Abstract
Background
Dysregulation and activation of Hedgehog (Hh) signalling may contribute to tumorigenesis, angiogenesis, and metastatic seeding in several solid tumours.
Objective
We investigated the impact of Hh inhibition on tumour growth and angiogenesis using in-vitro and in-vivo models of hepatocellular carcinoma (HCC).
Methods
The effect of the Hh pathway inhibitor GDC-0449 on tumour growth was investigated using an orthotopic rat model. Effects on angiogenesis were determined by immunohistochemical staining of von Willebrand factor antigen and by assessing the mRNA expression of several angiogenic factors. In vitro, HCC cell lines were treated with GDC-0449 and evaluated for viability and expression of vascular endothelial growth factor (VEGF). Endothelial cells were evaluated for viability, migration, and tube formation.
Results
In the orthotopic HCC model, GDC-0449 significantly decreased tumoral VEGF expression which was accompanied by a significant reduction of microvessel density and tumour growth. In HCC cells, GDC-0449 had no effect on cell growth but significantly reduced target gene regulation and VEGF expression while having no direct effect on endothelial cell viability, migration, and tube formation.
Conclusions
Hh inhibition with GDC-0449 downregulates tumoral VEGF production in vitro and reduces tumoral VEGF expression, angiogenesis, and tumour growth in an orthotopic HCC model.
Keywords: Angiogenesis, GDC-0449, hedgehog, hepatocellular carcinoma, VEGF
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and the third most common cause of cancer-related mortality.1 Since cancer-related symptoms often emerge late, more than 50% of all patients are diagnosed at an advanced stage.2,3 These patients have a poor prognosis since they are not amenable to surgical or interventional treatments.4,5 Until now, the multikinase inhibitor sorafenib is the only drug therapy that could improve survival of this poorly served group of patients. However, the prolongation of median survival was only moderate (2.8 months) compared to placebo.6 Thus, there is still an urgent need for improvements regarding treatment of advanced HCC.
The hedgehog (Hh) signalling pathway plays a crucial role in cell differentiation, regeneration and stem cell biology. In brief, binding of the Hh ligand to the receptor patched (Ptch) leads to the release of the seven-transmembrane signalling protein smoothened (Smo). Smo inhibits suppressor of Fused (SuFu) resulting in the nuclear translocation of the transcription factors glioma-associated oncogene (Gli1, Gli2, Gli3). Gli in turn activates transcription of several genes involved in cell growth and differentiation.7
Dysregulation of Hh signalling may contribute to tumorigenesis, angiogenesis, and metastatic seeding in several solid tumours.8–13 In the liver, the Hh pathway is silent under normal conditions14 but becomes reactivated during liver injury.15,16 Hh ligands act as trophic factors which promote tissue repair and liver regeneration. However, excessive Hh signalling triggers epithelial-to-mesenchymal transitions and thereby contributes to liver fibrogenesis.15 Additionally, Hh signalling promotes the accumulation of liver progenitor cells which may become tumour initiating cells for HCC.17 The fact that components of the Hh pathway are also frequently overexpressed in human HCC samples suggests an involvement of Hh signalling in hepatocarcinogenesis18–20 and makes this pathway an attractive candidate for pharmacological interventions.
GDC-0449 is an oral systemic hedgehog antagonist that binds to Smo and has already been tested in solid tumours in phase I and II trials, including basal cell carcinoma and medulloblastoma.21–24 Based on the encouraging results of a phase II study,24 GDC-0449 became recently approved by the US Food and Drug Administration for advanced basal cell carcinoma and represents the first drug on the market to target the Hh pathway.25
The aim of this study was to explore the impact of pharmacological Hh inhibition with GDC-0449 on tumour growth in an orthotopic HCC rat model. Since growing evidence suggests an involvement of Hh signalling in angiogenesis,11,26,27 which is a hallmark of cancer,28 we hypothesized that potential effects of Hh inhibition may primarily target angiogenesis.
Materials and methods
Drugs
GDC-0449, kindly provided by Genentech (South San Francisco, CA, USA), was dissolved in 0.5% methylcellulose with 0.2% Tween 80 for in-vivo experiments and in DMSO for in-vitro experiments.
Orthotopic rat model
Experiments were performed in ACI rats (Harlan, Indianapolis, USA). Animals received human care in accordance with the regulations for laboratory animals. The study was approved by the animal ethics committee of the Medical University of Vienna.
We used an orthotopic HCC model as published previously.29,30 In short, subcutaneously injected Morris Hepatoma 3924A (MH) cells (5 × 106) in a syngeneic ACI rat led to formation of a tumour within 14 days. Tumour inocula were prepared by mincing the excised subcutaneous tumour into equal cubes of 1 mm3. One cube per rat was then immediately surgically implanted into the liver. After randomization (1 week after tumour implantation), treated rats received GDC-0449 40 mg/kg b.i.d. per oral gavage (n = 9) or vehicle control (n = 6). After 5 weeks of treatment, animals were harvested and tumour volume was calculated after sacrificing (tumour volume = 4/3 × π × r1 × r2 × r3).
