Abstract
Background
Gastro-oesophageal reflux has been suggested to play a role in eosinophilic oesophagitis (EoO). Oesophageal acid exposure decreases baseline intraluminal impedance, a marker of mucosal integrity, in patients with gastro-oesophageal reflux disease (GORD).
Objectives
The aim of this study was to assess oesophageal baseline impedance levels in EoO patients and to investigate their relationship with oesophageal acid exposure.
Methods
Ambulatory 24-h pH-impedance monitoring was performed in 11 EoO patients and in 11 healthy controls with matched oesophageal acid exposure. We assessed baseline impedance levels in the distal, mid-, and proximal oesophageal impedance channels.
Results
Baseline impedance levels in EoO patients were markedly lower compared to controls in the distal oesophagus (median (interquartile range): 988 (757–1978) vs. 2259 (1767–2896) Ω, p = 0.015), mid-oesophagus (1420 (836–2164) vs. 2614 (2374–3879) Ω, p = 0.003), and proximal oesophagus (1856 (1006–2625) vs. 2868 (2397–3439) Ω, p = 0.005). Whereas baseline impedance decreased from proximal to distal in healthy subjects (p = 0.037), no such gradient was seen in EoO patients (p = 0.123).
Conclusions
Throughout the oesophagus, baseline impedance values are decreased in EoO patients, indicating impaired mucosal integrity. Our findings suggest that factors other than acid reflux are the cause of low baseline impedance in EoO.
Keywords: Aetiology, ambulatory 24-h ph-impedance monitoring, atopy, baseline impedance, EoO, follow up, gastro-oesophageal reflux, mucosal integrity, pathophysiology, RDQ
Introduction
Eosinophilic oesophagitis (EoO) has been increasingly diagnosed over the past decade.1 Patients with EoO most frequently report symptoms of dysphagia and/or food impaction, and sometimes heartburn. Eosinophilic infiltration of the mucosa throughout the oesophagus is a key feature of EoO.2 A local T-helper 2 (TH2) type inflammation is present in the oesophagus of EoO patients, similar to the inflammatory pattern seen in asthma and atopic dermatitis patients.3 EoO is therefore considered an allergic disease, and most patients indeed have an atopic constitution.4 Gastro-oesophageal reflux has also been suggested to play a role in EoO, but data is scarse.5,6 Only a few reports on reflux characteristics have been published in EoO patients, showing no difference in oesophageal acid exposure compared to controls.7–10
Intraluminal impedance monitoring can be used to detect gas and liquid reflux by measuring changes in electrical conductivity.11 Between meals and reflux episodes, the impedance levels return to a baseline level. This baseline impedance level is dependent on characteristics of the collapsed oesophageal wall. It has been shown that baseline impedance levels are decreased in gastro-oesophageal reflux disease (GORD) and achalasia.12 Distal baseline impedance values are correlated with oesophageal acid exposure in pediatric and adult GORD patients, and treatment with proton pump inhibitors (PPIs) significantly increases baseline impedance.13,14 A study in rabbits has shown that oesophageal impedance correlated with the transepithelial resistance measured in vitro, which is a marker of oesophageal epithelial integrity.15 Together, these studies have led to the conclusion that oesophageal acid exposure decreases baseline impedance and that baseline impedance is a marker of oesophageal mucosal integrity.13,16,17
Several authors have suggested that the oesophageal mucosal integrity is impaired in patients with EoO, in line with other allergic diseases such as atopic dermatitis and asthma.5,6
We hypothesized that oesophageal baseline impedance is decreased in non-treated EoO patients. Therefore, the aim of this study was to assess oesophageal baseline impedance levels in EoO patients and in controls and to investigate whether the baseline impedance levels are related to oesophageal acid exposure.
Materials and methods
Study subjects
We prospectively included 11 adult EoO patients, defined as having a history of dysphagia and/or food impaction and the presence of ≥15 eosinophils per high-power field, confirmed by histopathology. None of these patients showed symptomatic response to PPI treatment. PPI treatment was stopped at least 1 week before oesophageal pH-impedance measurements. Furthermore, any dietary or steroid treatments were discontinued at least 2 months prior to pH-impedance measurement. We also measured 20 healthy controls without gastrointestinal symptoms or a history of major abdominal surgery. From these 20 controls, 11 measurements were selected that could be matched to EoO patients by total acid exposure time. All study subjects filled out the reflux disease questionnaire (RDQ).18 Written informed consent of study subjects was obtained and the study was approved by the medical ethical committee of our institution.
