Abstract
The female perspective on reproductive strategies remains one of the most active areas of debate in biology. Even though a single mating is often sufficient to satisfy the fertilization needs of most females and the act of further mating incurs costs, multiple paternity within broods or clutches is a common observation in nature. Direct or indirect advantage to females is the most popular explanation. However, the ubiquity of this explanation is being challenged by an increasing number of cases for which benefits are not evident. For the first time, we test possible fitness correlates of multiple paternity in a marine turtle, an organism that has long attracted attention in this area of research. Contrary to the wide-spread assumption that multiple mating by female marine turtles confers fitness benefits, none were apparent. In this study, the environment played a far stronger role in determining the success of clutches than whether paternity had been single or multiple. A more likely explanation for observations of multiply sired clutches in marine turtles is that these are successful outcomes of male coercion, where females have conceded to superfluous matings as a compromise. Thus, multiple matings by female marine turtles may be a form of damage control as females attempt to make the best of a bad job in response to male harassment.
Mating once or many times is a critical decision that may have significant impact on an individual's fitness (1). The advantage to males of mating as often as possible is obvious. A more critical aspect that is required in understanding the evolution of reproductive strategies is the female's choice to mate multiple times. It is an interesting problem, because, for females, the advantage of multiple mating is less understandable. A single mating is often sufficient for fertilization and does not limit female reproductive success (2), whereas the act of mating is risky and costly to females. Nevertheless, female promiscuity is sufficiently common in nature that it is now widely assumed that there are fitness benefits resulting in positive selection for this behavior. Explanations range from fertilization assurance, sperm selection, and trading-up, to indirect benefits such as increasing the genetic variation of offspring (see hypotheses and associated references in refs. 1 and 3). Indeed, benefits to females have been demonstrated for a variety of species (3–5).
These explanations are compelling, but cases are increasing in which benefits to females cannot be found. Indeed, male mating strategies can be a powerful force in influencing patterns of multiple paternity within clutches (6). An alternative hypothesis based on sexual conflict better addresses occurrences of polyandry that are not beneficial for females. This hypothesis suggests that some instances of female multiple mating are in fact driven by male pressure to copulate as often and with as many females as possible. Females should be reluctant to mate more than is required for fertilization. However, because male coercion also incurs costs, a threshold of harassment may exist beyond which females would choose to give in to male pressure despite the costs of these superfluous matings, in a strategy of “making the best of a bad job” (7). Therefore, observing that females choose to mate multiple times does not necessarily indicate that they gain greater fitness benefits than those achieved by single mating.
Concerning the question of female promiscuity, marine turtles have attracted much attention. They appear to be ideal examples. Males do not contribute to reproduction beyond the provision of sperm, and observations of courtship behavior strongly suggest the likelihood of female multiple mating. The first study to confirm the occurrence of multiple paternity within clutches of marine turtle offspring (8) immediately prompted speculation that females gained fitness benefits from polyandrous behavior (9). Ever since, many more studies have documented the incidence of multiply fathered clutches for a variety of marine turtle populations: Caretta caretta (loggerhead) (10, 11), Lepidochelys olivacea (olive ridley) (12), Lepidochelys kempi (Kemp's ridley) (13), Dermochelys coriacea (leatherback) (14–18), and Chelonia mydas (green) (19–22). In cases where multiple paternity had been detected, benefit to females has been the most common explanation.
In this study, we determined the frequency of multiple paternity among clutches of offspring for the green turtle population of Ascension Island. Previously, with a limited sample, we had established that multiple paternity occurred in this population (22), but here we provide the first extensive dataset together with measures of reproductive success. Because female green turtles control mating and males cannot force copulation (23), multiple paternity is only possible where the female has chosen to mate more than once. Such choices would suggest that females might benefit from polyandry. The association between multiple paternity and indicators of reproductive success was therefore investigated. This study is an attempt in ascertaining the fitness correlates of multiple mating by female marine turtles in a wild population. It is a critical advance on previous work because other studies have invoked the hypothesis of benefits to females as the most likely explanation for the observed incidence of multiple paternity in marine turtles, but none had empirical evidence to support this hypothesis.
Materials and Methods
Green turtles migrate to Ascension Island (7°57′S, 14°22′W), an isolated peak on the mid-Atlantic ridge, to breed and lay their eggs. Samples were collected during two breeding seasons: January to April 1999 and December 1999 to April 2000. In addition to paternity, other factors may influence reproductive success. We have shown that the thermal properties of the sand found on the beach can influence hatchling phenotype (24). Darker sand leads to warmer conditions for egg incubation (25). Adult female turtles and their offspring were thus sampled at three beaches: Long Beach (LB), North East Bay (NEB), and South West Bay (SWB) (26), where NEB has darker sand (warmer) than LB and SWB (cooler). Also, studies of other reptiles have found associations between clutch and female sizes (27). Information on adult female size (curve carapace length) was also recorded (see ref. 24 for method), in addition to clutch sizes and hatching and fertilization successes.