Immunohistochemistry
Paraffin-embedded tissue samples were cut into 3 µm thin slices. After deparaffinization with Neo-Clear (Merck, Darmstadt, Germany), endogenous peroxidase was blocked by immersing slides in 2.5% H2O2 diluted in pure methanol. For von Willebrand factor (vWF) staining, antigen retrieval was performed by digestion in 0.1% protease type XIV (Sigma Aldrich, St Louise, MO, USA). Unspecific staining was blocked by incubation with goat serum diluted in TRIS-buffered saline (pH 7.4). Endogenous biotin was blocked with a Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA, USA). Slides were incubated with vWF primary antibody (1:600 dilution in goat serum; Abcam, Cambridge, UK) overnight at 4℃. Negative control slides were incubated with polyclonal Rabbit IgG Control Antibody (RnD Systems, Minneapolis, MN, USA) at corresponding dilutions. Excess antibody was removed by washing in Tris-buffered saline followed by incubation of slides with biotinylated goat anti-rabbit secondary antibody (1:300 dilution, Vector Laboratories) for 30 minutes at room temperature. Slides were washed again and incubated with avidin/biotin/peroxidase complex (Vectastain Elite ABC Kit, Vector Laboratories) for 30 minutes at room temperature.
Finally, slides were developed with diaminobenzidine diluted in a mixture of Tris-buffered saline and 0.03% H2O2 for 10 minutes. Nuclei counterstaining was performed with Gill’s haematoxilin solution (Merck).
An experienced senior pathologist (HPD) who was blinded regarding treatment groups performed the assessment of tumour microvessel density. VWF-antigen positive vessels ≥50 µM in diameter were counted in four representative fields (magnification ×100).
Cell lines
The MH cell line was obtained from the German Cancer Research Center (DKFZ; Heidelberg, Germany) and was cultured in RPMI 1640 medium supplemented with 20% fetal bovine serum (FBS), l-glutamine, and penicillin/streptomycin (all from Life Technologies, Paisley, UK). Rat aortic endothelial cells were a kind gift of Professor Dufour (Bern, Switzerland) and cultured in DMEM (Life Technologies) supplemented with 10% FBS and penicillin/streptomycin as well as in appropriate flasks/plates coated with 1% gelatine (Sigma-Aldrich). The human HCC cell line Huh-7 was purchased from Riken Cell Bank (Tsukuba, Japan) and maintained in DMEM supplemented with 10% heat-inactivated FBS, MEM Non Essential Amino Acids (Life Technologies), and penicillin/streptomycin.
Western blot
Preparation of total cell lysates and protein determination were performed according to standard procedures.31 For nuclear protein preparation, cells were harvested and resuspended in hypotonic buffer containing 10 mM HEPES (pH 7.9), 1.5 mM MgCl2, and 10 mM KCl, supplemented with appropriate protease and phosphatase inhibitors. Cells were lysed by addition of 0.16% Igepal CA-630 (Sigma Aldrich). After centrifugation, the nuclei containing pellet was resuspended in extraction buffer consisting of 20 mM HEPES (pH 7.9), 1.5 mM MgCl2, 300 mM KCl, 0.2 mM EDTA, and 25% glycerin, supplemented with protease and phosphatase inhibitors. Nucleic proteins were extracted by incubation at 4℃ under harsh agitation and subsequent centrifugation. Nucleic protein content in the supernatant was determined by means of Bradford assay (Pierce, Rockford, IL, USA). Immunoblotting of total cell lysates and nuclear protein preparations was performed according to standard methods, as previously described in detail.31 Protein expression was assessed with antibodies against Gli1 (Rockland Immunochemicals Gilbertsville, PA, USA) and Gli2 (Sigma Aldrich). Antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Enzo Life Sciences, Farmingdale, NY, USA) and proliferating cell nuclear antigen (Abcam) were used as loading controls for total cell lysates and nuclear lysates, respectively. Anti-Rabbit IgG horseradish peroxidase (Santa Cruz, Santa Cruz, CA, USA) and anti-mouse IgG horseradish peroxidase (Pierce) were used as secondary antibodies.
Cell viability
Cell growth was determined by neutral red assay.32 Equal numbers of cells (10,000 cells/well) in the logarithmic phase of growth were plated in complete medium in 24-well plates and allowed to attach overnight. The following day, GDC-0449 (1–1000 nM)/DMSO was added. Cells were then incubated for 72 hours at 37℃ in a humidified atmosphere containing 5% CO2 and then analysed according to manufacturer’s recommendations. At least two independent experiments were run in triplicate.
Real-time reverse-transcription PCR
For in-vivo experiments, tissue samples were snap frozen in liquid nitrogen immediately after resection and stored at −80℃ until RNA isolation. For RNA isolation, frozen tissue samples were placed into precooled tubes containing Trizol (Life Technologies) and homogenization beads, then agitated using a homogenizer. RNA extraction and isolation were performed according to the manufacturer’s instructions.