Oesophageal pH-impedance measurements
In EoO patients and healthy controls, pH-impedance measurements were performed off medication that could influence gastric acid secretion. Measurements were performed using a combined pH-impedance catheter assembly that consisted of six impedance segments and one ISFET pH-electrode (Unisensor AG, Attikon, Switzerland), which was placed at 5 cm from the upper border of the manometrically localized lower oesophageal sphincter. Impedance recording segments were located at 2–4, 4–6, 6–8, 8–10, 14–16, and 16–18 cm above the upper border. Impedance and pH signals were stored on a digital datalogger (Ohmega Medical Measurement Systems, Enschede, The Netherlands), using a sampling frequency of 50 Hz. Distal, mid-, and proximal oesophageal baseline impedance values were determined at 3, 9, and 17 cm above the lower oesophageal sphincter, respectively.
Data analysis
Baseline impedance levels were analysed according to previously published criteria.13 In summary, baseline impedance levels were assessed every 2 hours during a 30-second time period. The median baseline impedance level during all 2-hour periods was considered to be the baseline impedance level for the measurement. Investigators were blinded for the subject’s status while analysing the measurements. Reflux episodes were analysed according to previously published consensus criteria.19
Statistical analysis
Data are presented as median (interquartile range, IQR). Statistical analysis was performed using Prism software version 5 (GraphPad Software, La Jolla, CA, USA). Data of EoO patients and healthy controls were compared using the Mann–Whitney U-test. Distal and proximal baseline impedance data were compared using Wilcoxon’s test for paired measurements. For correlation analysis we used Spearman’s correlation statistics. Differences were considered statistically significant when p < 0.05.
Results
Subject characteristics
Eight of 11 (73%) EoO patients and all controls (100%) were male (p = 0.062). In EoO patients, study procedures were performed at median (IQR) 0.7 (0.4–2.0) years after EoO diagnosis. RDQ scores showed that EoO patients perceived more heartburn (4 (2–7) vs. 0 (0–0), p < 0.001) and regurgitation (3 (0–5) vs. 0 (0–0), p = 0.003) than controls. Consequently, patients had higher total RDQ scores than controls (10 (7–13) vs. 0 (0–0), p < 0.001).
Reflux characteristics
Because patients and controls were matched, upright, supine, and total acid exposure time were not different (Table 1). Comparison between patients and controls showed no differences in the total number of reflux episodes, acidic reflux episodes, and weakly acidic reflux episodes. Proximally extending reflux episodes were not more common in patients than controls. Furthermore, bolus clearance time and acid clearance time did not differ between patients and controls.
Table 1.
Reflux characteristics of EoO patients and controls
| EoO patients | Healthy controls | |
|---|---|---|
| Acid exposure time (%) | 4.7 (2.0–9.2) | 4.8 (4.4–5.6) |
| Upright | 6.4 (3.4–10.9) | 6.8 (4.2–8.1) |
| Supine | 2.2 (0.0–5.4) | 1.0 (0.0–2.8) |
| No. of reflux episodes/24 h | 50 (21–74) | 56 (41–71) |
| Acidic | 44 (17–51) | 44 (29–49) |
| Weakly acidic | 9 (5–10) | 14 (9–22) |
| Reaching mid-oesophagus | 38 (10–57) | 41 (33–58) |
| Reaching proximal oesophagus | 13 (1–21) | 31 (22–58) |
| Bolus clearance time (s) | 11.3 (7.5–13.5) | 17.9 (11.5–22.5) |
| Acid clearance time (s) | 72.0 (41.3–156.4) | 66.0 (56.1–98.3) |
Data are median (IQR). There were no statistically significant differences.
EoO, eosinophilic oesophagitis.