Field Methods. Adults were sampled by tissue biopsies. Biopsies were taken by using a 6-mm sterile skin biopsy punches (Steifel Laboratories, High Wycombe, U.K.) along the trailing edge of the foreflipper. Tagging ensured that duplicate samples were not taken. All adult females sampled were those that had come up onto beaches for nesting purposes. Blood or tissue had also been taken from the offspring of some females. Locations of incubating clutches were marked, and these locations were excavated at the first sign of hatching (see refs. 24 and 28 for methods). Nondestructive blood sampling of hatchlings was carried out according to established protocols (29). No more than 0.1 ml of blood was taken from the dorsal cervical sinus. Blood samples were preserved in an approximately equal volume of buffered solution (50 mM EDTA/2% SDS/10 mM NaCl/50 mM Tris·HCl at pH 8) at room temperature in the field and later stored frozen. Tissue samples were also taken from dead hatchlings found in the nest and from dead embryos of eggs that had failed to hatch. These samples were fixed in absolute ethanol in the field and later frozen.
Microsatellite Genotyping. Microsatellite data for five loci were obtained for 18 clutches of offspring and their mothers. Other adult females were also genotyped to provide population allele frequencies (see details in Supporting Text, which is published as supporting information on the PNAS web site). Clutches were sampled from all three beaches in 1999, but only from LB and NEB in the next breeding season (Table 1). Green turtle clutches are large (e.g., 42–170 eggs in this study) and could not been sampled to completion. Between 20% and 94% of offspring in each clutch were genotyped (Table 1). For all clutches, some offspring were genotyped for all five loci, but in some instances several offspring had been genotyped for only two loci (Table 1). The model of Neff and Pitcher (30) was used to assess the statistical power of detecting multiple paternity in each clutch. This model takes into account the number of offspring and loci sampled, the genotype of the known parent, and the population allele frequencies of the loci in question.
Table 1. Sample sizes (total N) for genotyped offspring.
| Nest code | Beach | Total N | Clutch genotyped, % | PrDM for 2 loci (N) | PrDM for 5 loci (N) |
|---|---|---|---|---|---|
| 1999 | |||||
| TP36 | LB | 32 | 29 | — | 1.000/1.000 (32) |
| TP39 | LB | 35 | 27 | 0.921/0.878 (8) | 1.000/0.999 (27) |
| TP5 | NEB | 55 | 52 | — | 1.000/1.000 (55) |
| TP44 | NEB | 44 | 27 | — | 1.000/0.999 (44) |
| TP51 | NEB | 31 | 94 | — | 1.000/0.999 (31) |
| TP53 | NEB | 59 | 59 | — | 1.000/0.999 (59) |
| TP48 | SWB | 43 | 44 | 0.975/0.970 (20) | 1.000/0.999 (23) |
| Total | 299 | ||||
| 2000 | |||||
| TT1 | LB | 56 | 51 | — | 1.000/1.000 (56) |
| TT2 | LB | 31 | 29 | 0.966/0.959 (17) | 0.999/0.994 (14) |
| TT4 | LB | 50 | 51 | 0.968/0.969 (38) | 0.998/0.989 (12) |
| TT5 | LB | 26 | 26 | 0.951/0.927 (12) | 0.999/0.943 (14) |
| TT6 | LB | 28 | 24 | 0.911/0.869 (9) | 1.000/1.000 (19) |
| TT8 | NEB | 33 | 34 | — | 0.999/0.999 (33) |
| TT9 | NEB | 51 | 53 | — | 0.999/0.999 (51) |
| TT10 | NEB | 46 | 49 | — | 1.000/1.000 (46) |
| TT11 | NEB | 29 | 21 | 0.944/0.923 (13) | 0.999/0.997 (16) |
| TT13 | NEB | 27 | 23 | 0.947/0.921 (11) | 0.999/0.997 (16) |
| TT14 | NEB | 25 | 24 | 0.941/0.910 (11) | 0.999/0.993 (14) |
| Total | 402 |
“Clutch” here refers to the fertilized clutch, excluding unfertilized eggs. The probabilities of detecting multiple paternity (PrDM) for each clutch with respect to the number of offspring (N) and loci that had been sampled are shown. PrDM values were estimated for two fathers that had equal (0.5:0.5)/skewed (0.667:0.333) contributions.
DNA was extracted by using the PUREGENE DNA isolation kit (Gentra Systems) according to the manufacturer's instructions. DNA concentration was assessed with a Genequant spectrophotometer (Pharmacia). Five microsatellite loci previously characterized for use in green turtles were analyzed: CM58, CM3, CC7, CC117, and CM84 (21, 31). In general, up to 3 μl of extracted DNA (20 μg/μl) was used in 10-μl PCR mixes containing 50 ng of each primer, 0.2-mM concentrations of each dNTP (Amersham Pharmacia), 0.4 unit of Taq polymerase (ABgene, Epsum, Surrey, U.K.), 1 μlof10× PCR buffer (Buffer IV, ABgene), and either 1.5 (all except CM84) or 2.5 (CM84) mM MgCl2 (ABgene). The thermal conditions were an initial 95°C for 2 min followed by 30 cycles of 55°C (CC7), 62°C (CM58, CM3, and CC117), or 64°C (CM84) for 1 min, 72°C for 1 min, 95°C for 45 sec, and ending with a extension step of 72°C for 7 min. PCR products were separated, sized, and analyzed by using the CEQ8000 Genetic Analysis System (Beckman Coulter).
