meme.sdsc.edu/meme/website/meme.html). For AthLFY and LcrLFY, a pairwise alignment was generated that spanned the entire intergenic region 5′ of LFY. For IscLFY1 and IacLFY, it was only possible to align the ≈350 bp 5′ of the LFY start codon. Possible regulatory elements were detected by using the PLACE sequence scan web server (ref. 31; www.dna.affrc.go.jp/htdocs/PLACE) or manually in macclade version 4.05.
Results
LFY from Ionopsidium acaule Replaces A. thaliana LFY Function. One LFY ortholog, here named IacLFY, was present in Ionopsidium acaule [the two sequences reported previously, under the gene name vcLFY (24), seem to be alleles (data not shown)]. For five of the six IacLFY constructs, T1 transgenic plants showed rescue of the lfy mutant phenotype, as indicated by the production of flowers with petals and stamens in lfy-6 homozygous plants. No systematic differences were seen between these constructs. The sixth line, however, never rescued the lfy-6 phenotype, most likely because of a mutation introduced into the transgene during cloning. We focused on eight A. thaliana lfy-6 lines with high fertility, representing all five functional constructs. These lines showed some floral defects (data not shown), reminiscent of weak ap2 mutations (32), but showed a wild-type A. thaliana plant architecture (Fig. 2a), similar to control lines in which lfy-6 was rescued with the AthLFY transgene (data not shown). This result suggests that the IacLFY locus did not change significantly during the evolution of rosette flowering. Consistent with this result, the 5′ regulatory sequences of IacLFY drove reporter gene expression in a pattern (Fig. 3a) similar to that reported for A. thaliana (28) but quite different from the native expression of IacLFY, which includes the SAM (24). These data imply that normal expression of IacLFY in Ionopsidium acaule requires trans-acting regulatory elements. Thus, under the hypothesis that altered expression of IacLFY contributes to the development of rosette flowering (24), this effect must be mediated by genetic changes that lie upstream of LFY.
Fig. 2.
Phenotypes of A. thaliana lfy mutants containing LFY transgenes. (a) IacLFY replaces endogenous AthLFY function, resulting in a wild-type architecture. (b–d) Compared with inflorescences from control AthLFY transformants (b), inflorescence elongation was inhibited in IscLFY1 transformants (c), and bracts (arrows) are often formed (d). (e and f) IscLFY1 lines showed modified floral architecture demonstrating an apetal1-like phenotype (e) and a reduction in petal number to an average of 1.38 ± 0.50, often showing complete apetaly (f). (g) Aerial rosettes are produced on some secondary inflorescence shoots of IscLFY1 transgenic plants (arrow). (h) The structure of one dissected rosette (Upper) is diagrammed (Lower) to show that each fruit/flower (yellow) has a subtending bract (green) indicated by a linking red line. (i) LcrLFY rescued the lfy mutation but caused flowers with 6.65 ± 1.27 petals rather than the typical four petals (sepal and stamen number is unchanged). (j) LcrLFY modifies inflorescence architecture in A. thaliana. (k) All of the inflorescences are prematurely terminated by partial, terminal flowers, resulting in the release of determinate axillary shoots from the rosette.
Fig. 3.