Baseline impedance
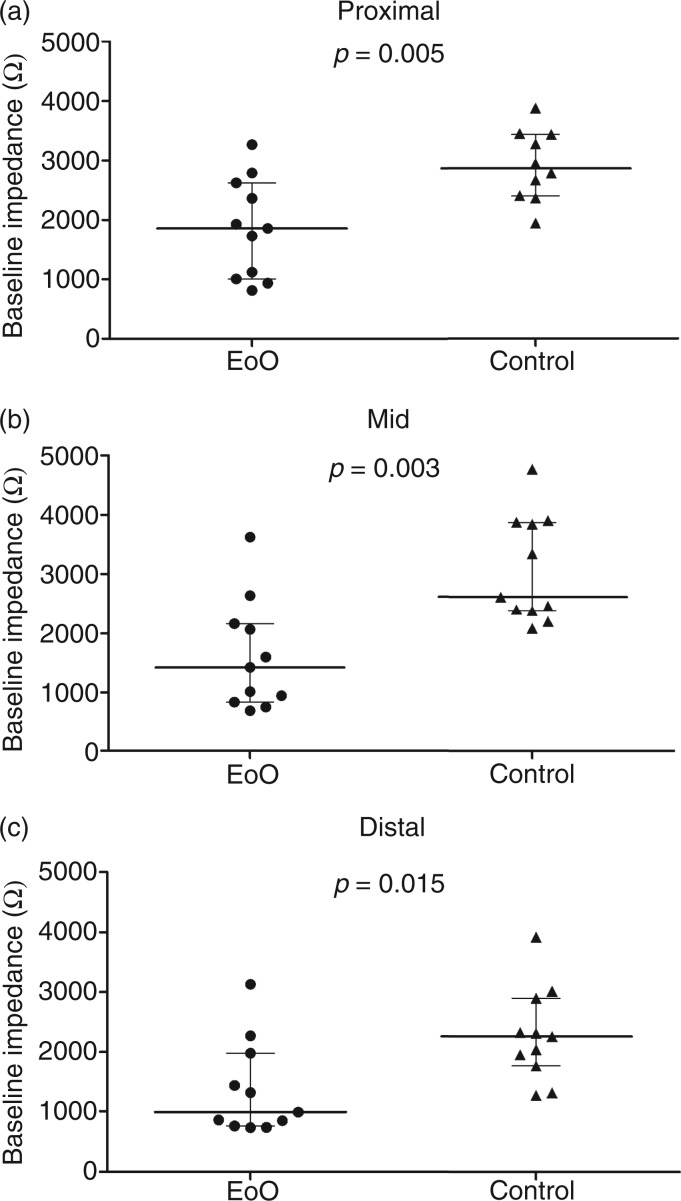
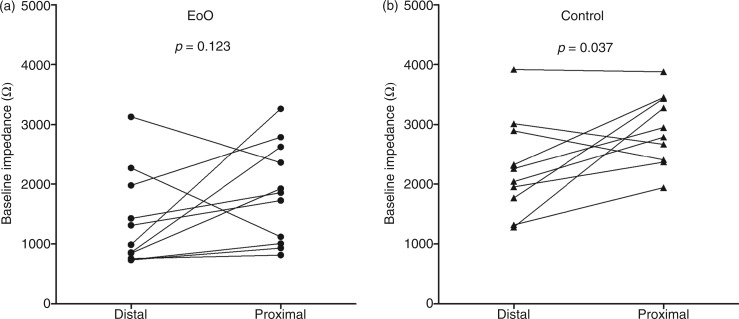
Baseline impedance levels (Figures 1 and 2) in EoO patients were markedly lower compared to healthy controls in the distal oesophagus (988 (757–1978) vs. 2259 (1767–2896) Ω, p = 0.015), mid-oesophagus (1420 (836–2164) vs. 2614 (2374–3879) Ω, p = 0.003), and proximal oesophagus (1856 (1006–2625) vs. 2868 (2397–3439) Ω, p = 0.005). In EoO patients, distal baseline impedance values were not significantly different from proximal values (p = 0.123; Figure 3). In controls, however, distal baseline impedance values were significantly lower than proximal values (p = 0.037).
Figure 1.
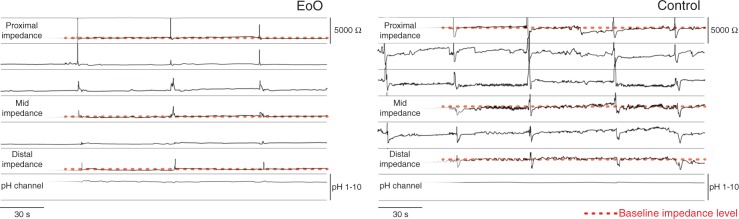
Example of a pH-impedance tracing in an EoO patient and a healthy control, showing baseline impedance levels in the proximal, mid, and distal oesophagus.
EoO, eosinophilic oesophagitis.
Figure 2.
Baseline impedance in the proximal (a), mid- (b), and distal (c) oesophagus in EoO patients vs. controls.
EoO, eosinophilic oesophagitis. Individual values and group medians are shown.
Figure 3.