Characterization of Microsatellite Loci. The homogeneity of genotype frequencies was assessed by using an exact probability test on allelic frequencies (32) with the program genepop (33). The data were further tested for deviation from Hardy–Weinberg equilibrium and genotypic disequilibrium. Deviations from Hardy–Weinberg equilibrium were assessed per locus by using the exact test with a Markov chain algorithm to estimate exact P values (34). Heterozygote deficiency was also tested in genepop (35). All calculations based on Markov-chain models performed with genepop used the default dememorization number (1,000), 500 batches, and 1,000 iterations per batch. To investigate linkage disequilibrium, the hypothesis that genotypes at one locus were independent from genotypes at another locus was tested by using Fisher's exact test on contingency tables for all pairs of loci with the method by Weir (36), as implemented in genepop. Observed and expected heterozygosities were those estimated by genepop (33). The frequency of null alleles was estimated with cervus (37). To assess the ability to identify individuals by the multilocus genotypes provided by the five microsatellites used in this study, probabilities of identity (PI) were estimated (38) (see Supporting Text).
Analyses of Parentage. Maternal genotypes were determined directly from the sampled female, and these could be observed in the offspring genotypes. Paternal alleles were inferred from offspring genotypes once maternal alleles were accounted for. Following the rationale described in ref. 21, multiple paternity in a clutch was only inferred when more than two paternal alleles were observed at more than one locus. When only two males are involved in fathering a clutch, it is straightforward to assign offspring to each father on the basis of shared paternal alleles. However, when more fathers are involved, assessing the number of fathers and manual assignment of offspring to each father is less simple. Two methods were used. The first used dadshare (www.zoo.cam.ac.uk/zoostaff/amos) to estimate the degree of relatedness between individuals (39) within a clutch and identify full-sib and half-sib groups by unweighted-pair group method with arithmetic mean clustering (40). Because mutations or mistyping may lead to overestimation of the number of full-sib clusters, sets of offspring were only confirmed to have different fathers where there were differences at more than one locus (individuals or sets of individuals were otherwise left as “unresolved”). The second method of inferring the minimum number of fathers from a progeny array was that implemented in the program gerud (41). Expected exclusion probabilities (42) were also calculated with gerud. Nonparametric analyses (spss version 11) were used to assess associations between paternity, female size, beach condition, and estimators of reproductive success. For these tests, data for all seasons were pooled because no significant associations with the year of study existed (data not shown).
Results
Analyses requiring a reference population were based on one of n = 53 for the 1999 breeding season, and another of n = 41 for 2000 (see Supporting Text). Analyses of the microsatellite data (see Supporting Text) confirmed that each locus could be treated as independent. These also showed no significant deviations from Hardy–Weinberg expectations, low null frequencies and probabilities of identity, and high probabilities of exclusion and of detecting multiple paternity, even for small sample sizes or few loci. (See Tables 4 and 5, which are published as supporting information on the PNAS web site.)
Offspring Dataset. Of 715 offspring genotyped, 14 were found to be unrelated to the others in the clutches with which they were sampled. These unrelated offspring were identified based on the absence of maternal alleles at some of the loci. None of these could be attributed to mutation (e.g., ref. 21) because absence of maternal alleles occurred in at least two different loci in all cases. Cross-contamination between clutches is possible because female adults may dig nests in areas where another turtle had already laid eggs (G. Hays, personal observation). The 14 unrelated samples were excluded from further analyses.
Multiple paternity was found for 61% of all clutches, where there was no significant difference in the frequency of multiple paternity in the two different years (Fisher's exact test, P = 1.000). In all cases, evidence of three or more paternal loci was found in at least three of the five loci (Table 2). No instances occurred where a maternal allele was lacking in only a single locus, which could signify a mutation. Mutation was therefore unlikely to have biased our estimates of multiple paternity. Among the multiply sired clutches, analyses indicated between two to five possible fathers (Table 2). Except for TT1 and TP5, both dadshare and gerud analyses provided identical results. TP5 could not be resolved by either method. dadshare analysis identified nine half-sib clusters for TP5; however, some clusters only differed in paternal alleles at a single locus, and mutation could therefore not be discounted as a potential factor. TT1 was only successfully resolved with dadshare. Three clusters of TT1 offspring clearly differed from each other at two or more loci, but a single individual could not be assigned to any cluster with certainty. Again, mutation or mistyping could not be discounted for this individual. With these two exceptions, paternal alleles of half-sib clusters in all other multiply sired clutches were confirmed to differ from other clusters in the clutch at two or more loci.
Table 2. The number of paternal alleles at each locus.
| Nest code | CM58 | CM3 | CC7 | CC117 | CM84 | Inferred no. of fathers |
|---|---|---|---|---|---|---|
| 1999 | ||||||
| TP36 | 2 | 2 | 2 | 2 | 1 | 1 |
| TP39 | 1 | 2 | 1 | 2 | 2 | 1 |
| TP5 | 5 | 2 | 4 | 3 | 5 | Unresolved |
| TP44 | 2 | 1 | 2 | 2 | 2 | 1 |
| TP51 | 4 | 3 | 4 | 4 | 5 | 3 |
| TP53 | 4 | 2 | 4 | 3 | 5 | 3 |
| TP48 | 3 | 2 | 3 | 4 | 4 | 2 |
| 2000 | ||||||
| TT1 | 2 | 2 | 4 | 3 | 5 | 3 |
| TT2 | 1 | 1 | 2 | 2 | 2 | 1 |
| TT4 | 4 | 3 | 3 | 3 | 3 | 2 |
| TT5 | 2 | 1 | 2 | 2 | 2 | 1 |
| TT6 | 3 | 2 | 4 | 4 | 1 | 2 |
| TT8 | 2 | 1 | 2 | 2 | 1 | 1 |
| TT9 | 5 | 3 | 5 | 6 | 3 | 3 |
| TT10 | 5 | 3 | 5 | 6 | 5 | 5 |
| TT11 | 3 | 2 | 4 | 3 | 4 | 2 |
| TT13 | 3 | 3 | 3 | 3 | 4 | 2 |
| TT14 | 2 | 2 | 2 | 2 | 2 | 1 |
Instances of multiple paternity is detected where there are more than three paternal alleles at a locus (in bold) for a clutch. In all instances, evidence of three or more loci were found for at least three loci.