For in-vitro experiments, equal numbers of cells (100,000 cells/well) were plated in medium containing 1% FBS in 6-well plates and allowed to attach overnight. The following day, GDC-0449 (10 nM) or DMSO was added. Cells were incubated for 24 hours at 37℃ in a humidified atmosphere containing 5% CO2 and then directly lysed in Trizol, and RNA extraction and isolation was performed. At least three independent experiments were run in triplicate.
For both in-vitro and in-vivo experiments, cDNA was synthesized with 2 µg of total RNA using High Capacity cDNA Reverse Transcription Kit (Life Technologies). Gene expression in tissue samples and various human and rat cell lines was determined by means of real-time PCR using 7500 Fast Real Time PCR System (Life Technologies), according to the manufacturers’ instructions. The following pre-designed primer/probe pairs were utilized: human Gli1 (hs01110766_m1), rat Gli1 (rn01504237_m1), rat Gli2 (rn01408890_m1), human vascular endothelial growth factor-A (VEGF-A; hs00173626_m1), rat VEGF-A (rn01511602_m1), rat angiopoietin-2 (Ang-2; rn01756774_m1), rat platelet-derived growth factor-B (PDGF-B; rn00585926_m1), rat basic fibroblast growth factor (bFGF; rn00570809_m1), human GAPDH (4333764F), rat GAPDH (4352338E; all primer/probe pairs by Life Technologies). The ΔΔCt method was used for gene expression calculation; human/rat GAPDH were used as endogenous control for normalization, respectively.
Cell migration assay
Cell migration was determined using modified Boyden chambers (Neuro Probe, Gaithersburg, MD, USA) equipped with 8 µm-pore filters (13 mm diameter; Whatman, Florham Park, NJ, USA) precoated with rat type I collagen (Becton Dickinson, Schwechat, Austria), as described previously.33 Briefly, rat aortic endothelial cells were serum starved overnight. Cells were then seeded in serum-free DMEM medium containing DMSO/GDC-0449 (10–1000 nM) into each upper well of the Boyden chamber. In the lower wells, serum-free medium containing DMSO/GDC-0449 was added and the cells were incubated for 6 hours at 37℃ in a humidified atmosphere containing 5% CO2. The cells that migrated and attached to the underside of the filter were fixed in 96% methanol and stained with Haemaquick stain solution (Tektron, Bornheim, Germany). Cells were viewed at ×40 magnification and counted in three randomly chosen fields. At least two independent experiments were performed.
Capillary tube formation assay
Twenty-four-well plates were coated with Matrigel (Becton Dickinson). Rat aortic endothelial cells were seeded at a concentration of 5 × 104 in DMEM supplemented with 1% FBS and penicillin/streptomycin. Cells were treated with GDC-0449/DMSO and incubated at 37℃ in a humidified atmosphere containing 5% CO2. Photographs were taken after 18 hours and tube area was quantified as the total number of pixels using ImageJ 1.45 software (National Institute of Health). At least two independent experiments were run in triplicate.
Enzyme-linked immunosorbent assay
VEGF-A protein was quantified in cell culture supernatants (after 48 h of treatment) by means of enzyme-linked immunosorbent assay (Human VEGF Quantikine ELISA Kit and Rat VEGF Quantikine ELISA Kit; RnD Systems, Minneapolis, MN, USA), according to the manufacturers’ instructions. At least three independent experiments were performed.
Statistics
Data are expressed as mean ± SEM if not indicated otherwise. Comparisons between groups were made using Student’s t-test or non-parametric Mann–Whitney U-test for continuous variables. A p-value <0.05 was considered significant. All statistical analyses were performed using the statistical software package SPSS version 17.0 (SPSS, Chicago, IL, USA).