Distal vs. proximal baseline impedance in EoO patients (a) and controls (b).
EoO, eosinophilic oesophagitis.
Correlation with acid exposure
In EoO patients, distal, mid-, and proximal oesophageal baseline impedance values were not correlated with acid exposure (r = 0.427, p = 0.190; r = 0.136, p = 0.689; and r = 0.027, p = 0.937, respectively). In controls, we also found no correlation between baseline impedance values and acid exposure (distal r = 0.214, p = 0.521; mid- r = −0.032, p = 0.926; and proximal r = −0.334, p = 0.345).
Overall, baseline impedance levels were not related to the number of reflux episodes. The total number of reflux episodes was not correlated with distal oesophageal baseline impedance in EoO patients (r = 0.164, p = 0.634). The number of mid-oesophageal reflux episodes was also not correlated to mid-oesophageal baseline impedance in EoO patients (r = 0.178, p = 0.595). Furthermore, no correlation was found between the number of proximal oesophageal reflux episodes and proximal baseline impedance in EoO patients (r = −0.273, p = 0.418).
Discussion
This is the first study in which oesophageal baseline impedance was evaluated in EoO patients. In this study we show that baseline impedance values are lower in EoO patients than in healthy controls with a similar oesophageal acid exposure. The observed differences in baseline impedance levels are present over the entire length of the oesophagus. No proximal-to-distal oesophageal gradient in impedance values was found in EoO patients, whereas such a gradient did occur in healthy controls.
It has been shown that with increasing degrees of oesophagitis (mucosal damage), GORD patients have an increasing number of reflux episodes and more acid exposure.20 Furthermore in GORD patients, baseline impedance is correlated with oesophageal acid exposure and PPI treatment increases baseline impedance.13,14 In-vitro animal data have shown that baseline impedance is correlated with the transepithelial resistance of the oesophageal epithelium, which is a marker of oesophageal epithelial integrity.15 These studies have led to the consensus that oesophageal acid exposure decreases baseline impedance and that baseline impedance is a marker of oesophageal mucosal integrity.13,16,17
In some patients with typical symptoms and signs of EoO, PPI seems to reduce inflammation en symptoms, and it partially restores the mucosal integrity.21 These patients are now diagnosed with proton pump inhibitor-responsive oesophageal eosinophilia (PPI-ROE), although differences between PPI-ROE patients and EoO patients are currently unclear.9 Perhaps in PPI-ROE patients, gastro-oesophageal reflux does play a role, whereas in EoO this is not the case. Furthermore, recent papers have suggested that PPIs may have anti-inflammatory effects besides their acid-suppressive effects.22,23 Response to PPI therefore does not automatically translate to presence of gastro-oesophageal reflux.
In atopic disease (e.g. asthma and atopic dermatitis), an impaired epithelial barrier function has been described.24,25 As in asthma and atopic dermatitis, the filaggrin gene is also a susceptibility gene in EoO, supporting the idea that impaired epithelial barrier function could play a fundamental role in EoO.26–28 In fact, the oesophageal mucosa of EoO patients is more permeable to molecules with the size of food allergens.21 In theory, this could enable passage of allergens through the mucosa which may cause immune activation. On the other hand, it could also very well be a result of the present T-lymphocytes and degranulating eosinophils and mast cells, causing epithelial remodelling and impaired mucosal integrity.2 It is currently unclear whether the oesophageal mucosal integrity is impaired due to a primary increased permeability of the mucosa or due to the allergic reaction. Although our study did not aim to answer this question, we speculate that in EoO the integrity changes may be caused by the allergic inflammation with its degranulating eosinophils and mast cells. In this study, we show that it is unlikely that the oesophageal inflammation starts with gastro-oesophageal reflux causing damage to the mucosa; however, in some EoO patients, other factors such as a loss-of-function mutation in the filaggrin gene may also cause integrity changes, which may facilitate allergen permeation. Regardless of the order of events, decreased baseline impedance may reflect decreased mucosal integrity in EoO.
In theory, the differences in baseline impedance values between patients with EoO and healthy controls could be caused by increased oesophageal exposure to gastric content in patients with EoO. However, in general, EoO patients do not have more (weakly) acidic reflux episodes than controls, and in our study patients and controls were matched for acid exposure time. No correlation was found between baseline impedance and acid exposure. Furthermore, baseline impedance was not only decreased in the distal oesophagus, which is more exposed to acid, but also in the mid-oesophagus and the proximal oesophagus of EoO patients. Distal, mid-, and proximal oesophageal baseline impedance values were not correlated with the numbers of reflux episodes reaching these levels of the oesophagus. Together, our findings suggest that other factors than gastro-oesophageal reflux are more likely responsible for the decrease in baseline impedance in EoO.