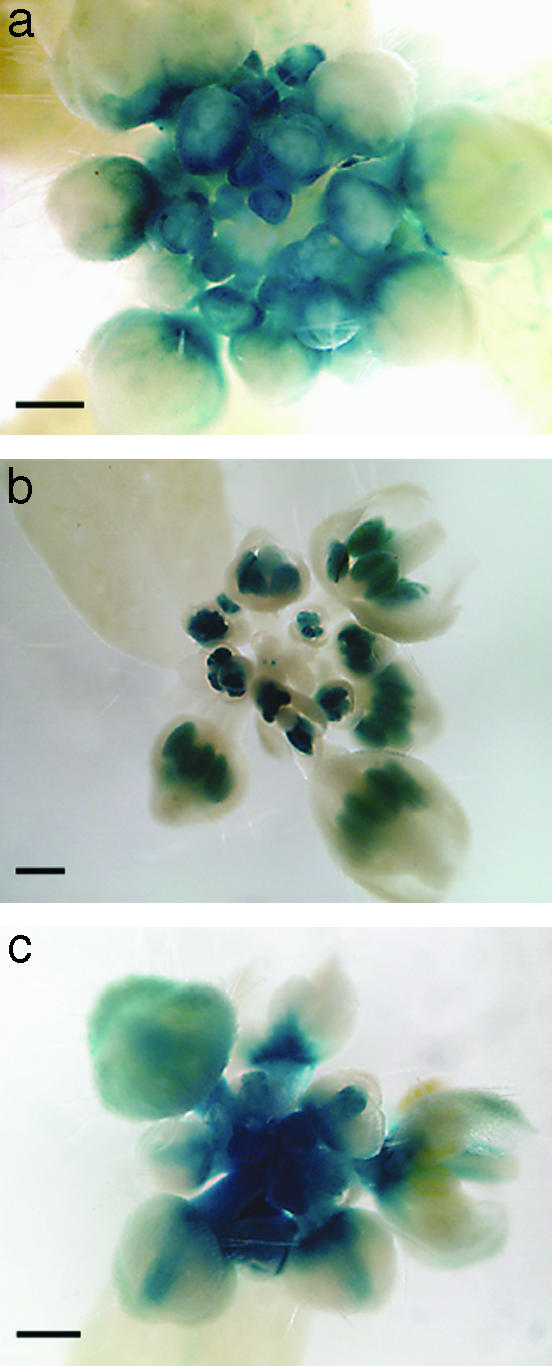
Expression of LFY:GUS transgenes in wild-type A. thaliana plants. Inflorescence apices are shown. (a) IacLFY::GUS activity is detected in flower primordia and maintained mainly in sepals until approximately stage 7. GUS activity is limited to the bases of the sepals in later stages of the development. Note the absence of IacLFY::GUS expression in the SAM. (b) IscLFY1::GUS activity is visible in the carpel and stamen primordia until stage 9 and persists mostly in stamens at later stages of floral development. IscLFY1::GUS expression is absent from the SAM. (c) LcrLFY::GUS is strongly expressed in the SAM and throughout floral primordia. Expression gradually declines from stage 5 flowers but is maintained in carpels and petals. Stages of flower development are according to Smyth et al. (40). (Scale bars = 0.5 mm.)
LFY from Idahoa scapigera Produced Aspects of Rosette Flowering in A. thaliana. There are two LFY genes in Idahoa scapigera (IscLFY1 and -2), but only IscLFY1 was studied here. We examined 16 independent A. thaliana lines derived from all six IscLFY1 constructs. T1–T3 plants homozygous for the lfy-6 allele but containing IscLFY1 transgenes produced stamens, indicating at least partial rescue (Fig. 2f). However, the IscLFY1 lines (unlike the control AthLFY lines) showed reduced petal number or complete apetaly, and most lines produced flowers resembling apetala1 (ap1) mutants (33) (Fig. 2e). One interpretation is that rescue of the lfy-6 mutation was incomplete in the outer floral whorls, resulting in the maintenance of inflorescence identity in this region. This phenomenon likely results from a failure of the IscLFY1 upstream sequences to drive expression in the perianth: the IscLFY1 5′ regulatory sequences drive reporter gene expression only in the stamen and carpel whorl (Fig. 3b). It will therefore be interesting to determine whether the promoter region of the second Idahoa LFY gene, IscLFY2, drives a complementary expression pattern, with strong activity in the perianth.
Fourteen IscLFY1 lines produced some plants with altered inflorescence architecture: shoots had noticeably shorter internodes (compare Fig. 2 b and c), and in many cases leaves subtended these flowers (Fig. 1a and Fig. 2d). Furthermore, an average of 1.52 shoots per plant produced an extreme phenotype in which internode elongation was inhibited to such a degree that an aerial flowering rosette was formed (Fig. 2g). Superficially similar aerial rosettes have been reported in natural and mutant A. thaliana plants (34, 35). The latter structures comprise lateral inflorescences that make a rosette of vegetative leaves without flowers and, when a reproductive transition occurs, produce normal, elongated inflorescences with ebracteate flowers. In contrast, the aerial flowering rosettes of IscLFY1 lines are in a reproductive state, being composed of bracteate (leaf-subtended) flowers (Fig. 2h). Thus, whereas the aerial rosettes reported previously are architecturally equivalent to inflorescence-flowering plants, the aerial flowering rosettes we observed resemble entire rosette-flowering plants. These observations suggest that a change at the IscLFY1 locus might have contributed to the evolution of rosette flowering by suppressing internode elongation and derepressing bracts. The fact that IscLFY1 converts only secondary shoots to rosette flowering rather than the whole plant implies that additional interacting genetic factors (possibly IscLFY2) also changed during the evolution of rosette flowering.