Results
GDC-0449 reduces tumour growth, angiogenesis, and tumoral VEGF expression in an orthotopic rat model of HCC
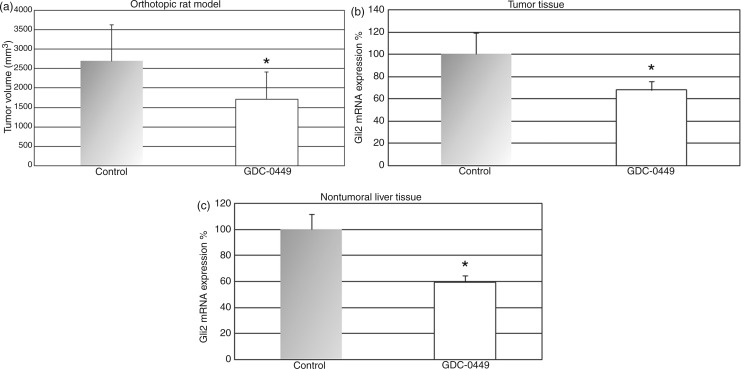
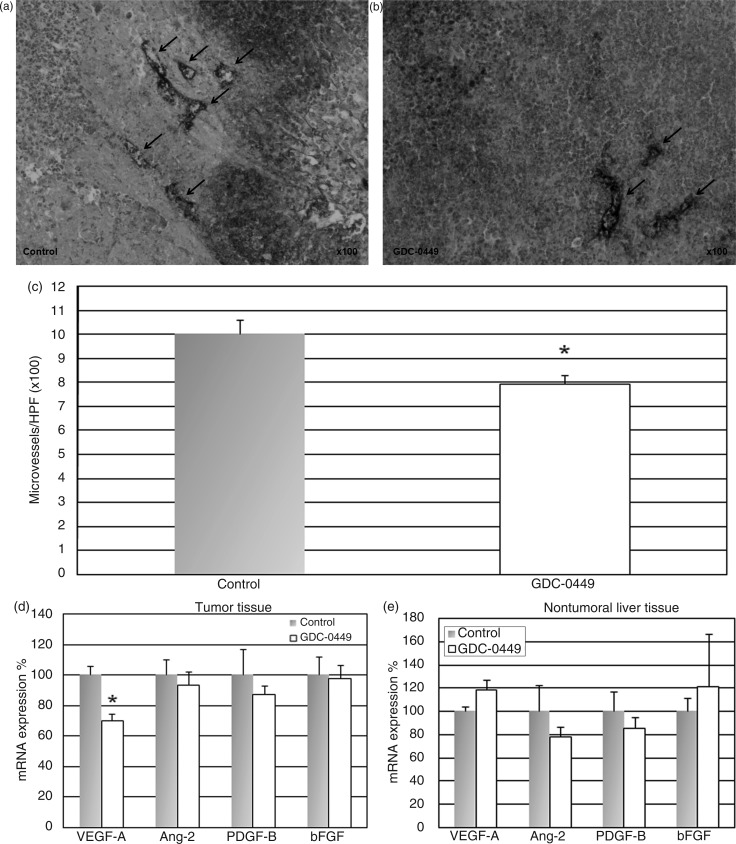
GDC-0449 was well tolerated, and no weight loss or other drug-related toxicities were observed. Tumour volume was significantly reduced by 36% in GDC-0449-treated rats compared to vehicle controls (tumor volume + standard deviation (SD), 1720 ± 696 mm3, range 302–2865 mm3 vs. 2696 ± 922 mm3, range 1898–4320 mm3; p < 0.05; Figure 1a). Treatment with GDC-0449 40 mg/kg b.i.d significantly reduced mRNA expression of the Hh target gene Gli2 in the tumour (Figure 1b) as well as in the nontumoral surrounding liver tissue (Figure 1c). Additionally, immunohistochemical analysis revealed that GDC-0449 also reduced the number of nuclear Gli2(+) cells in the tumour tissue which further indicates pathway regulation (Supplementary Figure 1a–c, available online). To investigate the effect of GDC-0449 on angiogenesis, the resected tumour tissues were immunohistochemically stained for vWF-antigen. GDC-0449 significantly reduced microvessel density by 21% compared to vehicle control (microvessels per high power field 7.9 ± 0.4 vs. 10.1 ± 0.5; p < 0.05; Figure 2a–c).
Figure 1.
GDC-0449 treatment reduces tumour growth and hedgehog target gene expression in an orthotopic rat model of HCC.
Treatment of ACI rats bearing surgically implanted orthotopic HCC with GDC-0449 40 mg/kg b.i.d p.o. significantly decreased tumour growth (a) as well as mRNA expression of the hedgehog target gene Gli2 in the tumour tissue (b) and nontumoral surrounding liver tissue (c) compared to vehicle-treated animals. *p < 0.05 vs. control animals. Error bars represent standard deviation (a) and SEM (b, c).
Figure 2.
GDC-0449 treatment reduces tumoral VEGF-A expression and angiogenesis in an orthotopic rat model of HCC.
ACI rats bearing surgically implanted orthotopic HCC were randomized to receive either GDC-0449 40 mg/kg b.i.d or vehicle control p.o. (a, b) Immunohistochemical staining for von Willebrand factor antigen to assess tumour microvessel density in control animals (a) and GDC-0449-treated rats (b); arrows indicate microvessels (magnification ×100). (c) GDC-0449 treatment significantly reduces von Willebrand factor antigen-positive microvessels compared to vehicle-treated rats. (d) GDC-0449 treatment significantly reduced mRNA expression of VEGF-A in the tumour tissue while other angiogenic factors significantly involved in liver tumour angiogenesis (Anf-2, PDGF-B, bFGF) were not regulated by GDC-0449. (e) In the nontumoral surrounding liver tissue, neither VEGF-A nor other angiogenic factors (Ang-2, PDGF-B, bFGF) were regulated by GDC-0449. *p < 0.05 vs. control animals.
We further investigated the effect of GDC-0449 on several angiogenic factors that play an important role in HCC, namely VEGF-A, Ang-2, PDGF-B, and bFGF. In the tumour tissue, we found a significant downregulation of VEGF-A mRNA expression in GDC-0449-treated rats compared to control animals, but no regulation of other angiogenic factors (i.e. Ang-2, PDGF-B, bFGF) (Figure 2d). In the nontumoral surrounding liver tissue, neither VEGF-A nor the other angiogenic factors were influenced by GDC-0449 (Figure 2e).