Baseline impedance values should be carefully interpreted in EoO patients, since they may be influenced by other factors than oesophageal mucosal integrity alone. For instance, the presence of exudate may decrease baseline impedance values as well. It has also been suggested that baseline impedance could be decreased due to altered oesophageal motility. Obviously, oesophageal motility abnormalities have been described in EoO.29 A study in patients with ineffective oesophageal motility showed that baseline impedance levels in patients with GORD were lower than those measured in controls.30 In that study, however, no information was presented about oesophageal acid exposure and since impaired motility is associated with pathological acid exposure, the latter could be a more plausible explanation for the observed low impedance levels. Moreover, if ineffective oesophageal motility were present in our study patients, one would expect increased bolus clearance time and acid clearance time; however, both parameters were not increased in EoO patients in our study. Furthermore, food impaction can interfere with the pH-impedance measurement, as it causes stasis of liquid in the oesophagus, which decreases baseline impedance. To avoid misinterpretation of impedance values, symptoms of dysphagia and food impaction should be reported during the measurement and these periods should be excluded from the analysis. In our study, none of the patients reported food impaction during the pH-impedance measurement. The abovementioned limitations will not occur using Ussing chambers to measure the transepithelial resistance in vitro, since these experiments do not require contact of an electrode with the mucosa.31 However, baseline impedance values have been shown to strongly correlate with the transepithelial resistance measured in vitro, suggesting that they reflect oesophageal mucosal integrity quite well.15
EoO patients perceived more GORD-related symptoms than healthy controls, despite the fact that reflux parameters were not increased in EoO patients. The increased acid perception may therefore be a result of acid hypersensitivity in EoO patients, as described in a previous study.32 Since in that study acid hypersensitivity was not correlated to increased sensitization of the central nerve system, the authors suggested that acid hypersensitivity may be related to abnormalities in the oesophageal tissue itself (e.g. mucosal integrity changes). Acid hypersensitivity in EoO patients may thus reflect decreased mucosal integrity; this could explain the presence of typical GORD-related symptoms in EoO patients.
The observed decrease in baseline impedance values may have clinical implications in the future. Baseline impedance could be a novel follow-up marker of disease activity in EoO patients. Currently, follow up of EoO patients requires frequent endoscopic evaluation, which is a costly and more invasive procedure. Further research evaluating the correlation between baseline impedance and endoscopic and histopathological signs of EoO is needed before baseline impedance can be used as a follow-up marker.
In conclusion, distal, mid-, and proximal oesophageal baseline impedance values are decreased in EoO patients and these baseline impedance values in EoO are not correlated to acid exposure. Our findings suggest that the decrease in impedance could be caused by other factors, such as impaired mucosal integrity.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. AJB is supported by the Netherlands Organisation for Scientific Research (NWO).
Conflict of interest
AJB has received research funding from AstraZeneca, Endostim, MMS, and Shire and has received speaker fees from MMS and Shire. The other authors declare that there is no conflict of interest.