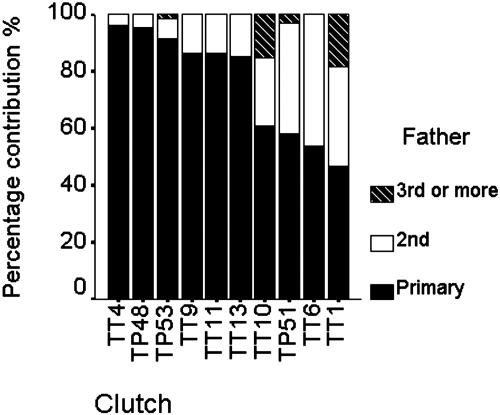
Among the clutches with multiple fathers, about half had more than two. With one exception (TT6, χ2 test = 0.143, df = 1, P = 0.705), all multiply sired clutches were significantly skewed from equal paternal contributions (χ2 tests, all P ≤ 0.001). TT6 had two fathers, each siring about equal numbers of offspring. In considering the contribution of the father that had sired the most compared with all other fathers of a clutch (primary father), all had contributed significantly >50% of the clutch with the exception of four clutches (TP51, TT1, TT6, and TT10; χ2 tests, all P ≥ 0.1). Two significantly different groups of primary fathers apparently exist in terms of their contribution: those siring almost all offspring of the clutch and those siring only about half (Mann–Whitney U, z = -2.558, P = 0.011) (Fig. 1). Therefore, these two groups of fathers were also used as separate grouping variables in subsequent analyses.
Fig. 1.
Contribution of different fathers to multiply sired clutches. TT4, TP48, TP53, TT9, TT11, and TT13 are clutches in which the primary father has contributed significantly >50% of the clutch (see text).
Relationships Among Paternity, Female Size, Beach Type, and Estimators of Reproductive Success. Clutch sizes among the 18 clutches under study ranged from 42 to 170. In comparing singly and multiply sired clutches (Table 3), none of the differences in clutch sizes, number of fertilized eggs, and proportions hatching and surviving to leave the nest were significant (Mann–Whitney U: z = -1.132, P = 0.258; z = -0.997, P = 0.319; z = -0.725, P = 0.468; z = -0.860, P = 0.390, respectively). Neither were clutch sizes, proportions of unfertilized eggs, fertilized clutch sizes, and proportions hatching and surviving to leave the nest significantly different in comparing the multiply sired clutches with primary fathers that fertilized only ≈50% with those sired mainly by primary fathers (Table 3) (Mann–Whitney U: z = -0.853, P = 0.394; z = 0.855, P = 0.392; z = -0.855, P = 0.392; z = -0.428, P = 0.669; z = 0.000, P = 1.000, respectively). Also, no significant correlation occurred between the proportions of the primary fathers' contribution and total clutch sizes (Spearman rank correlation coefficient = 0.201, P = 0.44), proportions of unfertilized eggs (Spearman rank correlation coefficient = 0.070, P = 0.789), fertilized clutch sizes (Spearman rank correlation coefficient = 0.150, P = 0.565), proportions hatched (Spearman rank correlation coefficient = 0.130, P = 0.620), or proportions surviving to leave the nest (Spearman rank correlation coefficient = 0.236, P = 0.361).
Table 3. Mean values (with standard deviations) for estimators of reproductive success and the size of the laying female (curve carapace length), with respect to clutch paternity and the type of beach used.
| Clutch size | Proportion unfertilized | Fertilized clutch size | Proportion hatched | Proportion survived | Female size, cm | |
|---|---|---|---|---|---|---|
| Total | 117.8 (25.29) | 0.100 (0.0688) | 106.6 (25.63) | 0.820 (0.180) | 0.800 (0.190) | 114.6 (5.03) |
| Paternity | ||||||
| Singly sired | 128.0 (21.22) | 0.095 (0.0623) | 116.1 (23.93) | 0.874 (0.136) | 0.865 (0.138) | 113.0 (2.45) |
| Multiply sired | 109.4 (27.00) | 0.093 (0.0722) | 100.1 (27.22) | 0.794 (0.217) | 0.772 (0.227) | 115.6 (6.04) |
| Primary father's contribution | ||||||
| >50% | 117.3 (16.05) | 0.074 (0.0727) | 108.5 (16.67) | 0.766 (0.240) | 0.741 (0.262) | 118.0 (5.33) |
| ≈50% | 97.5 (37.99) | 0.121 (0.0712) | 87.5 (37.50) | 0.836 (0.203) | 0.817 (0.188) | 112.3 (6.90) |
| Beach type | ||||||
| Cool | 117.6 (13.52) | 0.075 (0.0604) | 108.38 (10.45) | 0.953 (0.076) | 0.943 (0.074) | 115.1 (4.79) |
| Hot | 118.0 (32.64) | 0.119 (0.0717) | 105.20 (33.93) | 0.710 (0.176) | 0.691 (0.185) | 114.2 (5.43) |
The proportions that hatched and survived to leave the nest are with respect to the fertilized clutch. The proportion unfertilized, however, is the proportion of the total clutch.