Hh signalling is activated in HCC cell lines
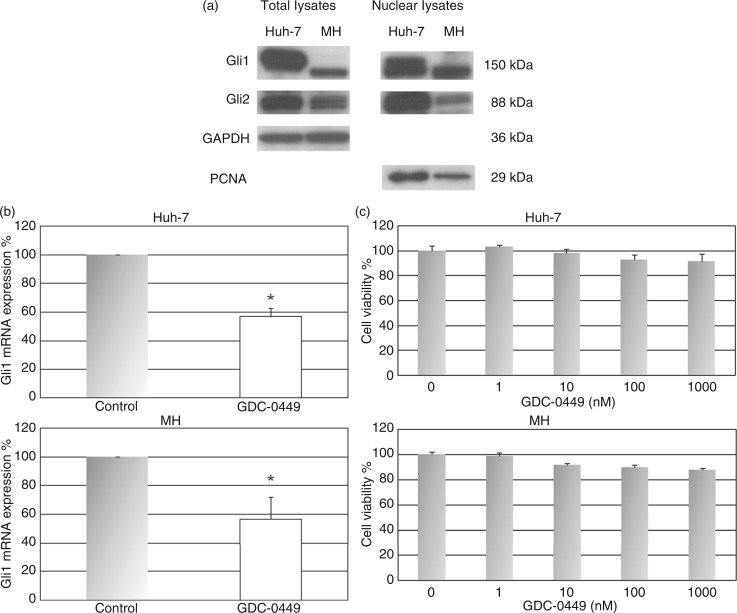
The transcription factors of the Hh pathway Gli1 and Gli2 are expressed in Huh-7 and MH cell lines (Figure 3A). Gli proteins are usually located in the cytosol and translocate into the nucleus when hedgehog signalling is activated. Therefore we investigated the expression of Gli1 and Gli2 in the nuclear fraction and found that these proteins are expressed in the nucleus (Figure 3A). This finding indicates that hedgehog signalling is active in these cell lines.
Figure 3.
GDC-0449 has no effect on tumour cell growth in vitro but reduces Hh target gene expression in HCC cell lines.
(a) Western blotting demonstrated that the transcription factors of the Hh pathway Gli1 and Gli2 are expressed in Huh-7 and MH cells. The expression of Gli1 and Gli2 in the nucleus indicated the nuclear translocation of these proteins, which represented a surrogate for active hedgehog signalling in these cell lines. (b, c) In Huh-7 and MH cells, treatment with GDC-0449 significantly reduced mRNA expression of the hedgehog target gene Gli1 (B) but had no effect on cell viability in vitro (c). *p < 0.05 vs. DMSO-treated controls.
GDC-0449 has no direct effect on cell growth in vitro but reduces Hh target gene expression in HCC cell lines
Gli1 and Gli2 are also target genes of the Hh pathway. Thus, Gli can serve as a readout for pathway activity and their mRNA expression levels are usually used to demonstrate pathway regulation.34 Treatment of Huh-7 and MH cells with 10 nM of GDC-0449 significantly reduced mRNA expression of the Hh target gene Gli1 compared to DMSO-treated controls (Figure 3B), indicating that GDC-0449 is active and regulates Hh signalling in these cell lines. Nevertheless, GDC-0449 treatment at concentrations of 1–1000 nM had no effect on the growth of Huh-7 and MH cells in vitro (Figure 3c). These findings suggest that active Hh signalling may not be mandatory for HCC cell growth and survival.
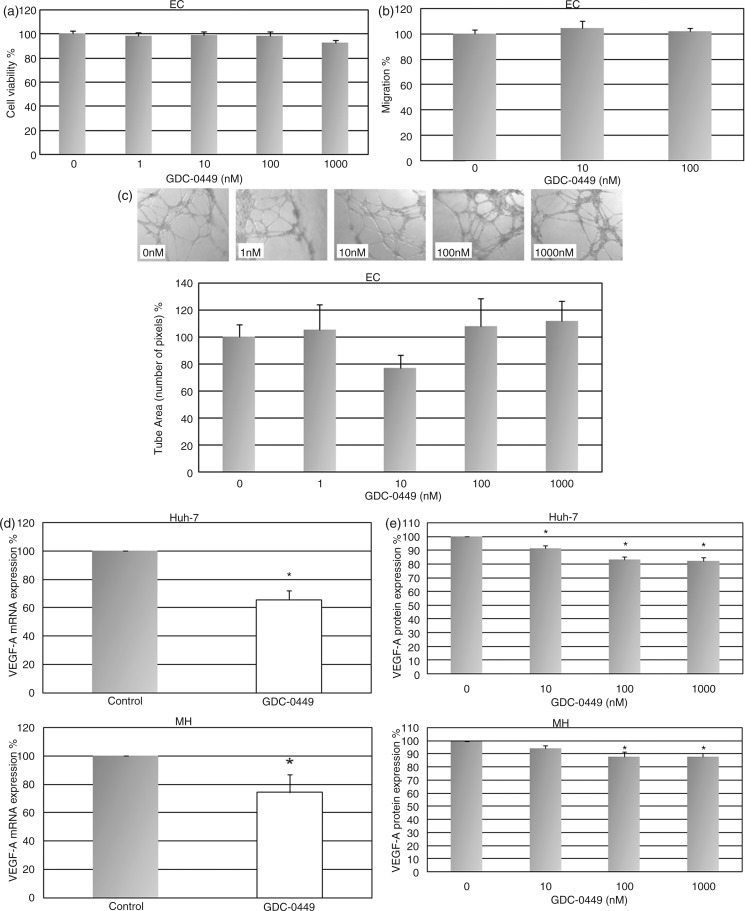
GDC-0449 has no effect on endothelial cell growth, migration, and tube formation
Since GDC-0449 significantly decreased angiogenesis in vivo, we investigated whether GDC-0449 directly affects endothelial cells. Western blot analysis revealed that endothelial cells express the hedgehog receptor Patched (Supplementary Figure 2) while Gli was not detectable. Consequently, treatment of endothelial cells with GDC-0449 at concentrations ranging from 1 to 1000 nM had no influence on cell growth, migration, and tube formation in vitro (Figure 4a–c). These results suggest that GDC-0449 exerts its anti-angiogenic effects not directly via endothelial cells.