References
- 1.Van Rhijn BD, Verheij J, Smout AJ, et al. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil 2013; 25: 47–52 [DOI] [PubMed] [Google Scholar]
- 2.Debrosse CW, Rothenberg ME. Allergy and eosinophil-associated gastrointestinal disorders (EGID). Curr Opin Immunol 2008; 20: 703–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straumann A, Bauer M, Fischer B, et al. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol 2001; 108: 954–961 [DOI] [PubMed] [Google Scholar]
- 4.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128: 3–20 [DOI] [PubMed] [Google Scholar]
- 5.Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol 2007; 102: 1301–1306 [DOI] [PubMed] [Google Scholar]
- 6.Hirano I. Eosinophilic esophagitis and gastroesophageal reflux disease: there and back again. Clin Gastroenterol Hepatol 2011; 9: 99–101 [DOI] [PubMed] [Google Scholar]
- 7.Rosen R, Furuta G, Fritz J, et al. Role of acid and nonacid reflux in children with eosinophilic esophagitis compared with patients with gastroesophageal reflux and control patients. J Pediatr Gastroenterol Nutr 2008; 46: 520–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalby K, Nielsen RG, Kruse-Andersen S, et al. Gastroesophageal reflux disease and eosinophilic esophagitis in infants and children. A study of esophageal pH, multiple intraluminal impedance and endoscopic ultrasound. Scand J Gastroenterol 2010; 45: 1029–1035 [DOI] [PubMed] [Google Scholar]
- 9.Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9: 110–117 [DOI] [PubMed] [Google Scholar]
- 10.Rea F, Caldaro T, Tambucci R, et al. Eosinophilic esophagitis: is it also a surgical disease? J Pediatr Surg 2013; 48: 304–308 [DOI] [PubMed] [Google Scholar]
- 11.Bredenoord AJ, Tutuian R, Smout AJ, et al. Technology review: esophageal impedance monitoring. Am J Gastroenterol 2007; 102: 187–194 [DOI] [PubMed] [Google Scholar]
- 12.Kessing BF, Bredenoord AJ, Smout AJ. Erroneous diagnosis of gastroesophageal reflux disease in achalasia. Clin Gastroenterol Hepatol 2011; 9: 1020–1024 [DOI] [PubMed] [Google Scholar]
- 13.Kessing BF, Bredenoord AJ, Weijenborg PW, et al. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol 2011; 106: 2093–2097 [DOI] [PubMed] [Google Scholar]
- 14.Loots CM, Wijnakker R, van Wijk MP, et al. Esophageal impedance baselines in infants before and after placebo and proton pump inhibitor therapy. Neurogastroenterol Motil 2012; 24: 758–752 [DOI] [PubMed] [Google Scholar]
- 15.Farre R, Blondeau K, Clement D, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut 2011; 60: 885–892 [DOI] [PubMed] [Google Scholar]
- 16. Woodland P, Al-Zinaty M, Yazaki E, et al. In vivo evaluation of acid-induced changes in oesophageal mucosa integrity and sensitivity in non-erosive reflux disease. Gut 2012; Epub ahead of print 21 June 2012. DOI:10.1136/gutjnl-2012-302645. [DOI] [PubMed]
- 17.Loots CM, van Wijk MP, Smits MJ, et al. Measurement of mucosal conductivity by MII is a potential marker of mucosal integrity restored in infants on acid-suppression therapy. J Pediatr Gastroenterol Nutr 2011; 53: 120–123 [DOI] [PubMed] [Google Scholar]
- 18.Shaw MJ, Talley NJ, Beebe TJ, et al. Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol 2001; 96: 52–57 [DOI] [PubMed] [Google Scholar]
- 19.Sifrim D, Castell D, Dent J, et al. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut 2004; 53: 1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bredenoord AJ, Hemmink GJ, Smout AJ. Relationship between gastro-oesophageal reflux pattern and severity of mucosal damage. Neurogastroenterol Motil 2009; 21: 807–812 [DOI] [PubMed] [Google Scholar]
- 21.Van Rhijn BD, Weijenborg PW, Verheij J, et al. Acid suppression restores impaired esophageal mucosal integrity in patients with esophageal eosinophilia. Gastroenterology 2013; 144: S155–S155 [abstract] [Google Scholar]
- 22.Cheng E, Zhang X, Huo X, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut 2013; 62: 824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Cheng E, Huo X, et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One 2012; 7: e50037–e50037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Benedetto A, Rafaels NM, McGirt LY, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol 2011; 127: 773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev 2011; 242: 205–219 [DOI] [PubMed] [Google Scholar]
- 26.Blanchard C, Stucke EM, Burwinkel K, et al. Coordinate interaction between IL–13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol 2010; 184: 4033–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol 2004; 4: 978–988 [DOI] [PubMed] [Google Scholar]
- 28.Morar N, Cookson WO, Harper JI, et al. Filaggrin mutations in children with severe atopic dermatitis. J Invest Dermatol 2007; 127: 1667–1672 [DOI] [PubMed] [Google Scholar]
- 29.Roman S, Hirano I, Kwiatek MA, et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol Motil 2011; 23: 208–214, e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blonski W, Hila A, Vela MF, et al. An analysis of distal esophageal impedance in individuals with and without esophageal motility abnormalities. J Clin Gastroenterol 2008; 42: 776–781 [DOI] [PubMed] [Google Scholar]
- 31.Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 2009; 296: G1151–G1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krarup AL, Villadsen GE, Mejlgaard E, et al. Acid hypersensitivity in patients with eosinophilic oesophagitis. Scand J Gastroenterol 2010; 45: 273–281 [DOI] [PubMed] [Google Scholar]