LFY from L. crassa also Altered A. thaliana Architecture. L. crassa has a single LFY gene (LcrLFY). One of the constructs seemed to have acquired a null mutation in the LcrLFY region during the PCR or the subsequent cloning process, because the Basta-resistant lines all showed a lfy-6 phenotype. The remaining five constructs showed an ability to rescue the lfy-6 mutation; however, because of mortality in these lines (probably unrelated to the transgenes), we were only able to establish six independent transgenic lines (representing four constructs). These lines showed good rescue of the floral defects of the lfy-6 mutation, but three lines from two constructs produced flowers with additional petals (Fig. 2i). Two lines (from two constructs) showed a wild-type architecture, one line produced slow-growing and small plants with a wild-type architecture, and the other three lines (from two constructs) manifested a highly modified plant form. These plants produced partial terminal flowers (Fig. 2k) on the main axis after 6–9 lateral flowers had been produced. Secondary inflorescence branches also had terminal flowers, but the number of flowers produced before termination gradually increased during development (Fig. 2j). This phenotype somewhat resembles rosette flowering in that the axillary shoots in the rosette produce determinate rather than indeterminate axes (see Discussion).
The production of terminal flowers in LcrLFY lines resembles mutations in TFL1, a gene that acts to down-regulate LFY transcription in SAMs (21, 36, 37). Because the LcrLFY upstream sequences drive expression both in flower primordia and in the SAM (Fig. 3c), the observed phenotype could be due to a loss of TFL1-responsive cis-regulatory elements. Analysis of the upstream sequences revealed several potential regulatory elements that are missing in LcrLFY and should be considered as possible targets of TFL1-mediated repression in the SAM (Fig. 5, which is published as supporting information on the PNAS web site). For example, LcrLFY lacks the C box bZIP binding site that falls within the “distal fragment” of AthLFY that is required for normal expression (28).
Discussion
Introduction of LFY from the three rosette-flowering lineages into an A. thaliana lfy genetic background resulted in the rescue of lfy mutant phenotypes, at least with regard to stamen development and the production of intact flowers early in plant development (lfy mutants do not make flowers until late in development, and these lack both petals and stamens). This result implies a high degree of functional conservation in the LFY gene product, as suggested previously (e.g., ref. 22). With IscLFY1 and LcrLFY, however, several transgenic lines manifested an architecture that is distinct from weak lfy mutant phenotypes. These results cannot be caused by changes in gene copy number because they were not observed in control lines containing the AthLFY transgene introduced by the same protocol, and they do not resemble the effects of adding extra copies of LFY, which primarily involve changes in flowering time (38). The altered shoot morphology can therefore be attributed to changes at the LFY locus that have evolved since the divergence of A. thaliana and Idahoa/Leavenworthia. Although these changes could have occurred on any phylogenetic branch between the rosette-flowering species and A. thaliana, we would suggest that, if the transgenes confer aspects of rosette flowering, it is most parsimonious to assume that the causal genetic changes occurred coincidentally with the evolution of rosette flowering. This inference should be tested ultimately by conducting equivalent experiments with the LFY genes of inflorescence-flowering mustard species.