Figure 4.
Treatment with GDC-0449 has no effect on endothelial cells but reduces VEGF expression in tumour cell lines in vitro.
(a–c) GDC-0449 treatment failed to reduce endothelial cell viability (a), migration (b), and tube formation (c). (d, e) GDC-0449 significantly decreased the expression of VEGF in Huh-7 and MH cells at the mRNA (d) and protein level (e). *p < 0.05 vs. DMSO-treated controls.
GDC-0449 reduces VEGF-A expression in HCC cell lines
Finally, to confirm the GDC-0449-induced reduction of tumoral VEGF expression observed in vivo, we investigated the effect of Hh pathway inhibition on VEGF-A expression in vitro. We first analysed VEGF-A expression at the mRNA level and observed a significant reduction of VEGF-A in GDC-0449-treated Huh-7 and MH cells (Figure 4d). Furthermore, we also found a significant dose-dependent reduction of VEGF-A protein in supernatants of human and rat HCC cells treated with GDC-0449 (Figure 4e). Collectively, these results indicate that the in-vivo effects on angiogenesis after Hh inhibition may be mediated via repression of tumour cell VEGF expression.
Discussion
In the present study, we explored the efficacy of the Hh inhibitor GDC-0449 in an orthotopic HCC model and in vitro.
We found that treatment with GDC-0449 significantly decreased tumour angiogenesis, which was accompanied by a significant reduction of tumour growth in an orthotopic HCC model.
The antiangiogenic activity of GDC-0449 observed in our model corresponds well to the published literature, where an involvement of Hh signalling in angiogenesis has been shown.11,26,27 Notably, these studies reported that Hh signalling may not directly affect endothelial cells in terms of viability and migration but rather increases the expression of proangiogenic cytokines, in particular VEGF.11,26,27
Hence, we next evaluated the effect of GDC-0449 on the expression of key angiogenic factors (i.e. VEGF, Ang-2, PDGF, bFGF) that are crucially involved in liver tumour angiogenesis.35,36
In the tumour tissue, VEGF expression was significantly reduced in GDC-0449-treated animals compared to controls, while the expression of other angiogenic factors (i.e. Ang-2, PDGF, bFGF) was not changed. In the nontumoral surrounding liver tissue, neither VEGF nor the other angiogenic cytokines were markedly regulated by GDC-0449. In line with the published literature,11,26 GDC-0449 had no impact on viability, migration, and tube formation of endothelial cells in our in-vitro experiments but significantly reduced VEGF expression in HCC cells.
Based on our data, GDC-0449 had no direct cytostatic or apoptotic effect on HCC cells. We rather propose that the observed antitumor effect of GDC-0449 may derive from the blockage of VEGF production in HCC tumour cells leading to a reduction of angiogenesis. The significance of VEGF for HCC biology and its prognostic role in HCC has widely been investigated. Elevated or increasing VEGF levels are associated with more aggressive tumour characteristics and tumour progression.37–41 It has been shown that VEGF levels increase after transarterial chemoembolization (TACE).42,43 Elevated VEGF levels correlate with a worse outcome after surgery, radiofrequency ablation (RFA), or TACE.42,44–47 Moreover, high VEGF levels in HCC tissue samples are associated with rapid recurrence after resection.48–50 Finally, it was recently reported that the multikinase inhibitor sorafenib increases tumoral VEGF expression, which might represent a potential road to resistance.29,51 Hence, combination of RFA, TACE, or sorafenib with a drug that has the potential to reduce VEGF production, such as GDC-0449, might improve the efficacy of these treatment modalities. Adjuvant administration of GDC-0449 may also reduce recurrence rates after tumour resection. On the other hand, in tumours which show progression/resistance following exposure to anti-VEGF treatment, mechanisms of resistance are frequently VEGF-independent.52 Thus, a second-line therapy with GDC-0449 might not show significant antitumour activity in this specific setting. Further studies are needed to investigate this specific issue.