Fathers may be ranked in terms of their contribution in multiply sired clutches. In considering genotyped offspring that could be assigned to fathers, whether these hatched successfully was independent of their distribution among ranked fathers (likelihood ratio = 5.149, P = 0.272). In other words, fathers that had contributed more to a clutch did not also have a higher proportion of offspring hatching.
The size of the mother (Table 3) was not significantly correlated with any estimator of reproductive success (data not shown). Neither was it significantly different for single and multiply sired clutches (Mann–Whitney U: z = -1.454, P = 0.146), nor was it associated with the proportions of the primary fathers' contribution (Spearman rank correlation coefficient = -0.245, P = 0.344).
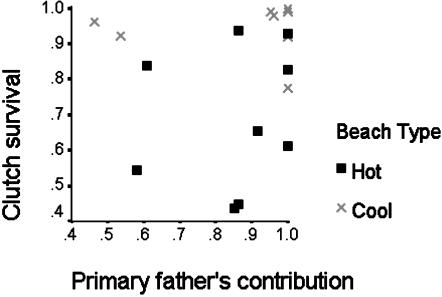
In contrast, the type of beach (cooler incubating conditions in LB and SWB and darker, hotter sands in NEB) was a significant factor in determining the proportions of offspring that hatched and survived to leave the nest (Mann–Whitney U: z = -2.843, P = 0.004, and z = -3.024, P = 0.002, respectively; means listed in Table 3). Fig. 2 illustrates the main findings: paternity appears to have little impact on the proportion of offspring that successfully leave the nest, whereas a larger number of successful clutches had been laid in the cooler light sands. Even when considering the data from each type of beach separately, the pattern of paternity still had no impact on nesting success (data not shown).
Fig. 2.
The proportion of fertilized eggs that had survived plotted against the proportion sired by the father with the highest paternity in the clutch (primary father). No association occurred between these variables. In contrast, more clutches laid in the cooler beaches had higher proportion of the clutch surviving than those in the warmer beach.
Discussion
Multiple paternity was evident in this nesting population of green turtles. However, no correlation occurred with any estimator of reproductive success. Neither was there indication that males fathering a greater proportion of a clutch were of better quality. Indeed, a striking result, consistent with previous work, was that nesting location had a strong effect on clutch success. The inevitable conclusion is that multiple mating was not significantly beneficial to the female green turtles of this population. Recent work on freshwater turtles (Chrysemys picta) reported similar results in which hatching success in multiply and singly sired clutches was not significantly different (27).
Benefits may have been too subtle to be detected in this study of a wild population, perhaps swamped by the stronger effect of beach quality. Many fitness parameters found to be positively associated with multiple mating in other organisms (e.g., see studies reviewed in ref. 43) are impossible to assess in this population of wild marine turtles; for example, female lifetime offspring production, offspring survival to adulthood, and subsequent reproductive successes. Because such integrated lifetime measures of reproductive success are very difficult to measure for wide-ranging, long-lived animals such as marine turtles, as with previous studies (27), we recorded only a more immediate measure of success, namely the survival of offspring through the incubation period. However, this measure is in itself most probably a key determinant of a mother's reproductive success and determines the suitability of different geographical areas as turtle rookeries (44). Thus, although every possible benefit of polyandry to female green turtles cannot be ruled out, obvious and immediate benefits were certainly not evident in this study.
Experimental manipulations of matings may provide more accurate measures of fitness. Such manipulations are not easy to implement for marine turtles, and no such work has been reported. However, studies showing a lack of benefits to females are also emerging from experimental data for other organisms. Examples include frogs (45, 46), newts (47), beetles (48, 49), and mites (50). Instead of benefits, these often show significant costs to females (e.g., refs. 45 and 48). In general, many different types of costs occur; these costs may include increased risk of mortality (45, 51), disease transmission (52) or predation (53–55), reduced fertilization (45, 48), interrupted foraging (56), and loss of time and energy (7). Many are caused by sexual harassment, as males attempt to influence female choice (57, 58). Female marine turtles also incur costs during mating. For example, they risk physical damage; females will be bitten on flippers, neck, and head, and wounds are left as open sores that require weeks to heal (44). Also, male harassment is costly for females. Female avoidance tactics (see next paragraph) are highly energetic, which is expensive because the turtles rely on stored energy for all their breeding and migration activities over the course of the >100-day, >4,400-km round journey from South America (59). Beaching as an avoidance tactic has an additional risk of injury or mortality; turtles trapped in rocks as they try to return to the sea may die of heat stress (59).