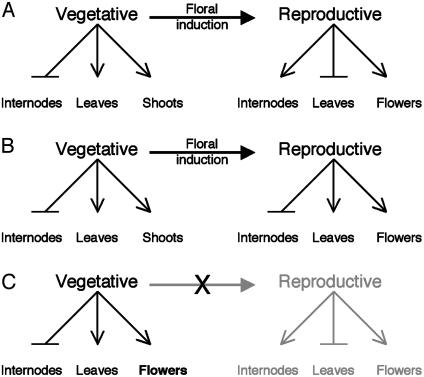
To provide a framework for deciding whether transgenic phenotypes can be considered “aspects of rosette flowering,” we present a simple developmental model. Inflorescence flowering in Brassicaceae can be represented by a two-stage program (Fig. 4A) involving (i) a vegetative phase during which the SAMs produce short internodes, expanded leaves, and axillary meristems with vegetative identity (paraclades) and (ii) a reproductive phase during which SAMs produce long internodes, lack leaves (bracts), and produce axillary meristems with a floral identity. Although this model is oversimplified (for example, internode elongation spreads into the vegetative zone, resulting in the production of elevated secondary inflorescences and cauline leaves; ref. 39), it provides a basis for identifying two distinct paths by which rosette flowering could evolve. The first possibility is that the reproductive phase was modified such that internodes no longer expand and bracts are no longer suppressed (Fig. 4B). The alternative possibility is that the transition to the reproductive phase is postponed indefinitely but that flowering takes places within the rosette as a result of the homeotic conversion of axillary SAMs into flowers (Fig. 4C). Because both scenarios would result in the same phenotypic end point, they can only be distinguished by reference to intermediate steps in the evolutionary process. Such intermediate steps might be uncovered by using a transgenetic strategy involving the transformation of A. thaliana plants with only one of several genes that changed during the evolution of rosette flowering. Thus, under the first scenario, transformation experiments could result in plants showing internode suppression and bract production within the inflorescence, whereas the second scenario predicts partial conversion of axillary rosette shoots to floral identity.
Fig. 4.
Developmental model for the evolution from inflorescence flowering (A) to rosette flowering (B and C). Bold arrows mark the transition (floral induction) from the vegetative to the reproductive phase. Within a developmental phase, an arrow indicates promotion of the indicated developmental unit, whereas bars indicate inhibition. Rosette flowering can evolve either by altering the rules of development during the reproductive phase (B), as inferred for Idahoa, or by arresting development in the rosette stage but producing ectopic flowers in place of axillary shoots (C). See text for explanation.
Introduction of IscLFY1 into Arabidopsis resulted in a tendency for internode compression and bract derepression within the inflorescence. This result suggests that the evolution of rosette flowering in Idahoa involved the developmental mechanism shown in Fig. 4B. The fact that an incomplete conversion to rosette flowering occurred shows that IscLFY1 is one, but not the only, gene that causes rosette flowering. An obvious candidate for partnership in the evolutionary transition is IscLFY2. It is plausible, therefore, that IscLFY1/IscLFY2 doubly transgenic A. thaliana would show a more extreme conversion to rosette flowering.
In the case of LcrLFY, we observed conversion of axillary meristems to a more determinate fate caused by ectopic accumulation of LFY transcript. Although this phenotype is consistent with the second developmental model for the evolution of rosette flowering, other genetic factors are clearly missing, as shown by the fact that the determinate structures are short shoots rather than single flowers. Furthermore, to explain the phenotype of wild-type Leavenworthia, which lacks terminal flowers, there must be mechanisms in Leavenworthia that prevent the production of terminal flowers on the primary shoot meristem.
In the case of Ionopsidium, the failure to alter plant architecture suggests that the evolution of rosette flowering did not involve changes at the LFY locus per se. Nonetheless, the fact that the native expression pattern of IacLFY (24) is similar to the expression driven by the LcrLFY promoter implies that Ionopsidium evolved rosette flowering via a similar developmental mechanism, but, in this case, trans-, rather than cis-, regulation was responsible. Candidate trans-regulatory loci in Ionopsidium include AP1 and TFL1.
Taken together, our data suggest but by no means prove that the IscLFY1 and LcrLFY loci contributed directly to the evolution of rosette flowering. Additionally, our study makes plausible the hypothesis that the evolution of rosette flowering in Ionopsidium acaule resulted from changes in the trans-regulation of IacLFY. Although it remains a formal possibility that changes at the LFY locus arose independently of the origin of rosette flowering (and only coincidentally mimic aspects of rosette flowering when placed in Arabidopsis), the level of certainty achieved in this study is far greater than what could have been achieved by using only comparative gene expression studies, illustrating the potential value of the evolutionary transgenic strategy.