Our study adds another piece of new information to the previously reported evidence about the significance of Hh signalling in chronic liver disease and HCC. Philips et al.53 investigated the effects of GDC-0449 on liver fibrosis and tumour growth in a Mdr2-deficient mouse model of advanced liver disease and HCC. These mice demonstrated increased production of Hh ligands and an accumulation of Hh-responsive cell types, which preceded the emergence of HCC. Treatment with GDC-0449 significantly improved liver fibrosis and reduced tumour growth and metastases in these genetically altered animals. Moreover, Xu et al.54 recently reported that a nanoparticle-encapsulated inhibitor of the Hh transcription factor Gli1 (NanoHHI) reduced tumour growth and inhibited systemic metastases in an HCC animal model.
A limitation of our study is the fact that we investigated the effect of hedgehog inhibition in an animal model consisting of a tumour growing in a non-cirrhotic liver. In the healthy liver, the Hh pathway is silent under normal conditions but becomes reactivated during liver injury and chronic inflammation.15,16 Thus, the effects of GDC-0449 observed in our study might be more pronounced in a tumour microenvironment with extensive hedgehog signalling (e.g. liver cirrhosis).
In conclusion, our findings demonstrate that pharmacological Hh inhibition with GDC-0449 decreases angiogenesis and tumour growth by downregulating tumoral VEGF-A production. Given the moderate efficacy of GDC-0449 observed in our preclinical study, clinical trials are needed to define the real value of Hh inhibition in HCC. In particular, a combination of GDC-0449 with other treatment modalities that were shown to increase VEGF expression (i.e. TACE, sorafenib) could be reasonable.
Acknowledgements
We thank Martha Seif and Hubert Hayden for their professional technical support.
Funding
This study was supported by research grants from the Medical Scientific Fund of the Mayor of the City of Vienna to Matthias Pinter (grant number: AP10081BGM) and Bayer Healthcare Pharma to Wolfgang Sieghart.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108 [DOI] [PubMed] [Google Scholar]
- 2.Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol 2004; 5: 409–418 [DOI] [PubMed] [Google Scholar]
- 3.Hucke F, Sieghart W, Schoniger-Hekele M, et al. Clinical characteristics of patients with hepatocellular carcinoma in Austria – is there a need for a structured screening program? Wien Klin Wochenschr 2001; 123: 542–551 [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer, EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–943 [DOI] [PubMed] [Google Scholar]
- 5.Peck-Radosavljevic M, Sieghart W, Kolblinger C, et al. Austrian Joint OGGH-OGIR-OGHO-ASSO position statement on the use of transarterial chemoembolization (TACE) in hepatocellular carcinoma. Wien Klin Wochenschr 2012; 124: 104–110 [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390 [DOI] [PubMed] [Google Scholar]
- 7.Rohatgi R, Scott MP. Patching the gaps in Hedgehog signalling. Nat Cell Biol 2007; 9: 1005–1009 [DOI] [PubMed] [Google Scholar]
- 8.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res 2007; 67: 2187–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochman E, Castiel A, Jacob-Hirsch J, et al. Molecular pathways regulating pro-migratory effects of Hedgehog signaling. J Biol Chem 2006; 281: 33860–33870 [DOI] [PubMed] [Google Scholar]
- 10.Yoo YA, Kang MH, Kim JS, et al. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis 2008; 29: 480–490 [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Tang T, Eastham-Anderson J, et al. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc Natl Acad Sci USA 2011; 108: 9589–9594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Sasajima J, Mizukami Y, et al. Hedgehog promotes neovascularization in pancreatic cancers by regulating Ang-1 and IGF-1 expression in bone-marrow derived pro-angiogenic cells. PLoS One 2010; 5: e8824–e8824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell 2008; 15: 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 2003; 425: 846–851 [DOI] [PubMed] [Google Scholar]
- 15.Omenetti A, Choi S, Michelotti G, et al. Hedgehog signaling in the liver. J Hepatol 2011; 54: 366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol 2008; 294: G595–598 [DOI] [PubMed] [Google Scholar]
- 17.Mishra L, Banker T, Murray J, et al. Liver stem cells and hepatocellular carcinoma. Hepatology 2009; 49: 318–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, He J, Zhang X, et al. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis 2006; 27: 1334–1340 [DOI] [PubMed] [Google Scholar]
- 19.Patil MA, Zhang J, Ho C, et al. Hedgehog signaling in human hepatocellular carcinoma. Cancer Biol Ther 2006; 5: 111–117 [DOI] [PubMed] [Google Scholar]
- 20.Sicklick JK, Li YX, Jayaraman A, et al. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis 2006; 27: 748–757 [DOI] [PubMed] [Google Scholar]
- 21.LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res 2011; 17: 2502–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med 2009; 361: 1173–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med 2009; 361: 1164–1172 [DOI] [PubMed] [Google Scholar]