Female green turtles are in control of mating. Females may aggregate in groups that exclude males (60). Males, however, will pursue females venturing outside the group. To avoid copulation, females may fold their hind flippers together, swim away rapidly, or circle to face the male, and respond aggressively with bites; finally, they may adopt a refusal position or beach themselves (60). In contrast to the reluctance of females, males are aggressive in their pursuit of matings and will try to mount any object that approximates the size and shape of a female, including debris, divers, and other male turtles. Males will most certainly attempt to mate many times and fertilize the clutches of several different females (ref. 44, and references therein). Satellite telemetry confirms that males are highly active in the mating grounds, resting only before migration (61). Females are often seen courted by groups of males, and copulating couples are usually harassed by attendant males (e.g., ref. 60). These observations imply that females do not lack potential mates if they were to actively seek them. Even in a female-biased population, up to five males would be seen to escort copulating pairs (60).
Hence, if multiple mating was indeed beneficial to females, then why does it not occur at a higher frequency, given the ready availability of males? To date, studies of various green turtle populations report low (21) to only moderately high (this study and ref. 20) frequencies of multiply sired clutches. If multiple mating confers significant fitness benefits to female turtles, then most females should seek to mate with many males and nearly all clutches should be multiply sired so long as mates are not a limiting factor (e.g., ref. 3). At Ascension Island, male-female encounters are certainly likely to be very high because the population is very large (many thousands of females nest each year), and turtles congregate in small areas of shallow water close to shore (62) so the density of turtles will be of the order of a thousand per square kilometer.
Perhaps females use male competition or aggressiveness as a means of selecting the best male. However, by definition, only a single “best” male should be mated, so this selection does not explain the occurrence of multiple paternity. Another possibility is that females require competing males or vigorous pursuit by multiple males as a cue to trigger female receptivity to mating. Indeed, this requirement will result in multiple paternity, but it does not adequately account for the occurrence of single mating or the observations of low levels of multiple paternity in some sea turtle populations (21). Alternatively, it may be argued that multiple mating will only occur if the female finds a second better-quality mate. Under this “trade-up hypothesis,” a female will mate initially to ensure that her eggs will be fertilized by at least one male. She will then choose to mate a second time only if she encounters a superior male where a mating will produce more genetically compatible offspring (63). This hypothesis would predict that multiple paternity may be common but by no means ubiquitous. However, the expectation for this hypothesis is that some aspect of multiply sired clutches will be “better” than singly fathered clutches. No evidence was found in this study that multiple paternity per se resulted in better-quality clutches.
A hypothesis more consistent with the results of this study is that females will generally resist mating more than once, unless the cost of resistance exceeds that of mating. Such “convenience polyandry” (64) has been demonstrated for some insects (7, 56, 65, 66) and suspected in a reptile (67). It is a reasonable explanation for cases where multiple mating incurs costs to females with little obvious benefits (e.g., refs. 45, 46, and 48). Here, females do not gain direct or genetic benefits from multiple mating. Instead, both multiple mating and reluctance to mate in the face of male harassment are costly for females. Females simply make the “best of a bad job” by opting for the less costly choice (7). If costs of mating were extremely high relative to costs of resistance, little or no multiple paternity would indeed occur. If, however, the costs of mating to females are sufficiently small that a moderate threshold exists in switching between resistance and submission to male coercion, this could explain the observation of low to intermediate levels of multiple paternity in green turtle populations.
To conclude, benefits of multiple mating to female marine turtles could not be detected, contrary to conventional expectation. Although not all possible benefits could be ruled out, any advantage would need to be considerable given that environmental factors create substantial variation in reproductive success. A more plausible explanation for polyandry in marine turtles is that multiple paternity is largely a result of male coercion, where females have given in to harassment as a means of reducing their overall costs.
Supplementary Material
Acknowledgments
We thank Annette Broderick, Robert Frauenstein, Fiona Glen, Brendan Godley, Julia Henshaw, many volunteers, and Darwin Initative Turtle Wardens for invaluable help with fieldwork; Tara George at the Ascension Island Conservation Centre for her support; administrators Roger Huxley and Geoffrey Fairhurst for permission to conduct fieldwork and for logistical help during our fieldtrips; Nancy Fitzsimmons for advice and information on marine turtle microsatellites; and Patty Parker and Bill Amos for advice and information on data analysis. This work was supported by an Early Career Project Grant from the British Ecological Society (reference no. 01/17) (to P.L.M.L.) and grants from the Department of the Environment, Transport and the Regions (Darwin Initiative), and Natural Environment Research Council (to G.C.H.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: LB, Long Beach; NEB, North East Bay; SWB, South West Bay.
References
- 1.Birkhead, T. (2000) Promiscuity: An Evolutionary History of Sperm Competition and Sexual Conflict (Faber & Faber, London).