What do our data say about parallelism? If for parallelism one requires that the same genetic changes occurred in different lineages, our data would refute parallelism. In only two of the three lineages is the LFY locus itself implicated, and in these two cases our data suggest alternate development mechanisms (Fig. 4). Nonetheless, we wish to propose that the evolution of rosette flowering is most readily understood within the framework of parallelism and developmental constraint. The possibility that LFY has played a role in three independent origins of the same phenotype (two involving changes at the LFY locus, one involving upstream changes) suggests that this gene could be situated at such a position in the developmental program that it can readily respond to selection for different plant architectures. If evolutionary constraint were minimal, it would be inconceivable that the same gene should be involved, even indirectly, in three of three independent origins of the same trait. Therefore, the repeated involvement of LFY implied by our data is incommensurable with a claim of strict convergence but rather suggests developmental constraint and hence parallelism (3). Thus, despite the obvious capacity to achieve the same end point by means of different genetic changes, our results show that the evolution of morphology is not random but highly structured by the intrinsic organization of developmental programs.
Supplementary Material
Acknowledgments
We thank R. Oldham, K. C. Walsh, and R. Warrich for technical assistance; F. Ausubel, A. B. Bleecker, C. Day, J. F. Doebley, D. Fernandez, J. C. Pires, E. A. Kellogg, L. C. Hileman, M.-C. Kim, E. Kramer, and D. Weigel for useful discussions; the late R. Rollins for seeds of L. crassa; R. Oldham, S. D. Smith, D. L. Stern, and three anonymous reviewers for comments on the manuscript; and C. Lipke and K. Elliot for assistance with photography and artwork. This research was supported by National Science Foundation Grants 0078161 and 0234118.
Abbreviation: SAM, shoot apical meristem.
Data deposition: The sequences reported in this article have been deposited in the GenBank database (accession nos. AY219226–AY219228).
References
- 1.Patterson, C. (1982) in Morphological Characters and Homology, eds. Joysey, K. A. & Friday, A. E. (Academic, London), pp. 21-74.
- 2.Maynard Smith, J., Burian, R., Kauffman, S., Alberch, P., Campbell, J., Goodwin, B., Lande, R., Raup, D. & Wolpert, L. (1985) Q. Rev. Biol. 60, 265-287. [Google Scholar]
- 3.Gould, S. J. (2002) The Structure of Evolutionary Biology (Belknap, Cambridge, MA).
- 4.Crandall, K., Kelsey, C., Imamichi, H., Lane, H. & Salzman, N. (1999) Mol. Biol. Evol. 16, 372-382. [DOI] [PubMed] [Google Scholar]
- 5.Bernasconi, P., Woodworth, A. R., Rosen, B. A., Subramanian, M. W. & Siehl, D. L. (1995) J. Biol. Chem. 270, 17381-17385, and erratum (1996) 271, 13925. [DOI] [PubMed] [Google Scholar]
- 6.Thompson, M., Shotkoski, F. & ffrench-Constant, R. (1993) FEBS Lett. 325, 187-190. [DOI] [PubMed] [Google Scholar]
- 7.Burke, A. C., Nelson, C. E., Morgan, B. A. & Tabin, C. (1995) Development (Cambridge, U.K.) 121, 333-346. [DOI] [PubMed] [Google Scholar]
- 8.Averof, M. & Patel, N. (1997) Nature 388, 682-686. [DOI] [PubMed] [Google Scholar]
- 9.Bharathan, G., Goliber, T. E., Moore, C., Kessler, S., Pham, T. & Sinha, N. R. (2002) Science 296, 1858-1860. [DOI] [PubMed] [Google Scholar]
- 10.Gompel, N. & Carroll, S. B. (2003) Nature 424, 931-935. [DOI] [PubMed] [Google Scholar]
- 11.Wittkopp, P. J., Vaccaro, K. & Carroll, S. B. (2002) Curr. Biol. 12, 1547-1556. [DOI] [PubMed] [Google Scholar]
- 12.Hoekstra, H. E. & Nachman, M. W. (2003) Mol. Ecol. 12, 1185-1194. [DOI] [PubMed] [Google Scholar]
- 13.Sucena, E., Delon, I., Jones, I., Payre, F. & Stern, D. L. (2003) Nature 424, 935-938. [DOI] [PubMed] [Google Scholar]
- 14.Eizirik, E., Yuhki, N., Johnson, W. E., Menotti-Raymond, M., Hannah, S. S. & O'Brien, S. J. (2003) Curr. Biol. 13, 448-453. [DOI] [PubMed] [Google Scholar]
- 15.Baum, D. A. (2002) in Developmental Genetics and Plant Evolution, eds. Cronk, Q. C. B., Bateman, R. M. & Hawkins, J. A. (Taylor & Francis, London), pp. 493-507.