- 24.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012; 366: 2171–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guha M. Hedgehog inhibitor gets landmark skin cancer approval, but questions remain for wider potential. Nat Rev Drug Discov 2012; 11: 257–258 [DOI] [PubMed] [Google Scholar]
- 26.Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 2001; 7: 706–711 [DOI] [PubMed] [Google Scholar]
- 27.Soleti R, Benameur T, Porro C, et al. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis 2009; 30: 580–588 [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70 [DOI] [PubMed] [Google Scholar]
- 29.Sieghart W, Pinter M, Dauser B, et al. Erlotinib and Sorafenib in an orthotopic rat model of hepatocellular carcinoma. J Hepatol 2012; 57: 592–599 [DOI] [PubMed] [Google Scholar]
- 30.Semela D, Piguet AC, Kolev M, et al. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol 2007; 46: 840–848 [DOI] [PubMed] [Google Scholar]
- 31.Sieghart W, Losert D, Strommer S, et al. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol 2006; 44: 151–157 [DOI] [PubMed] [Google Scholar]
- 32.Jurek D, Udilova N, Jozkowicz A, et al. Dietary lipid hydroperoxides induce expression of vascular endothelial growth factor (VEGF) in human colorectal tumor cells. Faseb J 2005; 19: 97–99 [DOI] [PubMed] [Google Scholar]
- 33.Carloni V, Romanelli RG, Pinzani M, et al. Focal adhesion kinase and phospholipase C gamma involvement in adhesion and migration of human hepatic stellate cells. Gastroenterology 1997; 112: 522–531 [DOI] [PubMed] [Google Scholar]
- 34.Lauth M, Bergstrom A, Shimokawa T, et al. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA 2007; 104: 8455–8460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu AX, Duda DG, Sahani DV, et al. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol 2011; 8: 292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012; 379: 1245–1255 [DOI] [PubMed] [Google Scholar]
- 37.Li XM, Tang ZY, Zhou G, et al. Significance of vascular endothelial growth factor mRNA expression in invasion and metastasis of hepatocellular carcinoma. J Exp Clin Cancer Res 1998; 17: 13–17 [PubMed] [Google Scholar]
- 38.Yamaguchi R, Yano H, Iemura A, et al. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology 1998; 28: 68–77 [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Tang ZY, Fan J, et al. Expression of platelet-derived endothelial cell growth factor and vascular endothelial growth factor in hepatocellular carcinoma and portal vein tumor thrombus. J Cancer Res Clin Oncol 2000; 126: 57–61 [DOI] [PubMed] [Google Scholar]
- 40.Poon RT, Ng IO, Lau C, et al. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg 2001; 233: 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 2008; 26: 2992–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 2004; 10: 2878–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 2008; 103: 914–921 [DOI] [PubMed] [Google Scholar]
- 44.Chao Y, Li CP, Chau GY, et al. Prognostic significance of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin in patients with resectable hepatocellular carcinoma after surgery. Ann Surg Oncol 2003; 10: 355–362 [DOI] [PubMed] [Google Scholar]
- 45.Poon RT, Lau C, Pang R, et al. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: importance of tumor biomarker in ablative therapies. Ann Surg Oncol 2007; 14: 1835–1845 [DOI] [PubMed] [Google Scholar]
- 46.Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci 2008; 99: 2037–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamesa T, Iizuka N, Mori N, et al. High serum levels of vascular endothelial growth factor after hepatectomy are associated with poor prognosis in hepatocellular carcinoma. Hepatogastroenterology 2009; 56: 1122–1126 [PubMed] [Google Scholar]
- 48.Cui J, Dong BW, Liang P, et al. Effect of c-myc, Ki–67, MMP–2 and VEGF expression on prognosis of hepatocellular carcinoma patients undergoing tumor resection. World J Gastroenterol 2004; 10: 1533–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeng KS, Sheen IS, Wang YC, et al. Prognostic significance of preoperative circulating vascular endothelial growth factor messenger RNA expression in resectable hepatocellular carcinoma: a prospective study. World J Gastroenterol 2004; 10: 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeng KS, Sheen IS, Wang YC, et al. Is the vascular endothelial growth factor messenger RNA expression in resectable hepatocellular carcinoma of prognostic value after resection? World J Gastroenterol 2004; 10: 676–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piguet AC, Saar B, Hlushchuk R, et al. Everolimus augments the effects of sorafenib in a syngeneic orthotopic model of hepatocellular carcinoma. Mol Cancer Ther 2011; 10: 1007–1017 [DOI] [PubMed] [Google Scholar]
- 52.Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005; 8: 299–309 [DOI] [PubMed] [Google Scholar]
- 53.Philips GM, Chan IS, Swiderska M, et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One 2011; 6: e23943–e23943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Y, Chenna V, Hu C, et al. Polymeric nanoparticle-encapsulated Hedgehog pathway inhibitor HPI-1 (NanoHHI) inhibits systemic metastases in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res 2012; 18: 1291–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]