- 2.Trivers, R. L. (1972) in Sexual Selection and the Descent of Man, 1871–1971, ed. Campbell, B. (Aldine-Atherton, Chicago), pp. 136-179.12209574
- 3.Newcomer, S. D., Zeh, J. A. & Zeh, D. W. (1999) Proc. Natl. Acad. Sci. USA 96, 10236-10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans, J. P. & Magurran, A. E. (2000) Proc. Natl. Acad. Sci. USA 97, 10074-10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calsbeek, R. & Sinervo, B. (2002) Proc. Natl. Acad. Sci. USA 99, 14897-14902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamudio, K. R. & Sinervo, B. (2000) Proc. Natl. Acad. Sci. USA 97, 14427-14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson, P. J., Arnqvist, G. & Stallmann, R. R. (1998) Am. Nat. 151, 46-58. [DOI] [PubMed] [Google Scholar]
- 8.Harry, J. L. & Briscoe, D. A. (1988) J. Hered. 79, 96-99. [DOI] [PubMed] [Google Scholar]
- 9.Galbraith, D. A. (1993) Herpetol. J. 3, 117-123. [Google Scholar]
- 10.Bollmer, J. L., Irwin, M. E., Rieder, J. P. & Parker, P. G. (1999) Copeia 1999, 475-478. [Google Scholar]
- 11.Moore, M. K. & Ball, R. M. (2002) Mol. Ecol. 11, 281-288. [DOI] [PubMed] [Google Scholar]
- 12.Hoekert, W. E. J., Neufeglise, H., Schouten, A. D. & Menken, S. B. J. (2002) Heredity 89, 107-113. [DOI] [PubMed] [Google Scholar]
- 13.Kichler, K., Holder, M. T., Davis, S. K., Márquez, R. & Owens, D. W. (1999) Mol. Ecol. 8, 819-830. [Google Scholar]
- 14.Reider, J. P., Parker, P. G., Spontila, J. R. & Irwin, M. E. (1998) in Proceedings of the 16th International Symposium on Sea Turtle Biology and Conservation, eds. Byles, R. & Fernandez, Y. (National Oceanic and Atmospheric Administration, Washington, DC), pp. 120-121.
- 15.Curtis, C. (1998) Master's thesis (Drexel University, Philadelphia).
- 16.Curtis, C., Williams, C. & Spotila, J. (1998) Proceedings of the 17th Annual Sea Turtle Symposium, U.S. Department of Commerce National Oceanic and Atmospheric Administration Technical Memo, NMFS-SEFSC-415, p. 170.
- 17.Dutton, P., Bixby, E. & Davis, S. K. (2000) in Proceedings of the 18th International Symposium on Sea Turtle Biology and Conservation, eds. Abreu-Grobois, F. A., Briseño-Dueñas, R., Márquez, R. & Sarti, L. (National Oceanic and Atmospheric Administration, Washington, DC), p. 156.
- 18.Crim, J. L., Spotila, L. D., Spotila, J. R., O'Connor, M., Reina, R., Williams, C. J. & Paladino, F. V. (2002) Mol. Ecol. 11, 2097-2106. [DOI] [PubMed] [Google Scholar]
- 19.Parker, P. G., Waite, T. A. & Peare, T. (1996) in Molecular Genetic Approaches in Animal Conservation, eds. Smith, T. B. & Wayne, R. K. (Oxford Univ. Press, New York), pp. 413-423.
- 20.Peare, T., Parker, P. G. & Irwin, M. E. (1998) Proceedings of the 16th Annual Symposium of Sea Turtle Biology and Conservation, National Oceanic and Atmospheric Administration Technical Memo, NMFS-SEFSC-412, 116.
- 21.FitzSimmons, N. N. (1998) Mol. Ecol. 7, 575-584. [DOI] [PubMed] [Google Scholar]
- 22.Ireland, J. S., Broderick, A. C., Glen, F., Godley, B. J., Hays, G. C., Lee, P. L. M. & Skibinski, D. O. F. (2003) J. Exp. Mar. Biol. Ecol. 291, 149-160. [Google Scholar]
- 23.Berry, J. F. & Shine, R. (1980) Oecologia 44, 185-191. [DOI] [PubMed] [Google Scholar]
- 24.Glen, F., Broderick, A. C., Godley, B. J. & Hays, G. C. (2003) J. Mar. Biol. Assoc. U. K. 83, 1183-1186. [Google Scholar]
- 25.Hays, G. C., Ashworth, J. S., Barnsley, M. J., Broderick, A. C., Emery, D. R., Godley, B. J., Henwood, A. & Jones, E. L. (2001) Oikos 93, 87-94. [Google Scholar]
- 26.Godley, B. J., Broderick, A. C. & Hays, G. C. (2001) Biol. Conserv. 97, 151-158. [Google Scholar]
- 27.Pearse, D. E., Janzen, F. J. & Avise, J. C. (2002) Behav. Ecol. Sociobiol. 51, 164-171. [Google Scholar]
- 28.Broderick, A. C., Godley, B. J. & Hays, G. C. (2001) Physiol. Biochem. Zool. 74, 161-170. [DOI] [PubMed] [Google Scholar]
- 29.Owens, D. W. & Ruiz, G. W. (1980) Herpetologica 36, 17-20. [Google Scholar]
- 30.Neff, B. D. & Pitcher, T. E. (2002) J. Fish Biol. 61, 739-750. [Google Scholar]