- 16.Wheeler, D. A., Kyriacou, C. P., Greenacre, M. L., Yu, Q., Rutila, J. E., Rosbash, M. & Hall, J. C. (1991) Science 251, 1082-1085. [DOI] [PubMed] [Google Scholar]
- 17.Nasrallah, M. E., Liu, P. & Nasrallah, J. B. (2002) Science 297, 247-249. [DOI] [PubMed] [Google Scholar]
- 18.Koch, M., Haubold, B. & Mitchell-Olds, T. (2001) Am. J. Bot. 88, 534-544. [PubMed] [Google Scholar]
- 19.Koch, M., Haubold, B. & Mitchell-Olds, T. (2000) Mol. Biol. Evol. 17, 1483-1498. [DOI] [PubMed] [Google Scholar]
- 20.Galloway, G., Malmberg, R. & Price, R. (1998) Mol. Biol. Evol. 15, 1312-1320. [DOI] [PubMed] [Google Scholar]
- 21.Bradley, D., Ratcliffe, O., Vincent, C., Carpenter, R. & Coen, E. (1997) Science 275, 80-83. [DOI] [PubMed] [Google Scholar]
- 22.Weigel, D. & Nilsson, O. (1995) Nature 377, 495-500. [DOI] [PubMed] [Google Scholar]
- 23.Weigel, D., Alvarez, J., Smyth, D. R., Yanofsky, M. F. & Meyerowitz, E. M. (1992) Cell 69, 843-859. [DOI] [PubMed] [Google Scholar]
- 24.Shu, G., Amaral, W., Hileman, L. C. & Baum, D. A. (2000) Am. J. Bot. 87, 634-641. [PubMed] [Google Scholar]
- 25.Coen, E. S., Romero, J. M., Doyle, S., Elliott, R., Murphy, G. & Carpenter, R. (1990) Cell 63, 1311-1322. [DOI] [PubMed] [Google Scholar]
- 26.Parcy, F., Nilsson, O., Busch, M. A., Lee, I. & Weigel, D. (1998) Nature 395, 561-566. [DOI] [PubMed] [Google Scholar]
- 27.Ratcliffe, O. J., Amaya, I., Vincent, C. A., Rothstein, S., Carpenter, R., Coen, E. S. & Bradley, D. J. (1998) Development (Cambridge, U.K.) 12, 1609-1615. [DOI] [PubMed] [Google Scholar]
- 28.Blazquez, M. A. & Weigel, D. (2000) Nature 404, 889-892. [DOI] [PubMed] [Google Scholar]
- 29.Clough, S. J. & Bent, A. F. (1998) Plant J. 16, 735-743. [DOI] [PubMed] [Google Scholar]
- 30.Maddison, D. R. & Maddison, W. P. (2002) macclade (Sinauer, Sunderland, MA), Version 4.05.
- 31.Higo, K., Ugawa, Y., Iwamoto, M. & Korenaga, T. (1999) Nucleic Acids Res. 27, 297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jofuku, K. D., den Boer, B. G., Van Montagu, M. & Okamuro, J. K. (1994) Plant Cell 6, 1211-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irish, V. F. & Sussex, I. M. (1990) Plant Cell 2, 741-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grbic, V. & Bleecker, A. B. (1996) Development 122, 2395-2403. [DOI] [PubMed] [Google Scholar]
- 35.Poduska, B., Humphrey, T., Redweik, A. & Grbic, V. (2003) Genetics 163, 1457-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shannon, S. & Meeks-Wagner, D. R. (1991) Plant Cell 3, 877-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradley, D., Vincent, C., Carpenter, R. & Coen, E. (1996) Development (Cambridge, U.K.) 122, 1535-1544. [DOI] [PubMed] [Google Scholar]
- 38.Blázquez, M., Soowal, L., Lee, I. & Weigel, D. (1997) Development (Cambridge, U.K.) 119, 721-743. [DOI] [PubMed] [Google Scholar]
- 39.Hempel, F. D. & Feldman, L. J. (1995) Plant J. 8, 725-731. [DOI] [PubMed] [Google Scholar]
- 40.Smyth, D. R., Bowman, J. L. & Meyerowitz, E. M. (1990) Plant Cell 2, 755-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.