- 31.FitzSimmons, N. N., Moritz, C. & Moore, S. S. (1995) Mol. Biol. Evol. 12, 432-440. [DOI] [PubMed] [Google Scholar]
- 32.Raymond, M. & Rousset, F. (1995) Evolution (Lawrence, Kans.) 49, 1280-1283. [DOI] [PubMed] [Google Scholar]
- 33.Raymond, M. & Rousset, F. (1995) J. Hered. 86, 248-249. [Google Scholar]
- 34.Guo, S. W. & Thompson, E. A. (1992) Biometrics 48, 361-372. [PubMed] [Google Scholar]
- 35.Rousset, F. & Raymond, M. (1995) Genetics 140, 1280-1283. [Google Scholar]
- 36.Weir, B. S. (1990) Genetic Data Analysis: Methods for Discrete Population Genetic Data (Sinauer, Sunderland, MA). [DOI] [PubMed]
- 37.Marshall, T. C., Slate, J., Kruuk, L. E. B. & Pemberton, J. M. (1998) Mol. Ecol. 7, 639-655. [DOI] [PubMed] [Google Scholar]
- 38.Paetkau, D., Calvert, W., Stirling, I. & Strobeck, C. (1995) Mol. Ecol. 4, 347-354. [DOI] [PubMed] [Google Scholar]
- 39.Queller, D. C. & Goodnight, K. F. (1989) Evolution (Lawrence, Kans.) 43, 258-275. [DOI] [PubMed] [Google Scholar]
- 40.Blouin, M. S., Parsons, M., Lacaille, V. & Lotz, S. (1996) Mol. Ecol. 5, 393-401. [DOI] [PubMed] [Google Scholar]
- 41.Jones, A. G. (2001) Mol. Ecol. Notes 1, 215-218. [Google Scholar]
- 42.Dodds, K. G., Tate, M. L., McEwan, J. C. & Crawford, A. M. (1996) Theor. Appl. Genet. 92, 966-975. [DOI] [PubMed] [Google Scholar]
- 43.Arnqvist, G. & Nilsson, T. (2000) Anim. Behav. 60, 145-164. [DOI] [PubMed] [Google Scholar]
- 44.Miller, J. D. (2003) in The Biology of Sea Tturtles, eds. Lutz, P. L., Musick, J. A. & Wyneken, J. (CRC, Boca Raton, FL), pp. 51-81.
- 45.Byrne, P. G. & Roberts, J. D. (1999) Proc. R. Soc. London Ser. B 266, 717-721. [Google Scholar]
- 46.Byrne, P. G. & Roberts, J. D. (2000) Evolution (Lawrence, Kans.) 54, 968-973. [DOI] [PubMed] [Google Scholar]
- 47.Garner, T. W. J. & Schmidt, B. R. (2003) Proc. R. Soc. London Ser. B 270, 619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orsetti, D. M. & Rutowski, R. L. (2003) Anim. Behav. 66, 477-484. [Google Scholar]
- 49.Pai, A. & Yan, G. Y. (2003) Can. J. Zool.-Rev. Can. Zool. 81, 888-896. [Google Scholar]
- 50.Kolodziejczyk, M. & Radwan, J. (2003) Behav. Ecol. Sociobiol. 53, 110-115. [Google Scholar]
- 51.Blanckenhorn, W. U., Hosken, D. J., Martin, O. Y., Reim, C., Teuschl, Y. & Ward, P. I. (2002) Behav. Ecol. 13, 353-358. [Google Scholar]
- 52.Loehle, C. (1997) Ecol. Modell. 103, 231-250. [Google Scholar]
- 53.Kelly, C. D., Godin, J. G. J. & Wright, J. M. (1999) Proc. R. Soc. London Ser. B 266, 2403-2408. [Google Scholar]
- 54.Magnhagen, C. (1991) Trends Ecol. Evol. 6, 183-185. [DOI] [PubMed] [Google Scholar]
- 55.Lima, S. L. & Dill, L. M. (1990) Can. J. Zool.-Rev. Can. Zool. 68, 619-640. [Google Scholar]
- 56.Rowe, L. (1992) Anim. Behav. 44, 189-202. [Google Scholar]
- 57.Clutton-Brock, T. H. & Parker, G. A. (1995) Anim. Behav. 49, 1345-1365. [Google Scholar]
- 58.Magurran, A. E. & Seghers, B. H. (1994) Proc. R. Soc. London Ser. B. 255, 31-36. [Google Scholar]
- 59.Hays, G. C., Broderick, A. C., Glen, F. & Godley, B. J. (2002) Can. J. Zool. 80, 1299-1302. [Google Scholar]
- 60.Booth, J. & Peters, J. A. (1972) Anim. Behav. 20, 808-812. [Google Scholar]
- 61.Hays, G. C., Broderick, A. C., Glen, F., Godley, B. J. & Nichols, W. J. (2001) Mar. Biol. 139, 395-399. [Google Scholar]
- 62.Mortimer, J. A. & Portier, K. M. (1989) Copeia 1989, 962-977. [Google Scholar]
- 63.Pitcher, T. E., Neff, B. D., Rodd, F. H. & Rowe, L. (2003) Proc. R. Soc. London Ser. B 270, 1623-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thornhill, R. & Alcock, J. (1983) The Evolution of Insect Mating Systems (Harvard Univ. Press, Cambridge, MA).
- 65.Weigensberg, I. & Fairbairn, D. J. (1994) Anim. Behav. 48, 893-901. [Google Scholar]
- 66.Cordero, A. & Andrés, J. A. (2002) J. Insect Sci. 2, 14. Available online: insectscience.org/2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garner, T. W. J., Gregory, P. T., McCracken, G. F., Burghardt, G. M., Koop, B. F., McLain, S. E. & Nelson, R. J. (2002) Copeia 2002, 15-23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.